MAGNETIC RESONANCE IMAGING FINDINGS IN HORSES WITH SEPTIC ARTHRITIS
Abstract
Fourteen horses with septic arthritis underwent high-field (1.5 T) magnetic resonance imaging (MRI). Septic arthritis was diagnosed based on results from historical and clinical findings, synovial fluid analyses and culture, and radiographic, ultrasonographic, arthroscopic, and histopathologic findings. MR findings included diffuse hyperintensity within bone and extracapsular tissue on fat-suppressed images in 14/14 horses (100%), joint effusion, synovial proliferation, and capsular thickening in 13/14 horses (93%), bone sclerosis in 11/14 horses (79%), and evidence of cartilage and subchondral bone damage in 8/14 horses (57%). Intravenous gadolinium was administered to five of the 14 horses and fibrin deposition was noted in all horses. Other findings after gadolinium administration included synovial enhancement in 4/5 (80%) horses, and bone enhancement in 1/5 (20%) horses. The MR findings of septic arthritis in horses were consistent with those reported in people. MRI may allow earlier and more accurate diagnosis of septic arthritis in horses as compared with other imaging modalities, especially when the clinical diagnosis is challenging. It also provides additional information not afforded by other methods that may influence and enhance treatment.
Introduction
Septic arthritis in the horse can have life-threatening consequences. In foals, mortality and morbidity rates of 5.2% and 27.4%, respectively, have been reported. These rates are lower in mature horses and the prognosis for survival is good if prompt diagnosis and treatment occurs.1–4 Equine septic arthritis in foals usually arises hematogenously.2,5 Infection occurs in the first 30 days of life and is often due to failure of passive transfer of immunoglobulins.1,3,4,6 In mature horses septic arthritis results typically from trauma, intraarticular injections, or postoperatively; infection may also be idiopathic.4,5,7 Standard bred horses and horses receiving an intraarticular injection are at a higher risk for development of septic arthritis.6,8
Septic arthritis is often diagnosed from historical and physical findings, and synovial fluid analysis. Radiographs should be performed in all horses with suspected septic arthritis.4 Ultrasonography allows a more detailed evaluation of a periarticular wound, joint effusion, possible foreign bodies, and synovial inflammation. Nuclear scintigraphy can assist in the differentiation of infected vs. aseptic osteochondral diseases but it has poor specificity.4,9,10 Computed tomography is excellent for delineating osteomyelitis with bone destruction and sequestration but lacks soft tissue contrast resolution, which is helpful for definition of soft tissue and changes, such as inflammation and hemorrhage.11
Magnetic resonance imaging (MRI) is used most often in horses with lameness that cannot be diagnosed by other imaging techniques.12–15 In people, MRI is an important tool for evaluation of musculoskeletal infectious diseases.11,16–23 It is sensitive for early detection of bone marrow abnormalities and fluid collections. Its main strength is the early and accurate determination of osteochondral damage and concurrent soft tissue injury.19,20,21,24 MRI is the gold standard for the diagnosis of human septic arthritis, acute osteomyelitis, and soft tissue infection.11,19–21,25,26
Understanding the MRI features of equine septic arthritis will lead to a better understanding of the disease in horses and allow for more accurate and prompt diagnosis as compared with other imaging modalities.19,20,24,25,27 Our goal was to describe the MRI characteristics of equine septic arthritis.
Materials and Methods
Fourteen horses with confirmed septic arthritis that underwent MRI at 1.5 T between December 2005 and March 2009 were evaluated. Horses were examined and images acquired at one of three equine referral hospitals. Age ranged from 2 months to 11 years of age (median 3 years). Breeds were six Thoroughbreds, five American Quarterhorses, two Standardbreds, and one Warmblood. There were six geldings, two colts, two stallions, two fillies, and two mares. Affected joints included four metacarpo/metatarsophalangeal joints, three distal interphalangeal joints, three tarsocrural joints, two proximal interphalangeal joints, one tarsometatarsal joint, and one coxofemoral joint.
Diagnosis of septic arthritis was based on historical and clinical findings as well as the results from synovial fluid cytologic analyses in horses where synovial fluid was available. Synovial fluid evidence of infection was a total white blood cell count >30,000 cells/μl or a differential with >80% neutrophils.4 Synovial fluid aspiration was attempted in all horses. If synovial fluid could not be obtained, radiographic, ultrasonographic, arthroscopic, and histopathologic findings, as well as response to antibiotic therapy were evaluated and a diagnosis of septic arthritis was made if these findings were consistent with synovial sepsis or the horse responded successfully to antibiotic therapy.28,29
Radiographs were made of the affected joint in all 14 horses. Ultrasonography was performed in eight horses and arthroscopy in seven horses. Three horses were euthanized and histopathologic results were available in all three.
All MR examinations were performed in a 1.5 T magnet1, 1 with the horses under general anesthesia and in either lateral or dorsal recumbency. Images were obtained using a dedicated transmit-receive extremity coil (FOV=12–17 cm) or a flexible body array coil (FOV=13–22 cm), except for the pelvic examination where a spine and body matrix coil was used (FOV=28–35 cm). Matrix sizes used were 384 × 288, 320 × 320, 256 × 256, and 256 × 182. Section thickness varied from 4 to 1.5 mm. All limbs were imaged in transverse, sagittal, and dorsal planes using proton density (PDw) (TR 3000–5500/TE 12–42), T2-weighted fast spin-echo (T2w) (TR 4500–6300/TE 90–115), and short tau inversion recovery (STIR) (TR 3800–9400/TE 28–70) sequences. Some horses were also imaged with proton density with fat-saturation (PDw-FS) (TR 2800–5300/TE 36–54), 3-D T1w volumetric interpolated breath-hold examination (VIBE) (TR 8.6–9.5/TE 3.4–3.7), and T1-weighted (T1w) (TR 670–920/TE 11–12) sequences before and after intravenous gadolinium administration (T1w-GAD) (TR 670–920/TE 11–12). For T1w-GAD studies, either 20 ml (5,740 mg) or 30 ml (8610 mg) of gadodiamide (Omniscan)‡ was given intravenously and T1w sequences were acquired 5 min following administration.
MR images for each horse were evaluated by two board certified equine veterinary surgeons and one board certified radiologist. All reviewers were experienced in equine musculoskeletal MR image interpretation. All limbs were evaluated for MR findings consistent with septic arthritis including synovial effusion, synovial proliferation, joint capsule thickening, cartilaginous defects, subchondral bone hyperintensity on fat-suppressed images, soft tissue swelling, abnormal or loss of subchondral bone, and fibrin deposition within the joint.
Results
Arthrocentesis was attempted in all horses, with successful synovial fluid retrieval in 10 of 14. Of these 10 samples, findings were diagnostic of septic arthritis in eight horses. Six horses had synovial fluid white blood cell counts >30,000 cells/μl. In the remaining four samples with <30,000 cells/μl, two samples had >80% neutrophils on cytological differential. Two of 10 horses had inconclusive synovial fluid analysis.
Septic arthritis was diagnosed based on clinical findings in all horses. Clinical findings were severe joint swelling, heat, and pain on flexion (12/14 or 86%), severe lameness (4 or 5 out of 5 on AAEP Lameness scale) without evidence of a fracture (11/14 or 79%), wounds with communication to a joint (3/14 or 21%), and hematogenous spread as a result of bacteremia (1/14 or 8.0%).30 All horses in this study met the inclusion criteria based on clinical findings or synovial fluid analysis.
Four of the 14 horses had radiographic signs suggestive of septic arthritis, and one horse had a fistulogram performed showing communication with a joint. Seven of 14 horses had ultrasonographic signs of effusion with severe synovial thickening. Fibrin within the synovial fluid was present in four horses. All seven horses undergoing arthroscopy had findings consistent with septic arthritis. Severe synovial thickening, fibrin deposition, and cartilage damage were the most common arthroscopic findings.
The most common MR findings were extracapsular and bone hyperintensity in 14 of 14 horses (100%) on fat-suppressed images (1, 2). The hyperintensity within the bone adjacent to the affected joint was diffuse and mottled in appearance in STIR and PDw-FS images. Joint effusion, marked synovial proliferation, and capsular thickening was noted in 13 of 14 horses (93%) (1, 2). With synovial proliferation, the affected joint had moderate to marked intermediate to low-signal tissue within or surrounding high-signal joint fluid on T2w and fat-suppressed images. Low-signal intensity within subchondral bone, consistent with bone sclerosis, was identified on T2w and PDw images in 11 of 14 horses (79%) (Fig. 3). Eight of 14 horses (57%) had marked subchondral bone damage and loss and bone sequestration (4 of 14 horses), which was seen as low-signal intensity focal areas surrounded by high-signal intensity on T2w, PDw, PDw-FS, and STIR images (2, 4, 5). These findings were indicative of subchondral osteomyelitis.
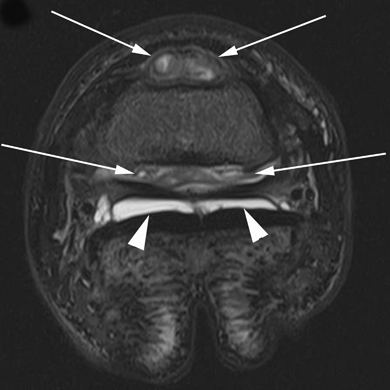
Transverse short tau inversion recovery (STIR) image of the right front distal interphalangeal joint. There is severe synovial effusion and synovial proliferation (white arrows) in the dorsal and palmar pouches of the joint. In addition, there is diffuse high signal throughout the middle phalanx. The right collateral sesamoidean ligament separates the inhomogenous intermediate septic joint fluid and severe synovial proliferation (white arrows) within the palmar pouch of the distal interphalangeal joint from the nonseptic homogenous high-signal joint fluid without synovial proliferation within the navicular bursa (white arrowheads).
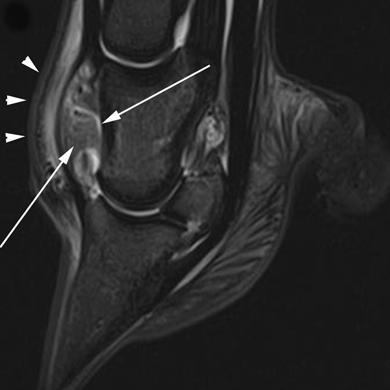
Sagittal short tau inversion recovery (STIR) image of a horse with septic arthritis of the right distal interphalangeal joint. There is severe synovial effusion and synovial proliferation (small white arrows) in the dorsal and palmar pouches of the distal interphalangeal joint. There is also extracapsular hyperintensity (white arrowheads). There is diffuse hyperintensity throughout the medullary region of the proximal and distal phalanx as well as the navicular bone.
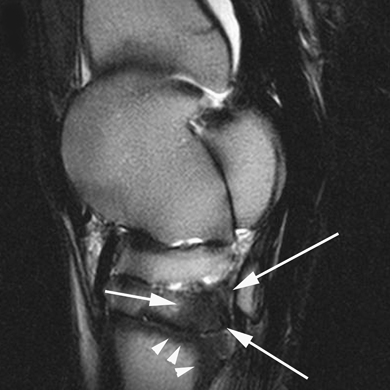
Sagittal T2-weighted image of the tarsus of a horse with septic arthritis of the left tarsometatarsal joint. There is low signal of the plantar aspect of the third tarsal bone (white arrows) and proximal aspect of the third metatarsal bone (white arrowheads).

A dorsal proton-density fat-suppressed (PDw-FS) image of a horse with septic arthritis of the right front distal interphalangeal joint. A bone sequestrum is present in the distal articular surface of the navicular bone (white arrows) that appears as a low-signal core lesion surrounded by a peripheral high signal.
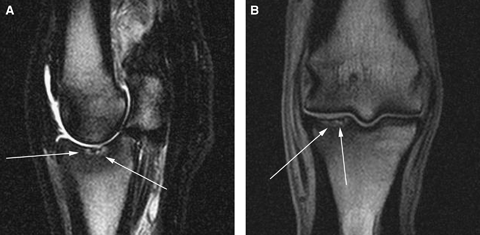
Sagittal T2w image (A) and dorsal PD image (B) of a horse with full thickness cartilage damage (white arrows) in the proximal aspect of the proximal phalanx. There is a focal irregularity in the cartilage as well as a heterogeneous signal within the articular surface. Subchondral bone loss is present below the articular surface and there is also diffuse low signal sclerotic bone surrounding the area of cartilage damage and subchondral bone loss.
Focal disruptions along the articular surface indicative of full thickness cartilage erosions were detected in 8 of 14 (57%) horses. Cartilage erosions were best seen on PDw and VIBE images. In all images, cartilage erosions were evident as an irregularity, cartilage thinning and/or heterogeneous signal within the articular surface (Fig. 5).
Marked contrast enhancement of vasculature and synovium was noted in four of the five cases receiving gadolinium (6, 7). Subchondral or bone marrow contrast enhancement was evident in one horse. Unenhanced low to intermediate signal tissue, indicative of fibrin within joint fluid, was present in all five horses given gadolinium (Fig. 6). MR findings for all cases are summarized (Table 1).

Transverse PD image of the tarsus of a horse with severe effusion and synovial proliferation in the plantar and dorsal pouches of the septic right tarsocrural joint. There is a focal area of low to intermediate signal in the plantarolateral aspect of the right tarsocrural joint (white arrows in A). Pregadolinium and postgadolinium T1w images of the same horse (B, C). There is marked synovial contrast enhancement along the plantarolateral and dorsal aspects of the right tarsocrural joint (white arrows in C). Gadolinium enhancement helps to decipher between vascular synovium (white arrows in C) and avascular fibrin deposition (white arrows in A).
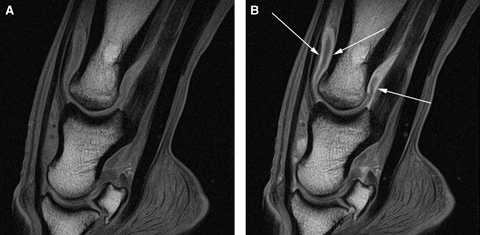
Sagittal pregadolinium (A) and postgadolinium (B) T1w images of a horse with a septic proximal interphalangeal joint. In (B) there is marked synovial enhancement throughout the dorsal and plantar pouches of the proximal interphalangeal joint (arrows).
| Extracapsular edema | High-signal bone | Effusion | Synovial proliferation | Capsular thickening | Subchondral bone loss | Sequestrum formation | Cartilage damage | Sclerosis | |
|---|---|---|---|---|---|---|---|---|---|
| Total # horses | 14 | 14 | 13 | 13 | 13 | 8 | 4 | 8 | 11 |
Discussion
The MRI characteristics of septic arthritis in the horses in this study are similar to those in people.11,20,27 Diffuse hyperintensity in bone and extracapsular tissue on fat-suppressed images, joint effusion, synovial proliferation, and capsular thickening were the most common findings. With gadolinium administration, marked synovial enhancement, synovial proliferation and thickening, and fibrin deposition were noted most frequently. One horse given gadolinium did not have evidence of synovial proliferation, likely because the tarsometatarsal and distal intertarsal joints typically contain a small amount of synovial fluid and the joint space is very small. In humans with septic arthritis, joint effusion and synovial proliferation was noted in only 70% and 22% of patients, respectively.27 Smaller joints were over represented, which may explain the lower incidence of synovial effusion in that study.27
Synovial fluid analysis was not available or conclusive in all horses, with eight of the 14 having septic arthritis based on white blood cell count and cytological analysis. Arthrocentesis can be unsuccessful if the inflamed joint has a large amount of synovial proliferation and fibrin accumulation, leading to the needle becoming blocked. Furthermore, smaller, low-motion joints such as the distal tarsal joints and proximal interphalangeal joint have less synovial fluid making arthrocentesis more difficult. In one study, bacteria were isolated from only 32% of synovial cultures from confirmed septic joints in horses.31 In our study, positive synovial fluid culture occurred in only four cases (27%). If synovial fluid is not obtainable or analysis is equivocal, diagnosis of septic arthritis must be based on historical and clinical findings along with results of diagnostic imaging. In a clinical setting many horses are evaluated with extremely early synovial sepsis, or chronic sepsis with a very low-grade infection. Either of these cases can have equivocal culture and cytologic results. The early diagnosis of sepsis, and possible nidus of infection followed by aggressive treatment is essential to returning horses to athletic function.
MRI can be used to aid in early diagnosis of septic arthritis when arthrocentesis is unsuccessful or equivocal based on identification of diffuse hyperintensity within bone and extracapsular soft tissue on fat-suppressed images, joint effusion, and synovial thickening on T2w images, and synovial enhancement and fibrin deposition on T1w-GAD images. At this point, MRI features that distinguish septic from nonseptic arthritis are not known. However, it is likely that diffuse hyperintensity in adjacent bone and extracapsular tissue are indicative of septic arthritis rather than nonseptic arthritis, especially in the absence of soft tissue injuries. MRI alone does not provide a definitive diagnosis and trauma, developmental osteochondosis, and ischemic necrosis are differential diagnoses that should be ruled-out before a diagnosis of septic arthritis.
Radiography, ultrasonography, and arthroscopy were often performed in the horses in this study. Radiographic findings ranged from subtle signs of periarticular osteophyte formation to severe degenerative articular changes and loss of bone. No horses had conclusive radiographic evidence of septic arthritis. In one study, >95% of radiographs of human infectious musculoskeletal diseases were unremarkable on presentation.32 Following infection, it may take 2 weeks or more for radiographic changes to develop because approximately 50% of bone mineral content needs to be affected before lysis is detectable radiographically.11 In contrast, MR findings of septic arthritis have been identified within 24 h of onset.4,16,19–21,24 With regard to sonography, while the findings in this study were consistent with septic arthritis, the diagnosis could not be made with ultrasonography alone.
Joint effusion and synovial proliferation were easily identified on T2w and STIR images, as high signal and low to intermediate signal, respectively. The signal from joint effusion in infected joints was lower than normal synovial fluid as a result of increased cells and protein.24 Diffuse hyperintensity within subchondral bone and bone marrow was best noted on STIR or PDw-FS images; this is often referred to as bone edema. Bone marrow lesions in MR images have been related to various histologic features, including necrosis, edema, inflammation, fibrosis, and hemorrhage.33 More accurately, it is a replacement of marrow fat cells with various forms of osseous/fibrous proliferative cells.24 High signal may occur from both inflammation and infection, but definitive osseous infection generally has a mottled, inhomogenous increase in signal intensity on STIR and PDw-FS images.24 We hypothesized that the 14 horses in this study with hyperintensity within bone on STIR and PDw-FS images resulted from inflammation alone extending beyond the sites of infection. However, overt osteomyelitis was present in the three horses examined histologically. Whether other horses with diffuse hyperintensity within bone on STIR and PDw-FS images had osteomyelitis is not known as they were not examined histologically.
Acute osteomyelitis is difficult to diagnose definitively based on MR findings alone. On the other hand, bone sequestration and chronic osteomyelitis appear to have somewhat specific MRI characteristics.23,34 Sequestered bone appears as an area of low-signal intensity surrounded by a peripheral area of high-signal intensity within effected bone on T2w, STIR, PDw, and PDw-FS images.34 It is described as an amorphous fragment with signal intensity similar to that of the cortical bone inside the area of active infection.34
Two MR findings described as being consistent with chronic osteomyelitis are the rim sign and the penumbra sign.18,23,34 The rim sign is a well-defined rim of low-signal intensity surrounding active disease on T1w, T2w, and PDw images, and it is highly specific to chronic osteomyelitis.34 In people, 93% of patients with chronic osteomyelitis had the rim sign while only 1 in 38 patients with acute osteomyelitis had this sign.34 The penumbra sign is a discreet zone of transition of relatively hyperintense signal between the intermediate to low-signal intensity abscess cavity and adjacent hypointense or sclerotic bone marrow on unenhanced T1w images.18,23 In people, the penumbra sign had a 96% specificity but only a 23% sensitivity for osteomyelitis.23 No horse in this study had a penumbra sign or a rim sign. T1w sequences were not acquired in all horses, and osteomyelitis was considered acute as a result of extension from the septic joint. Therefore, there may not have been adequate time for a rim or penumbra sign to develop.
As inflammatory changes are nonspecific in MRI, contrast enhancement has been shown to be beneficial in some cases of septic arthritis. In nonseptic inflamed and even normal joints, synovium, and vasculature will enhance after gadolinium administration. Contrast medium administration is important for depicting increased, extensive hyperemia, and vascular permeability in septic arthritis in comparison to normal nonseptic joints.35 In an early study in humans, the sensitivity and specificity of intravenous gadolinium-enhanced MRI with fat suppression for the detection of septic arthritis were 100% and 86%, respectively.36 On the other hand, this study concluded that contrast enhancement could not be reliably used to distinguish infectious from noninfectious inflammatory conditions.36 Also, when T1w-GAD images were compared with T1w images in humans with septic arthritis, there was an 88% sensitivity and 93% specificity compared with 79% sensitivity and 53% specificity for unenhanced images.37 A more recent study comparing pre- and postcontrast images resulted in a sensitivity of 50% and 67% and a specificity of 98% and 98%, respectively.38 Intravenous contrast can show areas of devitalized tissue within soft tissue and bone. What is of greatest interest to surgeons is not only the extent of infectious involvement, but also the presence or absence of necrotic tissue, and the basis of surgical treatment is removal of devitalized tissue.39 Contrast MRI is not required for an accurate diagnosis in all cases of septic arthritis. In human studies, gadolinium enhancement is most useful for evaluation of septic arthritis and soft tissue infection with abscess formation. However, gadolinium has not been shown to be useful in patients where precontrast images are normal.38,40 We found contrast-enhanced MRI to be beneficial in further characterizing abnormalities present on unenhanced MR images. We also found contrast-enhanced images to be beneficial in therapeutic and surgical planning.
There were no peracute cases of septic arthritis in this study. A few horses were imaged to find the cause of lameness with little to no suspicion of sepsis. In fact, a definitive diagnosis could have been made without MRI in all cases with conclusive synovial fluid analysis. In most horses, MRI was indicated to determine if there was an ongoing infection, a nidus of infection or if the ongoing lameness was due to chronic synovitis and osteoarthritis. The only indication for imaging in the peracute phase is when synovial fluid cannot be obtained or analysis is inconclusive.
This study aimed to describe MRI findings in horses with septic arthritis. It was a useful imaging modality in all of the horses in this study by providing detailed information in the disease process and guided medical and surgical therapy. It was especially helpful in horses with unsuccessful or equivocal arthrocentesis results.