LAPAROSCOPICALLY IMPLANTED TISSUE EXPANDER RADIOTHERAPY IN CANINE TRANSITIONAL CELL CARCINOMA
Abstract
Organ motion and injury to adjacent structures limit curative treatment of intraabdominal tumors with external beam radiotherapy. We evaluated the use of Laparoscopically Implanted Tissue Expander Radiotherapy (LITE-RT) to exclude critical structures during irradiation of the urinary bladder in two dogs with transitional cell carcinoma (TCC) using helical tomotherapy. Dogs had histologically confirmed bladder TCC with no metastasis. A custom-shaped tissue expander was placed between the colon and bladder laparoscopically in one dog and during laparotomy in the other. The prescribed radiation dose was 45 Gy to 98% volume of the bladder in 18 fractions of 2.5 Gy. Tumor response and normal tissue effects were monitored with cystoscopy and colonic biopsies before treatment and 3, 6, and 15 months after treatment. Based on treatment plans from inflated vs. deflated tissue expander CT images, there was a mean dose reduction to the colon of 53% and 31% for the two dogs. Interfractional target repositioning was possible by using volumetric megavoltage computed tomography helical tomotherapy. Both dogs had no clinical signs of chronic colitis but did experience mild cystitis during treatment. Tissue expanders became detached, requiring an additional surgery for reattachment, in both dogs. One dog developed a fibrous adhesion resulting in bladder rupture during inflation, which necessitated early device removal. One dog was euthanized for tumor-associated ureteral obstruction at 8 months while the other is alive at 21 months. We conclude that LITE-RT shows promise in treatment of canine bladder TCC due to lack of acute colitis and enteritis.
Introduction
Prognosis for canine transitional cell carcinoma (TCC) of the urinary bladder following treatment with radiation is guarded.1 Median survival following external beam and/or intraoperative radiation therapy (RT) ranges from 4 to 15 months.2–4 Single-dose intraoperative RT led to severe late radiation toxicity and euthanasia in 36% of patients.3 Fractionated RT in conjunction with chemotherapy was well tolerated with no overt radiation toxicity, but local tumor control was poor. However, the total radiation dose was only 34.5 Gy.4 Inability to limit dose to surrounding normal tissues with external beam RT may result in colitis and bowel perforation.5 Reducing the dose per fraction to minimize normal tissue toxicity results in the need to increase the number of fractions delivered, which can be problematic in older patients.
Interfractional variation in bladder size, and position of adjacent normal tissues, are other problems associated with bladder irradiation with external beam techniques. This leads to a large planning target volume (PTV). One way to reduce the size of the PTV is the use of a tissue expander, which provides increased separation between tumor and normal tissues. Inflated saline tissue expanders have been used to displace the colon and small bowel in human patients undergoing pelvic radiotherapy.6–8 More sophisticated, custom-shaped, tissue expanders are also available.9
Laparoscopically implanted tissue expander RT (LITE-RT) geometrically displaces surrounding normal organs from the target volume and isolates the target via inflation of a laparoscopically implanted, site-specific, custom-shaped tissue expander. Placement is through laparoscopic surgery to reduce surgical morbidity. The tissue expander is custom-shaped to the implant site and incorporates features to promote interfractional organ localization reproducibility. Specifically, relative to TCC of the bladder, the tissue expander is shaped to cradle and stabilize the bladder using lateral wings and suture tabs that attach to the prepubic tendon.
LITE-RT is composed of three elements: a custom-shaped tissue expander, laparoscopic surgery, and image-guided RT. These were previously developed specifically for treatment of canine TCC.9 The custom-shaped tissue expander was designed to provide colon–bladder separation and exclusion of small bowel during RT. The tissue expander was designed to promote accurate interfractional repositioning of the bladder despite daily inflation and deflation of the tissue expander. Suture tabs were attached to the tissue expander to provide fixation points to the abdominal wall. In addition, a laparoscopic placement protocol was developed.
Helical tomotherapy, an advanced form of image-guided, intensity-modulated RT (IG-IMRT), was used for radiation delivery due its integrated megavoltage CT imaging capabilities and highly conformal radiation dose distributions. The megavoltage CT acquired before delivery of each radiation fraction allows for three-dimensional verification of organ position after tissue expander inflation. Image verification is essential because inflation may displace and deform the target and surrounding tissues in all three dimensions. Combined use of the tissue expander and helical tomotherapy ensures target isolation and normal tissue displacement, thus maximizing dose delivery to target tissues and minimizing normal tissue dose.
Our aim was to report the clinical use of LITE-RT to treat spontaneously occurring canine bladder TCC. In preliminary work, we found a significant dose reduction to the small bowel and colon could be achieved with LITE-RT.9 We hypothesized that delivery of daily, fractionated radiotherapy to dogs with spontaneous bladder TCC using LITE-RT would result in reduced gastrointestinal morbidity. In the process, we sought to validate the accuracy of interfractional bladder repositioning using the custom-shaped tissue expander.
Materials and Methods
Two elderly neutered female dogs with confirmed bladder TCC underwent tumor evaluation via cystoscopy and ultrasound. Tumors were confined to the bladder trigone with no urethral or ureteral invasion. A 2.5 × 2.4-cm2 tumor surrounding the right ureter was present in one dog and a 5.5 × 5.5-cm2 tumor involving the majority of the ventral bladder wall and neck was present in the other.
Dogs were determined to be free of metastatic disease by abdominal ultrasound and thoracic radiography. Custom-shaped tissue expanders with 440 and 360-ml volumes* (Fig. 1) developed previously were used.9 Tissue expanders were placed between the urinary bladder and colon (Fig. 1) and secured to the prepubic tendon with full-thickness mattress sutures placed through suture tabs attached to the tissue expander. The injection port was passed through a subcutaneous tunnel and secured to the skin 5-mm lateral to the umbilicus. Laparoscopic placement was performed in one dog as described previously9 and via an open ventral-midline celiotomy in the other. Both dogs underwent a ventral midline cystopexy to further limit bladder motion.
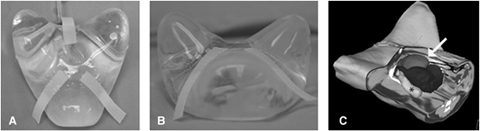
(A) Ventral view of the bladder specific, custom shaped tissue expander. (B) Cranial view of the tissue expander. (C) Three-dimensional rendering with a cutout depicting the location of the tissue expander in the abdomen. Bladder (medium gray, white arrow), tissue expander (dark gray), and colon (light gray, *) are shown.
Immediately after the tissue expander insertion procedure, dogs were placed in dorsal recumbency using a Vac-Lok™ positioning mattress,† with the pelvic limbs entering the CT gantry first. Kilovoltage CT scans were acquired with the custom-shaped tissue expander both inflated and deflated (Fig. 2). The tissue expander was inflated with a 15% concentration solution of saline and radio-opaque contrast medium, Hypaque®‡, to improve visualization on the subsequent megavoltage CT images. Radiation treatment plans were generated using the TomoTherapy treatment planning station (TPS)§ with contours imported from Pinnacle® TPS.10¶ Colon, spine, small bowel, bladder, and tissue expander were contoured. Tomotherapy treatment plans were generated on both the inflated and deflated kilovoltage CT image sets. The entire bladder was used as the target volume, and the treatment prescription was 45 Gy to 98% of the volume of the target in 18 fractions with a field width of 5.0 cm, pitch of 0.172, and modulation factor of 2.0.11 Treatment plans with the expander inflated were used for treatment delivery. Treatment plans with the expander deflated were used for dose reduction computation to the colon and small bowel as a result of tissue expander inflation. Treatment plans with the expander inflated and deflated were compared based on normalized prescription doses. The D5% (highest dose to 5% of the structure volume), was also recorded and provided a more robust measure of the high dose to structures, for it served to be more reproducible among plans. The utilization of a percentage volume to dictate the high dose eliminates the misrepresentation of the maximum dose to a given structure by excluding random single voxels, which may receive relatively high doses—a common phenomenon with IMRT. Hence, quoting the highest dose received by a small percentage of the structure serves to be more reproducible and may be better indicative of clinical outcome. Before delivery of each fraction, dogs were placed in the immobilization mattress. The tissue expander was inflated with the 15% saline/contrast medium solution to the same volume as used for the planning kilovoltage CT. A megavoltage CT of the pelvic region was acquired, and images were fused to the planning kilovoltage CT to ensure accurate organ positioning and avoidance of colon and small bowel. After radiation delivery, the tissue expander was deflated to a residual volume of 60 ml of the solution mixture. Total treatment time was typically 30 min. The megavoltage CT acquisition and fusion registration required 8–10 min while the radiation delivery was performed in 6–8 min. In addition to RT, both dogs received Piroxicam (0.3 mg/kg once daily).
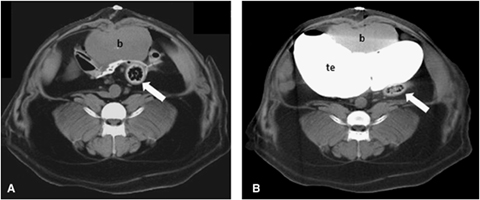
(A) Transverse computed tomography image of caudal abdomen with the deflated expander, note the bladder (b) overlying the colon (arrowhead). (B) Tissue expander (te) inflated with 306 ml of 0.9% saline and 54 ml of Hypaque® (360 ml total) displacing the bladder (b) from the colon (arrowhead).
Tumor response was monitored with cystoscopy. Colon biopsies were obtained before treatment, immediately posttreatment and at 3, 6, and 15 months after treatment. Cystoscopy was performed to assess tumor response to therapy and to obtain sample biopsies. Biopsies of the ventral colonic wall were obtained 9 cm orad of the anus with digital guidance. Toxicity was assessed by recording clinical observations throughout the course of RT and by clinical information provided by the owner at subsequent visits. Clinically, acute and late effects of radiation injury were graded using Veterinary Radiation Therapy Oncology Group (VRTOG) guidelines.12
Results
Both dogs received 18 fractions with minimal clinical evidence of radiation side effects. Tissue expander inflation displaced the colon away from the urinary bladder at the level of the mid bladder by 2.3 and 3.2 cm.
The radiation dose administered to the bladder, colon, and small bowel for the inflated and deflated treatment plans is summarized in Table 1. Minimal deviations in maximum and mean dose between inflated and deflated plans for the bladder indicate near equivalent target coverage. A maximum and mean dose reduction to the colon of 2% and 49%, and 53% and 31% were noted. A reduction in D5% of 13% and 38% for the two dogs was calculated. Similarly, significant dose reductions were achieved for the small bowel due to its cranial displacement with tissue expander inflation. A maximum and mean dose reduction to the small bowel of 74% and 100%, and 89% and 100% were noted for the dogs. For one dog the D5% dose to small bowel was reduced by 83% and for the other dog the entire small bowel was excluded from the radiation field.
| Structure | Dog I | Dog II | ||||
|---|---|---|---|---|---|---|
| Max.(Gy) | D5%(Gy) | Mean(Gy) | Max.(Gy) | D5%(Gy) | Mean(Gy) | |
| Deflated | ||||||
| Bladder | 47.6 | 46.3 | 46.8 | 45.5 | ||
| Colon | 39.5 | 23.9 | 12.9 | 41.6 | 27.6 | 9.4 |
| Small bowel | 45.4 | 31.5 | 8.4 | 43.8 | 26.5 | 5.9 |
| Inflated | ||||||
| Bladder | 48.0 | 46.7 | 46.8 | 45.4 | ||
| Colon | 38.7 | 20.9 | 6.1 | 21.4 | 17.1 | 6.5 |
| Small bowel | 11.7 | 5.4 | 0.9 | 0.0 | 0.0 | 0.0 |
- D5%, highest dose to 5% of the structure volume; max., maximum dose delivered to structure.
No signs of severe gastrointestinal disease were exhibited by either dog. Histopathologically, there was mild colitis in both pre- and poststudy samples, with no evidence of radiation-induced colitis. Pollakiuria and incontinence were mild in both dogs before treatment, worsened during treatment, and improved within 2 weeks after completion of treatment. One dog regained continence following treatment but developed pollakiuria at 12 months. The other dog had persistent mild incontinence and pollakiuria after treatment, and this became severe and progressed to stranguria at 6 months after treatment.
Tumor size reduction occurred in both dogs immediately after completion of radiotherapy (Fig. 3). A complete response was observed in one dog 3 months after treatment, but local extension of the tumor into the urethra was detected at this time. Progression of the urethral tumor was observed 6 months after treatment, but the primary tumor did not appear to be present. Tumor progression was observed on cystoscopy 15 months after treatment, and the tumor appeared to have spread to the bladder neck. This dog is alive 21 months after treatment with urinary continence but moderate pollakiuria. There are no signs of gastrointestinal toxicity.
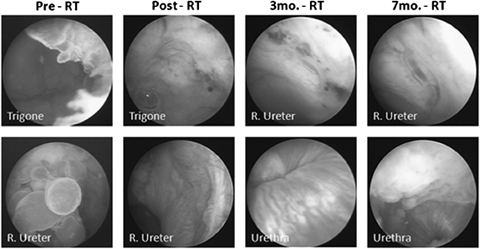
Cystoscopic images made at various time periods during treatment in one dog. Tumor was located at the bladder–urethra junction and at the right uretero-vesicle junction. Response of the primary tumor led to complete disappearance, but with tumor spread to the urethra can be seen in follow-up images (3 and 7 months—post-RT).
Stable disease was present in the other dog, but stenosis of the left ureterovesicular junction was noted on cystoscopic exam. Eight months after treatment, bilateral ureteral obstruction due to tumor invasion of the vesicoureteral junctions resulted in euthanasia of this dog. Severe bladder thickening, and bilateral hydroureter with hydronephrosis were found at necropsy. Severe fibrosis and tumor invasion of the bladder wall were observed histologically, but the colon appeared normal.
The tissue expander detached as a result of suture pulling out of the prepubic tendon in both dogs. This required surgical reattachment. A local reaction entailing fibrosing steatitis was observed around the tissue expander in one dog. This necessitated tissue expander removal during the course of treatment due to bladder perforation. This appeared to arise from distention of the tissue expander, causing a tear in the bladder near an adhesion between the bladder and dorsal–lateral body wall. The tissue expander was removed and the bladder was repaired. The remaining seven fractions were adjusted to 2.8 Gy/Fx (from 2.5 Gy/Fx) to account for treatment delay and for potential tumor proliferation during the treatment delay, leading to a total bladder dose of 47.1 Gy.5 Carboplatin chemotherapy was administered to this dog to treat potential tumor seeding of the abdomen due to bladder rupture. The chemotherapy protocol in this dog was 210 mg of carboplatin given as a single dose intravenously every 3 weeks for a total of four treatments. Bacterial cystitis accompanied by pyelonephritis occurred 14 days postchemotherapy requiring hospitalization.
Discussion
Although only two dogs were treated, the absence of chronic colitis represents an improvement over previous reports of RT of bladder TCC in dogs.2,3,5 However, the poor tumor response indicates a need for protocol improvement. Improved tumor control might be gained by surgical tumor cytoreduction before RT or by use of combined RT and chemotherapy. In humans, using tri-modal bladder sparing therapy consisting of transurethral tumor debridement, fractionated external beam RT, and chemotherapy, survival time approached that of radical cystectomy with the benefit of bladder preservation.13,14
While radical cystectomy with creation of an orthotopic bladder remains the gold standard of treatment for muscle invasive bladder cancer in humans,15 this entails multiple daily catheterizations and surgical complications that most animal owners are unwilling to accept. Radical cystectomy with ureterocolonic anastamosis in 10 dogs with bladder TCC lead to a survival time of 1–5 months with tumor metastasis present in five of seven dogs at postmortem examination.16 Tumor recurrence or development of distant metastasis is common with surgical excision even when a complete tumor excision is achieved.1 Bladder sparing strategies may be the optimal treatment strategy in dogs to maintain continence and bladder function, but additional treatment such as radiation and chemotherapy may be necessary to achieve extended local and distant tumor control.
Optimal total doses and fractionation of RT to effectively treat canine bladder TCC while preserving bladder function are unknown. Current data are skewed by an inability to precisely target the bladder with external beam radiotherapy or by complications induced by pelvic irradiation. Delivery of 34.5 Gy in 6 weekly fractions of 5.75 Gy using cobalt photons resulted in no complications.4 With a total dose of 43–54 Gy, however, there were colonic side effects with higher dose per fraction schedules (2.7 vs. 3.3 Gy per fraction).5 In our study, we used a relatively low total dose and dose per fraction because this was a pilot study. Future dose escalation in dogs may be possible based on current human protocols where 60 Gy is delivered in 1.8–2 Gy fractions.17 However, prolonged fractionation schedules are sometimes difficult to implement in animals because of cost and the necessity of anesthesia for each fraction. The LITE-RT technique has the potential to allow delivery of higher total doses without adverse colonic side effects.
The efficacy of using a tissue expander in conjunction with IMRT is unknown. It is possible that reduction of dose to the small bowel and colon can be obtained with IMRT alone.8 Further normal tissue dose reduction may be possible with use of a tissue expander in conjunction with IMRT. Furthermore, use of the tissue expander permits a larger PTV to account for bladder expansion and motion.9 This was evident in the current study as the tissue expander allowed maintenance of a large treatment field even with a nonexpanded bladder.
One aspect of LITE-RT addressed in the current study was the accuracy of interfractional bladder reproducibility. Figure 4 illustrates a representative transverse slice of the planning kilovoltage CT along with pretreatment megavoltage CT images for the first, third, and seventh fractions. Based on the correlation between the kilovoltage CT and fractions 1 and 3, interfractional reproducibility within 5 mm was possible despite daily inflation and deflation of the tissue expander. Between the fifth and seventh fraction, the tissue expander became detached. This was noted by the large deviation between the pretreatment megavoltage CT and planning kilovoltage CT. The ability to detect this deviation reinforces the need for a form of volumetric image guidance before delivery of each fraction when using a tissue expander.
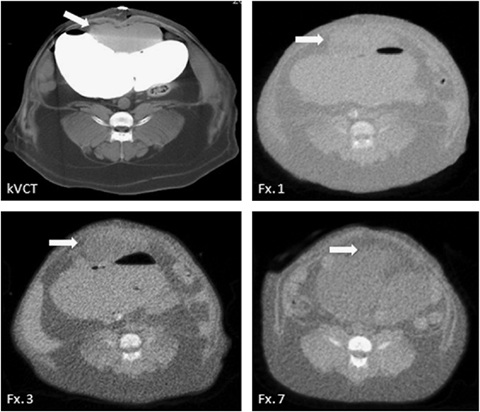
Pretreatment tomotherapy megavoltage computed tomography (CT) images of the abdomen with tissue expander inflation for various fractions. White arrows indicate the bladder. Adequate interfractional localization was possible during the first five fractions (see kilovoltage CT, Fx. 1, and Fx. 3). Between Fx. 5 and Fx. 7, the tissue expander detached from sutures and migrated cranially in the abdominal cavity (see Fx. 7).
Morbidity associated with the tissue expander must be compared with its overall benefit. Detachment of the expander was originally thought to be more likely when placed laparoscopically. However, in our study detachment occurred in the dog where open surgical placement of the expander was performed. The use of a mesh reinforced suture tab and application of a second prepubic tendon suture into each suture tab may reduce the chance for detachment. In humans, other complications noted when using a tissue expander included small bowel rupture, infection, protrusion through the surgical incision, deflation and paralytic ileus.18 A fibrous connective tissue capsule formed around an abdominal expander in 75% of dogs after 4 months.19 In humans, adhesion of small bowel to the expander capsule, after 3.5 months of implantation, resulted in perforation during removal of the expander.18 Adhesion formation and bladder rupture observed in one of our dogs may represent a similar complication.
The only late radiation effect was bladder fibrosis in one dog. While this may have been due to radiation, it may have also been due to tumor growth and invasion. Peritumoral desmoplastic reactions have been found in 38% of histologically classified canine bladder TCC.20 Bladder fibrosis in this dog may also have occurred in response to the presence of the expander, but the appearance of the tissues was consistent across the entire bladder, and specific changes were not observed in areas of contact with the expander. Regardless of cause, bladder fibrosis is an important finding because it directly affects bladder function.
Footnotes
ACKNOWLEDGMENTS
The authors gratefully acknowledge the support of the U.W. Biotechnology Training Program from the NIH National Cancer Institute Grant 5 T32 GM08349 and a grant from the University of Wisconsin Paul P. Carbone Comprehensive Cancer Center.