CHARACTERIZATION OF NORMAL FELINE RENAL VASCULAR ANATOMY WITH DUAL-PHASE CT ANGIOGRAPHY
This paper was presented at the joint annual scientific meeting of the American College of Veterinary Radiology and International Veterinary Radiology Association, August 7–11, 2006.
Abstract
Helical computed tomography angiography was used to evaluate the renal vascular anatomy of potential feline renal donors. One hundred and fourteen computed tomography angiograms were reviewed. The vessels were characterized as single without bifurcation, single with bifurcation, double, or triple. Multiplicity was most commonly seen for the right renal vein (45/114 vs. 3/114 multiple left renal veins, 0/114 multiple right renal arteries, and 8/114 multiple left renal arteries). The right kidney was 13.3 times more likely than the left to have multiple renal veins. Additional vascular variants included double caudal vena cava and an accessory renal artery. For the left kidney, surgery and computed tomography angiography findings were in agreement in 92% of 74 cats. For the right kidney, surgery and computed tomography angiography findings were in agreement in 6/6 cats. Our findings of renal vascular anatomy variations in cats were similar to previous reports in humans. Identifying and recognizing the pattern of distribution of these vessels is important when performing renal transplantation.
Introduction
In cats, renal transplantation is one alternative for animals with end-stage renal disease.1,2 Preoperative assessment of renal vascular and urogenital anatomy in donors is important to determine the presence or absence of renal arterial and venous variants, abnormalities of the renal parenchyma and collecting system, renal calculi, and other renal and extra-renal abnormalities. This information is used to help plan the surgical procedure and prevent complications.3,4 In humans, the existence of multiple renal arteries necessitates multiple renal artery anastomoses, thereby increasing anesthesia time, ischemia time, and operating time.5 Multiple renal anastomoses have also been associated with posttransplant graft thrombosis, renal stenosis, and renovascular hypertension.6 Having to perform multiple anastomoses also puts the donor at increased risk for bleeding and increases the need for blood transfusion. In addition, longer surgery time may result in more graft injury.7 These graft injuries might have consequences in the recipient such as increased inflammation, increased graft immunogenicity, more rejection episodes, and premature graft loss.8 Because of all these reasons, the vascular anatomy of the donor needs to be evaluated and characterized before surgery.
In humans, multislice computed tomography (CT) angiography is the most accurate method for preoperative vascular anatomic evaluation of potential renal donors.9–11 CT angiography provides precise preoperative information about renal vascular anatomy, with a reported accuracy of >97% for arteries and 96–100% for veins.11 In humans, multiple renal arteries are more common on the left and multiple renal veins more common on the right. In prior preliminary work, we noted some variation in feline renal vascular anatomy.12 Reference information on the variation of feline renal vasculature is limited, and there are no published data regarding the CT angiographic appearance of feline renal vessels.13,14 The purpose of this study was to characterize variations of renal vasculature in potential feline donors using dual-phase CT angiography.
Materials and Methods
Images from cats that underwent renal dual-phase CT angiography between 1999 and 2007 were reviewed. The cats were part of the renal donor colony and were maintained under animal welfare regulations and guidelines for the care and use of animals in research (Institutional Animal Care and Use Committee of the University of Pennsylvania). A third-generation single-slice helical CT unit* and a previously described CT angiography protocol were used.12 The CT angiography protocol was adjusted slightly, in that the slice thickness was changed from 2 mm to 1 mm with a pitch of 2. To be included, images of adequate diagnostic quality were required.
Transverse, dorsal, and sagittal reformatted images, and 3D reconstructed images (when available), were reviewed† to compare with surgical findings. Information regarding vessel length was not included in our study, although this is routinely measured using reformatted images and electronic calipers. Information collected from the reports included kidney shape, renal vascular anatomy, and variations of the aorta and caudal vena cava at the level of the kidneys. The vascular pattern described originally by the radiologist was the one used for statistical analysis. For each kidney, the vessels were characterized as single with no bifurcation, single with bifurcation (when a single vessel originates or terminates from/at the aorta or caudal vena cava but divides into two or more branches before the hilus), double, or triple. When the renal extremity of a vessel was not the hilus, the vessel was qualified as accessory. Renal arteries and veins were characterized as double or triple when there were two or three separate aortic origins or vena cava terminations.
The mean±standard error and a 95% confidence interval of each anatomic variant for each vessel were calculated. The proportion of each anatomic subtype was compared with proportions available from a large-scale anatomic study using a one-sample Student's t-test for proportions.14 The null hypothesis was no difference between the proportions of each anatomic variant found in our cats and the proportions found in the anatomic study. The level of statistical significance was set at P=0.05. All calculations were performed using statistical package software available commercially (Intercooled Stata 8.0‡).
If suitable as a renal donor based on renal vascular anatomy defined by CT angiography and other clinical findings, nephrectomy and transplant were performed days to weeks after the initial imaging. When available, surgical information on renal vascular anatomy was collected and compared with the CT angiography findings.
Results
All studies were of diagnostic quality with good opacification of the aorta, caudal vena cava, the kidney parenchyma, and vessels. Renal vascular anatomy was evaluated in 228 kidneys in 114 cats. Based on retrospective subjective review of reformatted images in different planes, useful information was not added in any cat. The proportion of each anatomic variant is shown in Fig. 1 and Table 1. Multiplicity was more common in the right vein compared with the left vein or either artery (45/114 multiple right renal veins vs. 3/114 multiple left renal veins, 0/114 multiple right renal arteries and 8/114 multiple left renal arteries; Fig. 2). The relative risk for the right kidney to have multiple veins compared with the left was 13.33. The most common overall pattern for each vessel of both kidneys was single with bifurcation (Fig. 3).
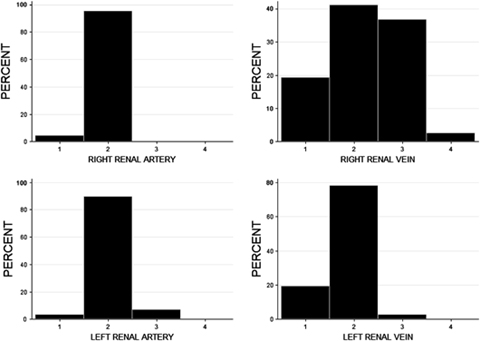
Histograms showing the distribution of each anatomic variant for each type of renal vessel. 1, single vessel with no bifurcation; 2, single vessel with bifurcation; 3, double vessel; 4, triple vessel; 79 × 58 mm (300 × 300 DPI).
| Vessel | Proportion ±Standard Error | 95% ConfidenceInterval |
|---|---|---|
| Right renal vein | ||
| Single | 0.19 ± 0.04 | (0.12, 0.28) |
| Single with bifurcation | 0.41 ± 0.05 | (0.32, 0.51) |
| Double | 0.37 ± 0.05 | (0.28, 0.46) |
| Triple | 0.03 ± 0.02 | (0.005, 0.075) |
| Right renal artery | ||
| Single | 0.04 ± 0.02 | (0.01, 0.10) |
| Single with bifurcation | 0.96 ± 0.02 | (0.90, 0.99) |
| Left renal vein | ||
| Single | 0.19 ± 0.04 | (0.13, 0.28) |
| Single with bifurcation | 0.78 ± 0.04 | (0.69, 0.85) |
| Double | 0.03 ± 0.01 | (0.005, 0.07) |
| Left renal artery | ||
| Single | 0.04 ± 0.17 | (0.01, 0.87) |
| Single with bifurcation | 0.89 ± 0.03 | (0.82, 0.94) |
| Double | 0.07 ± 0.02 | (0.03, 0.13) |
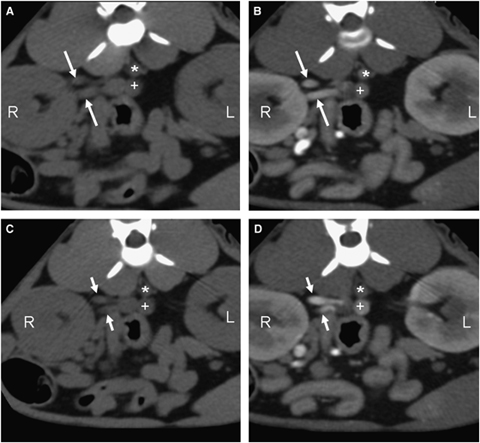
Transverse, contiguous CT renal angiogram precontrast and venous phase of the same cat at the level of the right kidney hilus. A and C represent the precontrast images, while B and D were obtained during the venous phase. Notice the two separate renal veins (white arrows) traveling toward the caudal vena cava. Right kidney (R), aorta (*), caudal vena cava (+); 93 × 88 mm (300 × 300 DPI).
(A) Transverse CT renal angiogram at the level of the right renal hilus showing a single artery with bifurcation (black arrow). (B) Three-dimensional oblique image of the same cat showing bilateral single arteries with bifurcation (black arrows). Right kidney (R), left kidney (L), aorta (*), spleen (S); 65 × 93 mm (300 × 300 DPI).
The vascular abnormalities found on CT angiography were similar to those reported in the anatomic study, with a statistically significant difference only for the subgroups: single vein with bifurcation (P=0.002), multiple veins (P=0.02), and multiple arteries (P=0.0002; Table 2).14
| Vessel | CT Results, Mean Proportion ± SEM | 95% CI | Anatomic Results | P-Value |
|---|---|---|---|---|
| Single vein no bifurcation | 0.19 ± 0.03 | (0.14, 0.25) | 0.22 | 0.18 |
| Single vein with bifurcation | 0.60 ± 0.03 | (0.53, 0.66) | 0.50 | 0.002* |
| Multiple veins | 0.21 ± 0.03 | (0.16, 0.27) | 0.27 | 0.02* |
| Single artery no bifurcation | 0.04 ± 0.01 | (0.02, 0.07) | 0.00 | NA |
| Single artery with bifurcation | 0.93 ± 0.02 | (0.88, 0.96) | 0.90 | 0.12 |
| Multiple arteries | 0.04 ± 0.01 | (0.015, 0.07) | 0.007 | 0.0002* |
- The proportions found in a large-scale anatomic study of 1000 cats; 2000 kidneys are shown for comparison. Significant differences are marked by an asterisk (*). Double and triple vessels are grouped together as multiple; NA, not applicable; CI, confidence interval; SEM, standard error of the mean.
The other associated findings were irregular kidneys (six cats), mesenteric lymphadenopathy (four cats), mineralization of the cortico-medullary junction or collecting system (two cats), nodular fat necrosis (one cat), splenomegaly (three cats), double caudal vena cava (three cats; Fig. 4), and an accessory renal artery (one cat; Fig. 5).
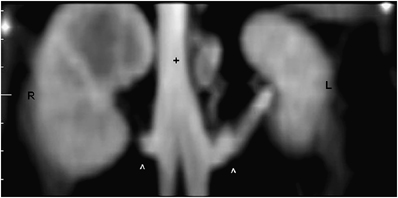
Dorsal reformatted image of the caudal vena cava (+) at the level of the kidneys. Cranial is to the top. Notice double caudal vena cava joining at the level just cranial to the renal veins (^). Right kidney (R), left kidney (L); 115 × 58 mm (300 × 300 DPI).
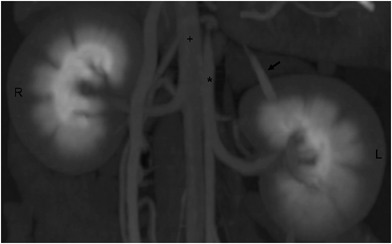
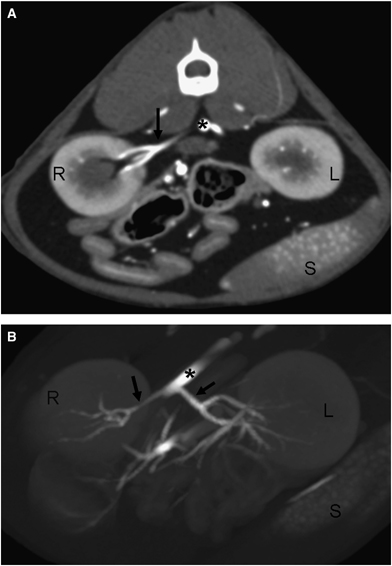
Postcontrast, 3D reconstructed image of the kidneys showing a left accessory renal artery to the cranial pole (black arrow). Right kidney (R), left kidney (L), aorta (*), and caudal vena cava (+); 100 × 62 mm (300 × 300 DPI).
Seventy-seven cats underwent surgery. In 67 out of the 77 cats, a left nephrectomy was performed and there was agreement between the CT angiography findings and the surgical findings.
In six cats, a right nephrectomy was performed. In one, a double left renal artery had been seen on CT angiography before surgery (Fig. 6) but that kidney was not examined to confirm this. In four others, a double left renal artery was found during surgery that had not been seen on CT angiography. In one other, an accessory artery was found in the left kidney and seen on CT angiography. In all six cats, there was agreement between the right renal vascular anatomy seen at surgery and observed on CT angiography.
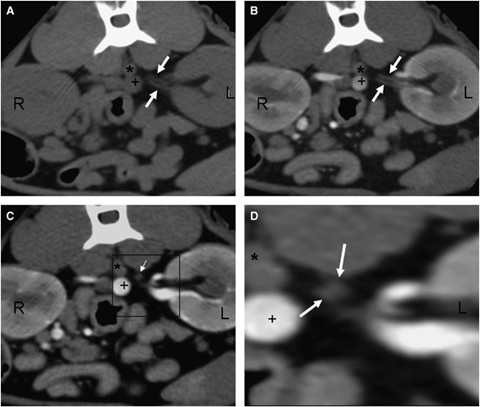
Transverse CT renal angiogram images at the level of the kidneys. (A and B) Pre- and post-IV contrast images at the level of the left kidney (L) showing double renal arteries (white arrows) traveling parallel to each other. (C) Late arterial–early venous phase at the level of the left kidney, cranial to images A and B and closer to the aortic origin. Notice the two renal arteries (white arrow) traveling to the aorta (*). (D) Close up of the double left renal arteries (white arrows) of the area showed within the black box in image 6C. Right kidney (R), left kidney (L), aorta (*), caudal vena cava (+); 88 × 75 mm (300 × 300 DPI).
In four of the 77 cats, transplantation was aborted [double left renal artery found on surgery and right kidney unsuitable for transplant (n=1), double left renal vein draining the left ureter and hemorrhage during manipulation of the right kidney (n=1), irregular kidneys found on surgery but not on CT (n=2)].
In the 74 cats in which the left kidney was dissected, there was full agreement with the CT angiography findings in 68 cats (92%).
Thirty-seven cats did not have surgery. In 18, the decision was based on CT angiography findings. This included vessels too short (n=1), double left renal artery (n=6), prolonged nephrogram (n=1), irregular kidneys (n=6), and lymphadenopathy (n=4). Eight of the 37 cats did not have surgery for medical complications that developed after CT angiography. The 11 remaining cats did not have record of surgery at the time of data collection.
Discussion
In our patients, multiple renal veins were more common on the right (relative risk of 13.33), which is in agreement with an anatomic study of 1000 cats, where the relative risk of multiple renal veins on the right was 4.8.14 Multiple renal veins on the right are four times more common than on the left in humans.15 This difference relates to the embryologic development of the renal veins. In embryos, the vessels that form the caudal vena cava are three pairs of symmetrical veins: the postcardinal, subcardinal, and supracardinal. The right renal vein forms entirely from the right sub- and supracardinal anastomosis, which contains two primitive renal veins; this forms the renal part of the caudal vena cava. The left renal vein forms after the degeneration of the left subcardinal vein and therefore is formed from only one venous primordium.16 Because of its unique origin, the left renal vein is less likely to be multiple than the right one.
A double caudal vena cava was found in three cats at the renal and postrenal levels. Two of them also had double right renal veins and one had double left renal veins. The embryology of the inferior vena cava in people is complex.16 It involves a combination of growth, regression, and fusion among the three longitudinal pairs of veins (postcardinal, subcardinal, and supracardinal). The normal inferior vena cava is composed of four main segments: hepatic, suprarenal, renal, and infrarenal segments. Duplication of the caudal vena cava results from persistence of both supracardinal veins. In humans with double caudal vena cava, the left caudal vena cava ends at the left renal vein, which crosses anterior to aorta in a normal fashion to joint the right caudal vena cava and forms one vein at the hepatic level.17 In our study, all three cats had this anatomic pattern. The incidence of double caudal vena cava in humans is between 0.02% and 3%.17,18 In patients with double caudal vena cava, the renal veins are short and may be abnormal.19
We found multiple renal arteries only in the left kidney. The same has been described in dogs20 and a similar distribution has been described in humans where multiple renal arteries are 1.5 times more common on the left. During development, embryonic kidneys migrate cranially from the pelvic area and therefore the arterial supply to the kidney changes as it travels cranially. More cranial renal arteries form while the most caudal ones disappear. The arterial supply to the kidney also has a dual embryologic origin, cranially from the adrenal artery and caudally from the iliac arteries. The development of the main renal artery is the result of anastomosis of aortic branches with degeneration of cranial secondary branches. Failure of one of this vessels to degenerate can cause multiple, accessory, or polar, renal arteries. Accessory renal arteries were not commonly found in this study. This is in accordance with the anatomic study in cats where no accessory arteries were found.14 Accessory arteries are more common in humans where they are seen in up to 25 percent of patients.21 The most common source of disagreement between CT angiography and surgery in humans is the inability to visualize small accessory arteries.22 In humans, the use of multislice CT angiography is preferred and has become standard because it offers a shorter image acquisition time, the ability to image large volumes without a decrease in the signal-to-noise ratio, a much better spatial resolution, and decreased tube heating.3,4 For the same reasons, multidetector CT should be advantageous to evaluate feline patients as well, especially because the vessels imaged are much smaller. There was a statistically significant difference between our study and the anatomic study for a few anomalies (Table 2).14 These differences could be due to differences in the sample size. Populations with a different genetic background could also be an explanation. Last, lack of spatial resolution could have led to an underestimation of the number of multiple vessels and an overestimation of the number of single vessels.
In comparison with operative findings, there was an agreement of 92% between CT angiography and surgical left renal vascular anatomy. There was disagreement in six out of 74 cats. The most common reason for this (5/6) was the inability to visualize double renal arteries when they originate in close apposition to each other from the aorta. In retrospect, three of these vessels were suspected from the transverse images and 3D reconstruction was helpful in one of them. In one cat, double left renal veins were not seen on the CT angiography because of mild motion artifact. The right renal vascular anatomy seen on CT angiography was consistent with surgery in all six cats, in which the right kidney was used for transplantation because of abnormalities in the left kidney.
Sixty-nine percent of cats excluded as donors were excluded because of CT angiography findings. The most common reasons for rejection were irregular kidneys and multiple left renal arteries. Interpretative errors could have been the result of inadequate review of the transverse or reformatted images, inexperience, or technical and patient errors. Insufficient spatial resolution might also have been a problem: a pitch of 2 with a slice thickness of 1 mm was used for all these patients. In theory, this may have contributed to the interpretative errors by increasing the width of the section sensitivity profile when compared with a lower pitch. In our previous renal CT angiography protocol, we used 2-mm slice thickness and a pitch of 2 and retrospectively evaluated reconstructed images with a 2-mm slice thickness and 1-mm slice interval.12 In this study, a 1-mm slice thickness was used instead to increase the spatial resolution, and similarly a pitch of 2 was used to maximize the area coverage, because the arterial contrast medium transit time is short. For the left kidney, surgery and CT angiography findings were in agreement in 92% of 74 cats, and in only six cats (7.8%) did the inaccuracy of CT angiography had surgical implications in terms of unsuitability of the left kidney for transplantation (double vessels identified as single). In the six cats where the right kidney was used, the right renal vascular anatomy was in agreement with the CT angiography findings in all cats. Although very good on average, our degree of agreement was less than the agreement between CT angiography and surgery reported in humans (96–100%). Certainly, the standard use of multislice CT angiography, with a much better resolution, and the significantly larger size of renal vessels account for the better performance of CT angiography in humans.
The limitations of this study include the incomplete surgical data for the right kidney of patients who underwent left nephrectomy and the lack of a gold standard comparison for the patients who did not undergo surgery. Nonetheless, based on the good accuracy of the CT angiography findings in animals that underwent surgery, we believe that the anatomic variations reported herein are a good representation of the actual anatomical variations in a population of cat donors.