IMAGING DIAGNOSIS—TRICUSPID ATRESIA IN AN ALPACA
Support: None.
Not previously presented.
Signalment
Eight-day-old female alpaca cria.
History
The cria was evaluated because of suspicion of pneumonia. The cria had been weak since birth, was not nursing well, and had intermittent collapse. Intravenous hyperimmune plasma and an unspecified course of intravenous potassium penicillin and ceftiofur had been administered. The cria initially responded to this treatment but relapsed after discontinuation of antibiotics. The cria was persistently tachycardic and tachypnic and had failed to gain weight despite supplementation with goat's milk.
Physical Exam
The cria was in a poor body condition weighing 9.8 kg, it could nurse but became exhausted after brief periods of standing. The rectal temperature was 104.4°F, the respiratory rate was 88 bpm, and the heart rate was 180 bpm; there was increased respiratory effort and nasal flare. Four fractured ribs and an associated flail chest were evident on the left. The bronchovesicular sounds were harsh over both hemithoraces. There was a rapid regular rhythm and a grade 6/6 holosystolic coarse crescendo–decrescendo murmur over the left heart base. The murmur radiated widely and was heard over the sternum and tricuspid valve region as a 4/6 holosystolic coarse crescendo–decrescendo murmur. The mucous membranes were pale and moist with a capillary refill time of 2–3 s. Peripheral arterial pulses were rapid and synchronous. There was severe resting hypoxemia with a PaO2 of 37.5 mmHg (normal=90–100 mmHg) and an oxygen saturation of 77.8% (normal=95–99%), a mild anemia with a packed cell volume (PCV) of 19% (normal=25–38%), and hemoglobin of 7.68 g/dl (normal=10.4–17.4 g/dl). The total protein was 4.8 g/dl (normal=5.1–6.0 g/dl) and albumin was 2.0 g/dl (normal=3.1–3.5 g/dl). The white cell count and differential were within normal limits but the fibrinogen was mildly elevated at 612 mg/dl (normal=100–400 mg/dl). Electrocardiographic examination revealed a sinus tachycardia. Sonographic evaluation of the thorax confirmed the presence of rib fractures and moderate bronchopneumonia in the cranial right hemithorax.
Echocardiography
Using two-dimensional echocardiography, right atrial dilation and hypertrophy, left atrial enlargement, left ventricular enlargement, a hypoplastic right ventricle, and a moderate amount of anechoic pericardial fluid were identified (Table 1). The tricuspid valve leaflets were absent; instead, a dense band of echoes was observed at the level of the tricuspid valve (1, 2). A large muscular ventricular septal defect was located within the mid portion of the muscular interventricular septum. The defect measured 1.50 cm in comparison to the aorta which measured 2.37 cm (3, 4). The interatrial septum was seen as a thin echogenic wall that bulged toward the left atrium during systole (Fig. 1). The aorta appeared normal in size with normal coronary arteries. The pulmonary artery was similar to the aorta in size, measuring 2.28 cm. The mitral, aortic, and pulmonary valves appeared subjectively normal. Using M-mode evaluation, there was absence of a tricuspid valve echo, a small right ventricle, a pattern of left ventricular volume overload, and a fractional shortening of 36%.
| Tricuspid Atresia (cm) | Normal (cm) | |
|---|---|---|
| LVIDd | 4.70 | 2.96 |
| LVIDs | 2.99 | 2.11 |
| IVSd | 0.55 | 0.76 |
| IVSs | 1.31 | 1.14 |
| LVFWd | 0.58 | 0.52 |
| LVFWs | 1.01 | 0.98 |
| LAD | 4.29 | 3.21 |
| PA | 2.28 | 1.81 |
| Aorta | 2.37 | 1.86 |
- Normal values were obtained by averaging echocardiographic data obtained from three clinically normal crias (ages 10–18 days).
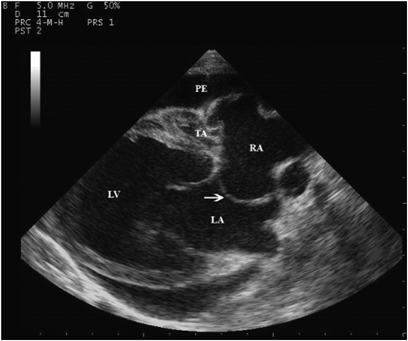
Two-dimensional echocardiographic right parasternal long axis view. Note the echogenic band of tissue at the level where the tricuspid valve should be TA; dilated left ventricle, LV; and pericardial effusion, PE. The interatrial septum (arrow) can be seen bulging toward the left atrium (LA). RA, right atrium.
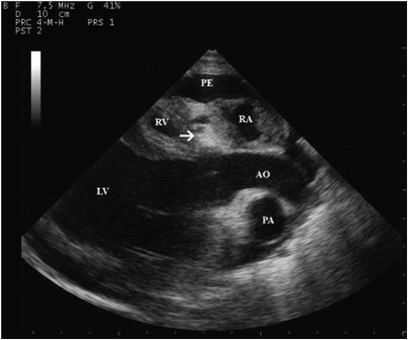
Two-dimensional echocardiographic right parasternal long axis view of left outflow tract. Note the echogenic band of tissue in the tricuspid valve region (arrow) and the hypoplastic right ventricle (RV). AO, aorta; PA, pulmonary artery; PE, pericardial effusion; LV, left ventricle; RA, right atrium.
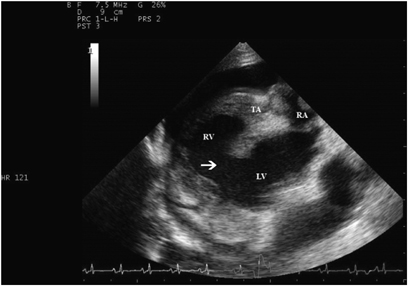
Two-dimensional echocardiographic right parasternal view. Note the ventricular septal defect (arrow) in the interventricular septum. RV, right ventricle; LV, left ventricle; RA, right atrium; TA, tricuspid valve region.
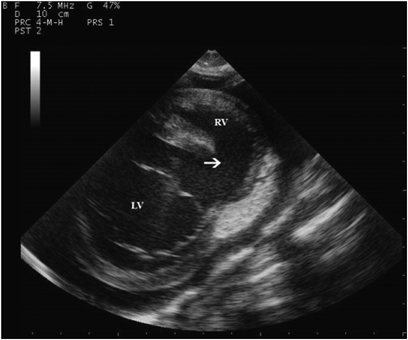
Two-dimensional echocardiographic short axis view at the level of the mitral valve. Note the ventricular septal defect (arrow) in the interventricular septum. RV, right ventricle; LV, left ventricle.
Based on color flow and spectral Doppler echocardiography, there was no flow across the muscular band of tissue separating the right atrium and right ventricle. There was no regurgitation at other valve sites. There was bidirectional turbulent flow across the ventricular septal defect with left to right flow during systole and right to left flow during diastole. The systolic shunt velocity was 2.05 m/s. A contrast echocardiographic study using an intravenous injection of agitated saline was performed and there was a complete lack of passage of contrast material from the right atrium to the right ventricle. Opacification of the right atrium was followed by opacification of the left atrium followed by the left ventricle. Simultaneous opacification of the right ventricle and aorta followed.
A diagnosis of tricuspid atresia with a large muscular ventricular septal defect, patent foramen ovale, and congestive heart failure was made. Septicemia, pneumonia, and multiple rib fractures were also diagnosed. Humane euthanasia was recommended. The owner removed the cria from the hospital against medical advice.
Discussion
Tricuspid atresia is the congenital absence of the tricuspid valve. It is a rare congenital abnormality in human neonates occurring in approximately 1 in 10,000 births without gender, geographic, or racial predilection. It is also the third most common cyanotic congenital cardiac defect.1 Tricuspid atresia has been sporadically described in animals, mostly in Arab or Arab cross foals, but also in other equine breeds and a lamb.2–8 To our knowledge, tricuspid atresia has never been reported in an alpaca or other camelid, although other complex cardiac defects have been described in llamas.9,10 The etiology of tricuspid atresia is most likely multifactorial, involving environmental and genetic factors. No specific risk factors have been identified in humans or animals.
Tricuspid atresia is classified according to the morphology of the valve, great artery relationship, and pulmonary artery lesions. The muscular type of tricuspid atresia is the most common type in humans and is characterized by a dimple or localized thickening at the site where the tricuspid valve would normally be. The muscular type occurs in 89% of affected humans and is the only type described in animals.5,6,11 Other less common forms in humans include the membranous type, Ebstein type, valvular type, atrioventricular canal type, and an unguarded type with a muscular shelf.12
After describing valve morphology, tricuspid atresia is further categorized according to the associated relationship of the great vessels. In type I, the great arteries are normally related; in type II, the great arteries are in a d-transposition relationship; in type III, the great arteries are otherwise malpositioned and subtyped 1–5; and in type IV, there is a truncus arteriosus. These types and subtypes are further subcategorized according to pulmonary artery lesions, with subgroup “a” having pulmonary atresia, subgroup “b” having pulmonary stenosis or hypoplasia, and subgroup “c” being normal.11 The status of the interventricular septum and the presence of other associated malformations complete the description. Classification is important in human patients, as this determines suitability for and type of surgical correction.
In tricuspid atresia, the lack of continuity between the right atrium and right ventricle prevents blood from entering the right outflow tract in the usual manner. Interatrial communication is absolutely necessary, either in the form of a patent foramen ovale or atrial septal defect, because venous blood returning to the right atrium can then be shunted to the left atrium and subsequently to the left ventricle and aorta. Connection with the right outflow tract and pulmonary circulation is usually accomplished by a ventricular septal defect. In the absence of a ventricular septal defect, a patent ductus arteriosus or aortopulmonary collaterals must be present. The magnitude of pulmonary blood flow is largely dependent on the degree of pulmonary outflow obstruction. With accompanying pulmonary atresia or valve stenosis, pulmonary blood flow is reduced, worsening the degree of cyanosis. In the absence of pulmonary artery lesions, the volume of blood to the lungs is increased, reducing the degree of cyanosis but potentially hastening the onset of left heart failure. In either instance, left ventricular volume overload and a poorly oxygenated coronary blood supply both eventually lead to the development of congestive heart failure. Numerous other cardiac defects can occur in association with tricuspid atresia and these defects further define the path of blood flow, pulmonary perfusion, and clinical picture. Foals with tricuspid atresia and d-transposition of the great arteries, persistent left cranial vena cava, main pulmonary artery atresia, coarctation of the aorta, anomalous drainage of the caudal vena cava into the left atrium, and/or patent ductus arteriosus have been described.2,5,8,13
The symptoms of tricuspid atresia manifest early in life and depend on the magnitude of pulmonary blood flow. Symptoms in human neonates are usually present within a few hours to a month after birth and consist of cyanosis, dyspnea, tachypnea, and tachycardia.11 Foals with tricuspid atresia also develop clinical signs very early in life (2 days to 10 weeks of age) with lethargy, weakness, and difficulty nursing. These foals are also tachycardic, tachypnic, and cyanotic and may collapse with minimal exertion.6 There is no characteristic murmur of tricuspid atresia but murmurs associated with a ventricular septal defect, pulmonic stenosis, or patent ductus arteriosus may be present. Systemic arterial desaturation is always present as a consequence of the obligatory mixture of the systemic, coronary, and pulmonary venous blood in the left atrium. The degree of cyanosis is dependent on the amount of pulmonary artery obstruction, as well as the presence and severity of associated defects. The alpaca in this report had signs similar to those reported in foals and human infants, although complicated by the septicemia, anemia, pneumonia, and rib fractures. In light of these other factors, the heart murmur was the most important localizing sign and most likely was associated with the ventricular septal defect. Cyanosis was not detected on physical examination despite arterial saturation being <80%. It is likely that the low hemoglobin content together with normal pulmonary artery formation contributed to the lack of clinically apparent cyanosis.
Contrast echocardiography with an injection of agitated saline permits confirmation of tricuspid atresia by delineating the lack of passage of bubbles from the right atrium to the right ventricle. Cardiac catheterization is primarily used in human patients to define details important in surgical management and is usually unnecessary for the diagnosis of tricuspid atresia.11 Two-dimensional echocardiographic findings that have been described in infants and foals include a thick echo in the region of the tricuspid valve that does not separate in diastole, a small right ventricle, an enlarged right atrium, right to left bulging of the interatrial septum, left atrial enlargement, and left ventricular enlargement.6,14,15 All of these echocardiographic findings were present in the alpaca in this report. In addition, color flow Doppler and contrast echocardiography confirmed the path of blood flow across a patent foramen ovale and ventricular septal defect. The low velocity of the left to right shunt across the muscular ventricular septal defect indicates a large, nonrestrictive defect. The diastolic right to left shunting across the ventricular septal defect indicates right ventricular diastolic pressure that exceeds left ventricular diastolic pressure. Appropriate coronary artery association and great artery attachment ruled out transposition of the great vessels. The pulmonic valve was normally formed and the main pulmonary artery was similar in size to the aorta ruling out significant main pulmonary artery lesions. Using the human classification scheme, this alpaca would have type Ic tricuspid atresia.
The treatment for infants with type Ic tricuspid atresia is corrective surgery. Without surgery, 50% of infants die by 6 months of age and fewer than 10% survive to 10 years of age.16 Palliative surgery aimed at increasing pulmonary blood flow, improving arterial saturation, and permitting growth is performed first. Creation of a communication between the venous return and the pulmonary artery (Fontan procedure) and closure of the atrial septal defect or patent foramen ovale is performed at a later date.11 Surgical correction of tricuspid atresia has not been reported in animals. A successful Fontan procedure has been performed in a dog with severe tricuspid stenosis, although the dog died a few months following surgery, presumably because of a cardiac dysrhythmia.17




