The effect of beta and kappa casein genes on milk yield and milk composition in different percentages of Holstein in crossbred dairy cattle
ABSTRACT
The objective of this study was to compare the genotype, and composite genotype frequency, and the association between beta and kappa casein genes and milk yield (MY), percentage of fat (%Fat), protein (%Prot), and solids non-fat (%SNF) between two groups of crossbred Holstein: G1 ≤ 87.5% Holstein = 89 cows and G2 > 87.5% Holstein = 142 cows. Five genotypes of beta casein gene were observed. A1A2 and A1B were the most and rarest frequency, respectively, in both groups. Five genotypes of kappa casein gene were found. The highest and the lowest frequency were AA, and BB and BE, respectively, in both groups. Composite genotype A1A2AA was the most frequent in both groups. Linkage disequilibrium (LD) between two genes was detected. Significant differences of frequencies between both groups of both genes were not found. The association of the genes and the traits was different between G1 and G2. Negative effects on the traits were found in both groups. In addition, the stronger effect of the beta casein gene was observed in most of the traits. The conclusions were that different %Holstein caused different significant effects of these genes. A study of the association of these genes within each percentage of Holstein is strongly recommended.
INTRODUCTION
The high content of milk protein affects the high content of solids non-fat (%SNF) which is important for profitability of the dairy industry in Thailand, since these traits have a high positive correlation (Hayes et al. 1984): if the content of protein increases the %SNF will also be increased.
Eighty percentage of milk protein is casein protein (Koczan et al. 1991) which is controlled by the casein gene family. They locate on bovine chromosome 6 at q31–q33 and composed with αs1, αs2, β and κ-casein genes (Mercier & Viloite 1993; Jann et al. 2004). With the polymorphism and the significant effect on milk yield (MY) and milk protein of the β, and κ-casein genes, they show the potential to be the gene markers for marker-assisted selection (MAS). However, although there are several previous studies which investigate the relationship between casein genes and milk production, and milk composition (Van Eenennaam & Medrano 1991; Bobe et al. 1999; Ikonen et al. 1999; Braunschweig et al. 2000, etc.), the effect of these genes and the association with the traits are not consistent. The explanation for this is that the unique genetic structure in each dairy cattle family, herd or breed causes the expression of these genes to interact at different loci (Bovenhuis et al. 1992). So that, if using β and κ-casein genes as MAS, analyses of the association between the genes and the traits within the cattle herd, are required.
Crossbred Holstein, Bos taurus × Bos indicus, in various percentages of Holstein from 75% to 98.75%, make up the main population in Thailand and Southeast Asia, since they have good heat tolerance (Milazzotto et al. 2008). However, the limitations of Thai native cattle are that have very low performance in milk production and milk composition, since they have very low genetic potential in these traits. When the genetics from both breeds were combined, the significance and the magnitude of casein gene effect may be changed (Bovenhuis et al. 1992), as mentioned in the previous paragraph. Simultaneously, Toosi et al. (2010) found that the linkage disequilibrium (LD) between genes can be changed, particularly, in crossbred populations, where the rate of decaying of LD will be faster than in purebred populations. Thus, the LD of casein genes themselves in crossbred Holstein remain unknown. In addition, at various percentages of Holstein crossbreeding, gene frequencies, LD and significant associations between genes and the traits of interest, and the effect of genes, may all be different.
The aim of this study was to investigate polymorphism, genotype and composite genotype frequencies, LD, the association between genes and milk production, and aspects of milk composition of beta and kappa casein genes in different percentages of Holstein crossbred cattle in Thailand. Consequently, the result will point to the potentials of both genes as MAS in crossbred Holstein cattle.
MATERIALS AND METHODS
Cattle and data
Crossbred milking cows were divided into two groups determined by the level of percentage of Holstein: G1 (89 cows, ≤ 87.5%) and G2 (142 cows, > 87.5%). The traits of milk yield per cow per day (MY), % fat (%Fat), % protein (%Prot), and % solids non-fat (%SNF) were used in this study, and the data records in each group numbered around 1000–1100. Table 1 shows the mean and standard deviation of each trait in each group.
| Traits | Mean (SD) of β − casein genotype | Mean (SD) of κ − casein genotype effect | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1A1 | A1A2 | A2A2 | A2B | AA | AB | AE | ||||||||
| G1 | G2 | G1 | G2 | G1 | G2 | G1 | G2 | G1 | G2 | G1 | G2 | G1 | G2 | |
| MY (kg/day) | 8.73 (2.89) | 9.6 (1.77) | 10.62 (3.89) | 10.4 (3.99) | 10.51 (3.83) | 10.28 (4.1) | 10.75 (3.9) | 9.51 (4.19) | 10.52 (3.89) | 10.95 (4.13) | 10.55 (3.90) | 9.28 (3.44) | 10.61 (3.37) | 10.42 (4.34) |
| %fat | 3.36 (0.72) | 3.56 (0.42) | 3.70 (0.70) | 3.69 (0.60) | 3.65 (0.64) | 3.69 (0.63) | 3.81 (0.75) | 3.85 (0.74) | 3.66 (0.70) | 3.70 (0.62) | 3.75 (0.67) | 3.71 (0.60) | 3.32 (0.76) | 3.52 (0.52) |
| %Prot | 2.84 (0.25) | 2.75 (0.22) | 3.13 (0.39) | 2.86 (0.33) | 3.14 (0.38) | 2.96 (0.37) | 3.19 (0.33) | 2.99 (0.40) | 3.10 (0.38) | 2.88 (0.36) | 3.19 (0.37) | 2.92 (0.31) | 3.01 (0.28) | 2.77 (0.35) |
| %SNF | 8.34 (0.39) | 8.06 (0.37) | 8.48 (0.51) | 8.19 (0.44) | 8.54 (0.44) | 8.26 (0.45) | 8.64 (0.46) | 8.37 (0.59) | 8.48 (0.48) | 8.17 (0.47) | 8.52 (0.50) | 8.31 (0.42) | 8.59 (0.33) | 8.08 (0.45) |
DNA extraction and genotyping
DNA was isolated from whole blood samples using Genomic DNA Mini Kit (Geneaid, Taipei, Taiwan). The DNA solution was quantified by spectrophotometer and diluted to 10 µg/µL concentration. The allele and genotype of β, and κ-casein gene were analyzed by allele-specific polymerase chain reaction (ASPCR) and PCR-restriction fragment length polymorphism (PCR-RFLP), respectively. These methods followed the methods of Cowan et al. (1992) and Aroondechachai et al. (2004), respectively.
Statistical analysis
Allele, genotype and composite genotype frequencies which significantly deviated from Hardy-Weinberg equilibrium (HWE) and linkage disequilibrium (LD) were analyzed by GENEPOP version 3.4 (Raymond & Rousset 1995).The relationship between genetic information (allele, genotype, and composite genotype) and MY, %SNF, %Prot, %Fat were analyzed by the regression model shown below, and ordinary least square (OLS) was used to estimate the effect of these genes on the traits by SPSS for window (Release 10.0; SPSS Inc., Chicago, IL, USA):
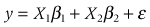
where y, β1, β2, ε are the phenotypic data (MY, %SNF, %Prot, %Fat), overall mean of each group (G1, G2), regression coefficient of contemporary group included with the number of lactation, herd-year season, and days in milk, regression coefficient of genotype, and composite genotype and residue effect, respectively. X1 and X2 are the incidence matrices which show contemporary group and genetic information (genotype, composite genotype), respectively.
RESULTS AND DISCUSSION
Gene polymorphism and frequency between groups
Table 2 shows the genotype and composite genotype frequencies of β-casein, κ-casein genes, and composite genotype, respectively. The significant differences of allele, genotype frequency between G1 and G2 were not found (P > 0.05). Three alleles and five genotypes of β-casein gene were observed. Allele A2 and B were the most and the rarest frequent, respectively. A1A2 and A1B were the most and rarest frequent, respectively. In κ-casein gene, three alleles and five genotypes were found. The most and the least frequent were A and E, respectively, and genotype AA was the most frequent, while BB and BE were the rarest; in particular, BB was absented in G2 (Table 2). The allele and genotype frequency affect to composite genotype frequency, A1A2AA, was the most frequent in both groups (Table 2). The pattern of gene frequency (the highest, and lowest genotype frequencies) in crossbred Holstein in Thailand is also similar to various dairy cattle breeds, such as: Red Danish and Black and White Danish (Bech & Kristiansen 1990); Swedish Red and White and Swedish Holstein (Lunden et al. 1997); Holstein (Ojala et al. 1997); Finnish Ayrshine (Ikonen et al. 1999); Crossbred Holstein (Aroondechachai et al. 2004); and Italian Holstein (Comin et al. 2008). However, they contrast with Jersey populations which were studied by Bech and Kristiansen (1990). Some of these studies and the results in this study show that the different strains of Holstein do not cause differences in milk protein gene frequency.
| Breed group | Frequency of β (above) and κ (below) casein gene | |||||||
|---|---|---|---|---|---|---|---|---|
| A1A1 AA | A1A2 AB | A1B AE | A2A2 BB | A2B BE | A1 A | A2 B | B E | |
| G1 (n = 89) | 0.056 | 0.528 | 0.034 | 0.315 | 0.067 | 0.337 | 0.612 | 0.051 |
| 0.584 | 0.360 | 0.022 | 0.022 | 0.011 | 0.775 | 0.208 | 0.017 | |
| G2 (n = 142) | 0.056 | 0.606 | 0.007 | 0.268 | 0.063 | 0.363 | 0.602 | 0.035 |
| 0.563 | 0.338 | 0.078 | 0 | 0.021 | 0.771 | 0.180 | 0.049 | |
| Frequency of composite genotype (higher than 0.03) | |||||
|---|---|---|---|---|---|
| A1A2AA | A1A2AB | A2A2AA | A2A2AB | A2BAB | |
| G1 (n = 89) | 0.348 | 0.169 | 0.214 | 0.101 | 0.067 |
| G2 (n = 142) | 0.380 | 0.148 | 0.148 | 0.120 | 0.064 |
Linkage disequilibrium
The significance of LD in β and κ-casein genes were found in both groups (P < 0.0001). The different percentages of Holstein did not seem to cause differences in LD in each group, but LD should be detected when the generation changes, since in the crossbred population the rate of decay of LD will be faster than in purebreds (Toosi et al. 2010). However, from the present result it can be implied that the composite genotype is the appropriate form for use as a genetic marker. However, the genotype effects were still used to observe the interaction effect between two loci.
The effect of loci between two groups
Both genotypes and composite genotypes which had average frequencies > 0.03 were used for analysis and the results are shown in Table 3. The direction (positive, negative) effect of each genotype were almost similar between G1 and G2, except for the effect of genotype A2A2 (β-casein) on %Fat, or effect of genotype AA (κ-casein) on %SNF, or the effect of genotype AB on MY. Further, the significance of effect between the two groups was different. The significant effect of A1A1 was found on all favorable traits in G1, while this genotype showed non-significant effects on MY in G2, for example. Similar results were observed again when the effect of composite genotypes was estimated. The significant effect of A1A2AA was found on all traits in only G2, but it had an effect only on %Fat and %SNF in G1. There are two possible reasons to explain the inconsistency of effect between these two groups. First, the limitation of the sample size in this study may cause an inconsistent effect, even when the repeated test-day data of all traits could increase the power of the test to 80–95%, which is high enough to detect real significance and non-significant effect of some genotypes in each group. In particular, this effect should be the real small effect; however, increasing sample size should be considered in the next study to improve inferences about results of crossbred Holstein populations with Thai native and Holstein. Second, the effect of genes may be different in each family or breed (Bovenhuis et al. 1992; Boettcher et al. 2004), since the association of the genes and the traits were due not only to the milk protein genes but also possibly to other loci (Boettcher et al. 2004), and simultaneously the linkage between milk protein gene and the other loci could be changed when breed, strain or family were changed. In this case, different percentages of Holstein may cause different associations. The results suggest that using the genes as genetic markers needs to be used within each percentage of Holstein in crossbred cattle.
| Trait | Effect of β (above) and κ (below) casein gene | |||||||
|---|---|---|---|---|---|---|---|---|
| A1A1 AA | A1A2 AB | A2A2 AE | A2B – | |||||
| G1 | G2 | G1 | G2 | G1 | G2 | G1 | G2 | |
| MY | −1.62** (0.57) | −0.60 (0.81) | 0.10 (0.42) | 1.50* (0.49) | 0.17 (0.45) | 1.01 (0.53) | 0 | 0 |
| 0.95 (0.57) | 0.66 (0.46) | 0.37 (0.58) | −0.88 (0.48) | 0 | 0 | – | – | |
| %Fat | −0.56*** (0.12) | −0.34* (0.14) | −0.22* (0.09) | −0.25** (0.08) | −0.27** (0.09) | 0.15 (0.09) | 0 | 0 |
| 0.24* (0.12) | 0.12 (0.08) | 0.41*** (0.12) | 0.16* (0.08) | 0 | 0 | – | – | |
| %Prot | −0.32*** (0.06) | −0.16* (0.07) | −0.02 (0.04) | −0.17*** (0.04) | −0.02 (0.05) | −0.03 (0.05) | 0 | 0 |
| 0.05 (0.06) | 0.09* (0.04) | 0.16** (0.06) | 0.14* (0.04) | 0 | 0 | – | – | |
| %SNF | −0.39*** (0.08) | −0.19* (0.10) | −0.22*** (0.06) | −0.23*** (0.06) | −0.14* (0.07) | −0.13* (0.07) | 0 | 0 |
| −0.12 (0.08) | 0.12* (0.06) | −0.07 (0.08) | 0.22*** (0.06) | 0 | 0 | – | – | |
| Trait | Effect of composite genotype of β and κ casein gene | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A1A2AA | A1A2AB | A2A2AA | A2A2AB | A2BAB | ||||||
| G1 | G2 | G1 | G2 | G1 | G2 | G1 | G2 | G1 | G2 | |
| MY | 0.31 (0.44) | 2.0*** (0.05) | −0.44 (0.49) | 0.70 (0.53) | 0.14 (0.48) | 2.30*** (0.57) | −0.84 (0.54) | −0.20 (0.58) | 0 | 0 |
| %fat | −0.27** (0.09) | −0.24** (0.09) | −0.06 (0.10) | −0.27** (0.09) | −0.29** (0.10) | −0.19 (0.10) | −0.22* (0.11) | −0.11 (0.10) | 0 | 0 |
| %Prot | −0.05 (0.04) | −0.16*** (0.04) | 0.07 (0.05) | −0.16*** (0.05) | −0.02 (0.05) | −0.03 (0.05) | 0.12 (0.05) | −0.03 (0.05) | 0 | 0 |
| %SNF | −0.23*** (0.06) | −0.23*** (0.06) | −0.23*** (0.07) | −0.19* (0.07) | −0.20** (0.07) | −0.18* (0.07) | −0.03 (0.08) | 0.07 (0.07) | 0 | 0 |
- * P < 0.05,
- ** P < 0.01 and
- *** P < 0.0001; values in parenthesis are SE.
The significant effect of genotype and composite genotype
The negative significant effect on favorable traits was observed in all genotypes of β casein except A1A2 variant which had a positive effect on MY. While the positive significant effect on %Fat, %Prot, and %SNF were shown in all genotypes of κ casein gene, this locus did not associate with MY (Table 3). When the composite genotype effects were estimated, the negative effect on %Prot and %SNF were observed in all variants, and only A1A2AA and A2A2AA showed positive effect on MY. In addition to the observed interaction effect between both genes, the effect of κ casein was dominated by β casein, particularly on %Prot and %SNF. The results of this study have both similarities with and differences from previous studies, such as Bobe et al. (1999); Van Eenennaam and Medrano (1991); Bovenhuis et al. (1992); Famula and Medrano (1994); Ron et al. (1994); Ojala et al. (1997); Freyer et al. (2003); Ikonen et al. (1999, 2001); Braunschweig et al. (2000); Boettcher et al. (2004) and Comin et al. (2008), in particular with regard to the subject of magnitude of effect, direction of the effect, homozygous or heterozygous effect and different contrasts between each variant. However, the explanation in this study is that crossbred Holstein ihave part of their genetic structure from Thai native cattle, which have a very low performance in milk production and milk composition. This may affect casein loci expression, and thus these genes may be insufficient to use as MAS in crossbred Holstein in Thailand. Fine quantitative trait loci (QTL) analysis, particularly on chromosome 6, which has been reported via QTL to be related with milk production traits (Ron et al. 2001; Freyer et al. 2003; Chen et al. 2006), may be necessary to study in this population and estimate the effect of these genes together with QTL-linked markers for use as MAS.
Conclusions
There are polymorphisms of β and κ casein in the cattle of crossbred Holstein in Thailand. The different percentages in the crossbreeds of Holstein did not cause different genotypes and their frequencies, but did cause different effects of these genes on favorable traits. Even negative effects were found on some traits, such as %Prot and %SNF, but it cannot be concluded that there is no potential for these genes to be used as gene markers. Association between the genes and the traits within percentages of Holstein with higher sample sizes is strongly recommended for the next study. In addition, the interaction effects between the genes were observed, showing a stronger effect of β casein. Consequently both of these genes may not be enough to use as gene markers in crossbred Holstein cattle; therefore, other loci and their interactions between each other, are needed for future study.
ACKNOWLEDGMENTS
The authors are grateful to Assoc. Prof Dr Vorawit Siripolwat for the useful suggestions, Chiyasarn farm, the private dairy cattle farm, and Suranaree University farm for providing the data and blood collection. Finally, the authors are also grateful to the Thailand research fund and the office of the higher education commission for supporting the fund.




