Effect of cellooligosaccharide or synbiotic feeding on growth performance, fecal condition and hormone concentrations in Holstein calves
ABSTRACT
We investigated the effect of cellooligosaccharide (CE) or a combination of dextran and Lactobacillus casei ssp. casei strain JCM1134T (synbiotic; SB) feeding on growth performance, fecal condition and hormone concentrations in Holstein calves. Fifty-two female Holstein calves were randomly assigned to three treatment groups: CE feeding group (n = 16), SB feeding group (n = 18), and control group (n = 18). Body weight at 90 days of age, as well as daily body weight gain (DG) and feed efficiency after weaning to 90 days of age were greater (P < 0.05) in the CE feeding group than in the control group. The total fecal score tended to be lower (P < 0.1) in the SB feeding group than in the control group. Plasma insulin concentration was higher (P < 0.05) in the CE feeding group than in the control group at 90 days of age. Our results indicate that CE feeding improved DG and feed efficiency in calves. On the other hand, there was less effect on growth performance and fecal Escherichia coli counts in calves fed SB.
INTRODUCTION
Diarrhea caused by pathogenic bacteria is one of the most common diseases in dairy calves (MAFF 2009). Prevention of diarrhea is important to promote the growth of calves. However, current measures utilized against diarrhea rely on antibiotic therapy. Overuse of antibiotics in calves can have serious consequences, such as the development of resistant populations of bacteria. Subsequent use of the same antibiotics for therapy may then be ineffective (Abe et al. 1995; Cruywagen et al. 1996; Donovan et al. 2002; Quigley et al. 2002). WHO (2000) has published recommendations for the use of antibiotics in food animals. Accordingly, many alternatives for the use of antibiotics have been tested.
Prevention of diarrhea in calves without resorting to antibiotics can be achieved by improving the intestinal microbial balance, such as decreasing Escherichia coli counts. Fructooligosaccharides and mannan oligosaccharides are included in prebiotics as nondigestible oligosaccharides (NDO). NDO prevent diarrhea and improve growth performance in calves (Donovan et al. 2002; Quigley et al. 2002; Heinrichs et al. 2003). NDO resist digestion in the small intestine and are potential substrates for bacteria that colonize the large intestine. Physiological effects of NDO include fermentation by bacteria colonizing the large intestine (Watanabe 1998; Van Loo et al. 1999; Otsuka et al. 2004). Cellooligosaccharide (CE) can be considered an NDO because of its beta-1–4 linkages (Satouchi et al. 1996). It was reported that CE feeding improved daily body weight gain (DG) in weanling pigs (Otsuka et al. 2004). In an in vitro study, CE affected the fermentation of organic acids by mixed ruminal bacteria (Callaway & Martin 1997; Lila et al. 2006). However, to date the effect of CE feeding in calves has not been clarified.
Addition of Bifidobacterium and Lactobacillus spp. to the diets of calves improved DG and fecal condition (Abe et al. 1995; Cruywagen et al. 1996). On the other hand, one study showed that the use of mixed microbial concentrate containing Lactobacillus spp. did not have a significant effect on calf performance (Jenny et al. 1991). Specific probiotics with regard to their energy sources include fructooligosaccharides, galactooligosaccharides and lactuloses, which are sources of nutrition for lactic acid bacteria, and can also be used by indigenous enterobacteria (Ogawa et al. 2005a). Therefore, inhibition of the growth of bacteria causing diarrhea, such as E. coli, is considered to be highly effective in combination with a lactic acid bacteria-specific substrate. In a recent study, Lactobacillus casei ssp. casei strains JCM1134T and JCM8129 showed an ability to utilize dextran (Ogawa et al. 2005a). Oral supplementation with L. casei ssp. casei strain JCM1134T together with dextran (synbiotic: SB) enhances humoral and cell-mediated immune responses in mice (Ogawa et al. 2005b), and increases milk production of Holstein dairy cows in heat conditions such as summer (Yasuda et al. 2007).
The objective of this study was to clarify the effects of oral CE or SB administration on the growth performance, feed intake, diarrhea, fecal enterobacterial counts and plasma hormone concentrations in Holstein calves.
MATERIALS AND METHODS
Animals, management and diets
Fifty-two female Holstein calves from the research organizations of six prefectures (Toyama, Chiba, Aichi, Ishikawa, Ibaraki and Kanagawa) were randomly assigned to three groups at birth in a balanced complete block design. All calves were given colostrum within 30 min of birth. Calves were divided into the CE feeding group (n = 16), SB feeding group (n = 18) and control group (n = 18). The experimental period was 90 days (0–90 days of age). Calves were cared for according to the Guide for the Care and Use of Agricultural Animals in Agricultural Research of the National Institute of Livestock and Grassland Science.
Calves were fed whole milk necessary for a daily body weight gain of 0.4 kg (AFFRC 1999) based on birth weight, divided into morning (09.00 hour) and evening (16.00 hour) feeds using a feeding bottle or a bucket with a nipple until weaning. The CE feeding group was fed CE (Nippon Paper Chemicals Co., Ltd, Tokyo, Japan) at 5 g/day by dissolving in whole milk. The SB feeding group was fed dextran 5 g/day and L. casei ssp. casei strain JCM1134T at 1.0 × 109 colony-forming units (CFU)/day (Meito Sangyo Co., Ltd, Aichi, Japan) by dissolving in whole milk. Post-weaning, CE or SB was dissolved in warm water. Calves had free access to water, calf starter without antibiotics (New Makestar, Zenkokurakunoshiryo Co., Ltd, Tokyo, Japan), and timothy hay (Cut 1, early bloom, Canada). They were weaned when the daily intake of calf starter reached 800 g, after which calf starter was given at a daily maximum of 2400 g. Calf starter and timothy hay samples were collected every other week and composited every other month for analysis of nutrient composition (Jikyushiryohinshitsuhyokakenkyukai 2001; Table 1).
| Item | Calf starter‡ | Timothy hay§ |
|---|---|---|
| Dry matter, % | 86.0 | 89.1¶ |
| Organic matter, % | 93.5 | 93.3¶ |
| Crude protein, % | 21.8 | 8.1¶ |
| Neutral detergent fiber, % | 20.5 | 63.5¶ |
| TDN††, % | 84.2 | 62.6‡‡ |
- † All values are expressed on a dry matter basis.
- ‡ ‡Calf starter: New Makestar, Zenkokurakunoshiryo Co., Ltd, Tokyo.
- § §Timothy Hay: Cut 1, early bloom, Canada.
- ¶ ¶The mean of values obtained in each research organization.
- †† ††TDN: total digestible nutrients.
- ‡‡ Standard Tables of Feed Composition in Japan [National Agricultural Research Organization (NARO) 2001].
Body weight was measured weekly before feeding in the morning (09.00 hour). Blood samples were also collected before feeding in the morning (09.00 hour) in heparin-containing sodium test tubes (Terumo Co., Ltd, Tokyo, Japan) via jugular vein puncture at 0, 7, 21 and 35 days of age, weaning time, and at 90 days. Blood samples were centrifuged at 20 000 × g for 30 min at 4°C. The plasma was then aspirated and stored at −20°C until analysis. Fecal samples were collected at 0, 35, 70 and 90 days of age, and stored at −20°C until use. Fecal consistency was scored and recorded daily according to the following definitions: 0 = firm; 1 = normal; 2 = soft but not runny; 3 = soft and runny; and 4 = watery (Cruywagen et al. 1996). Scores of more than 2 were considered to indicate diarrhea.
Analyses of fecal bacteria
The presence of fecal Lactobacillus sp. and E. coli were determined according to the methods of Liu et al. (2008) with certain modifications, and organism numbers were counted. In brief, 1 g of feces was suspended in 9 mL of sterilized physiological saline, and 10-fold serially diluted samples were used for analysis. For Lactobacillus sp., suspensions were spread on de Man Rogosa Sharpe agar medium (Becton Dickinson and Company, Franklin Lakes, NJ, USA) and anaerobically cultured at 37°C for 48 h. For E. coli, suspensions were spread on MacConkey agar medium (Eiken Chemical, Co., Ltd, Tokyo, Japan) and aerobically cultured at 37°C for 24 h. The number of bacteria in 1 g of feces was calculated from the number of colonies at the end of culturing. Microbial numbers were expressed as log10 CFU/g.
Blood sample analytical procedures
Plasma growth hormone (GH) concentration was determined by double antibody radioimmunoassay as described by Johke (1978). Plasma insulin concentration was measured using a commercially available radioimmunoassay kit (Insulin Eiken RIA kit; Eiken Chemical Co., Ltd) suitable for the measurement of bovine insulin concentrations (Itoh et al. 1997). Plasma concentrations of insulin-like growth factor I (IGF-I) were determined by double antibody radioimmunoassay (Hodate et al. 1990). All hormone concentrations were determined by the same assay to prevent interassay variation. Interassay coefficients of variation for GH, insulin and IGF-I radioimmunoassay were 7%, 3% and 5%, respectively.
Statistical analyses
Measurements were analyzed using a randomized block design by Fisher's protected least significant difference test. Differences were considered significant at P < 0.05. All measurements were analyzed using Stat View 5.0J (SAS Institute, Cary, NC, USA).
RESULTS
Changes in body weight are shown in Figure 1. Body weight at 56–90 days of age was greater (P < 0.05) in the CE feeding group than in the control group. At 56–63 days of age, body weight was greater (P < 0.05) in the CE feeding group than in the SB feeding group. At 70–90 days of age, body weight tended to be greater (P < 0.1) in the SB feeding group than in the control group, and at 84 days of age, it was greater (P < 0.05) in the SB feeding group than in the control group. Ninety-day body weight, DG and feed efficiency in the post-weaning period were greater (P < 0.05) in the CE feeding group than in the control group (Table 2). Differences in the days of weaning were not significant among the groups. The total fecal score tended to be lower (P < 0.1) in the SB feeding group than in the control group.
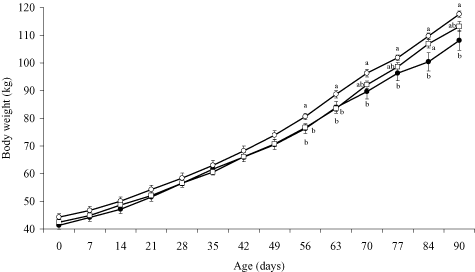
Changes in body weight in the control group (●), cellooligosaccharide (CE) feeding group (○), and synbiotic (SB) feeding group (□). Each point represents mean ± SEM. Different letters (a, b) indicate significant effects (P < 0.05) at the same age.
| Item | Control | CE† | SB‡ |
|---|---|---|---|
| Calves, no. | 18 | 16 | 18 |
| Body weight, kg | |||
| Initial | 40.5 ± 1.4 | 42.8 ± 1.1 | 42.2 ± 1.0 |
| Weaned time | 69.2 ± 1.5 | 72.4 ± 1.7 | 70.3 ± 1.2 |
| 90 days of age | 107.0 ± 3.3* | 116.3 ± 1.2* | 112.4 ± 1.8 |
| Daily gain, kg | |||
| Total period | 0.73 ± 0.03* | 0.82 ± 0.01* | 0.78 ± 0.02 |
| Pre-weaning period | 0.62 ± 0.02 | 0.6 ± 0.02 | 0.59 ± 0.02 |
| Post-weaning period | 0.88 ± 0.05* | 1.07 ± 0.02* | 1.01 ± 0.04 |
| Days of weaning | 46.3 ± 1.1 | 46.8 ± 1.0 | 47.5 ± 1.0 |
| Feed intake§, kg of dry matter | 128 ± 6 | 139 ± 5 | 132 ± 5 |
| Feed efficiency, daily gain per feed intake | |||
| Total period | 0.49 ± 0.01 | 0.51 ± 0.01 | 0.51 ± 0.01 |
| Pre-weaning period | 0.74 ± 0.03 | 0.70 ± 0.02 | 0.72 ± 0.03 |
| Post-weaning period | 0.39 ± 0.02* | 0.44 ± 0.01* | 0.43 ± 0.01 |
| Total fecal score¶ | 120 ± 6** | 115 ± 6 | 109 ± 5** |
| Diarrhea days†† | 9.2 ± 2.0 | 7.0 ± 1.8 | 5.5 ± 1.4 |
- * Means within a row with different superscripts differ significantly (P < 0.05).
- ** Means within a row with different superscripts tend to differ (P < 0.1). Each value is mean ± SEM.
- † †CE: The cellooligosaccharide (CE) feeding group.
- ‡ ‡SB: The synbiotic (SB) feeding group.
- § §Feed intake equals total dry matter of feed fed to calves, including milk, calf starter, and timothy hay.
- ¶ Recorded cumulative total for the experiment (0 = firm, 1 = normal, 2 = soft but not running, 3 = soft and running, 4 = watery).
- †† ††Total days of fecal score of more than 2.
Changes in fecal bacterial counts are shown in Figure 2. No differences were observed in fecal Lactobacillus sp. counts between different management regimens. Fecal E. coli counts were lower (P < 0.05) in the SB feeding group than in the control group at 70 days of age.
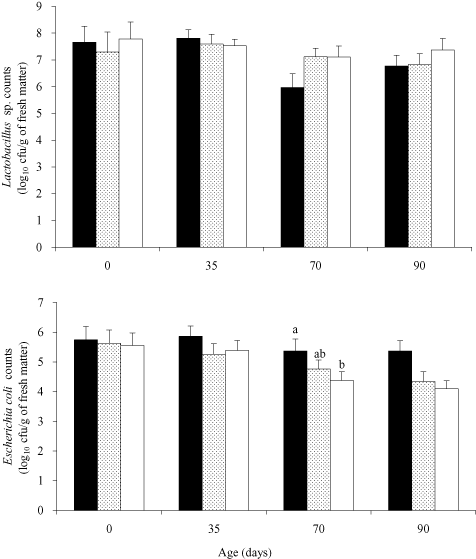
Changes in fecal Lactobacillus sp. and Escherichia coli counts in the control group ( ), cellooligosaccharide (CE) feeding group (
), cellooligosaccharide (CE) feeding group ( ), and synbiotic (SB) feeding group (□). Each point represents mean ± SEM. Different letters (a, b) indicate significant effects (P < 0.05) at the same age.
), and synbiotic (SB) feeding group (□). Each point represents mean ± SEM. Different letters (a, b) indicate significant effects (P < 0.05) at the same age.
Changes in plasma GH, insulin and IGF-I concentrations are shown in Figure 3. No differences were recognized in plasma GH concentration among the groups. Plasma insulin concentration was higher (P < 0.05) in the CE feeding group than in the control group at 90 days of age. Plasma IGF-I concentration tended to be higher (P < 0.1) in the CE feeding group than in the control group at 90 days of age.
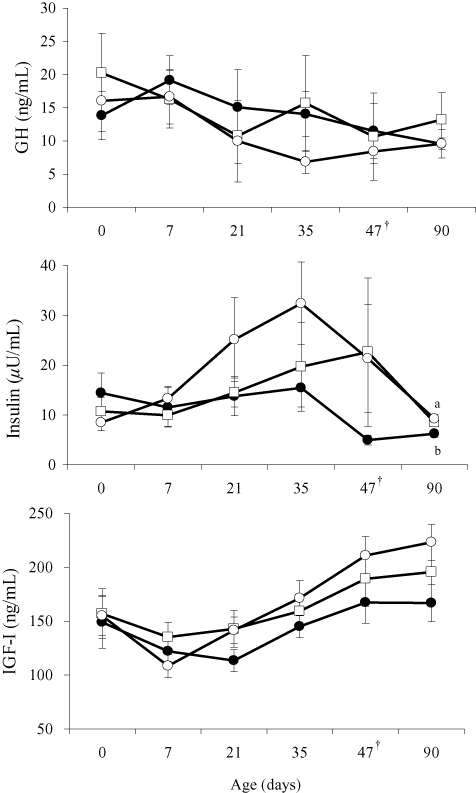
Changes in plasma growth hormone (GH), insulin, and insulin-like growth factor I (IGF-I) concentrations in the control group (●), cellooligosaccharide (CE) feeding group (○), and synbiotic (SB) feeding group (□). Each point represents mean ± SEM. Different letters (a, b) indicate significant effects (P < 0.05) at the same age. †Days of weaning.
DISCUSSION
Major effects of CE were observed on ruminal fermentation as increased propionate and total volatile fatty acid (VFA) levels, which suggests that CE affected the fermentation pattern by providing carbon and energy sources as well as by influencing electron disposal toward propionate production (Callaway & Martin 1997). Lila et al. (2006) also found that CE increased propionate and total VFA levels in vitro, and suggested that CE affected ruminal fermentation and digestibility. In addition, the increase in VFA production caused by CE would stimulate papillary growth in the rumen of calves (Tamate et al. 1962). Thus, the improvements of DG and feed efficiency in the CE feeding group during the post-weaning period observed here could be caused by increased VFA absorption from the rumen. Although total ruminal VFA contents were not measured in this study, it is possible that VFA production in the rumen started after the weaning period. Haga et al. (2008) reported that the plasma concentration of acetate and β-hydroxybutyrate increased after weaning in calves, which suggests the absorption of considerable amounts of VFA from the rumen.
In the intestine, CE mainly generates butyrate by intestinal bacterial fermentation (Watanabe 1998). Furthermore, the VFA content of the colon is likely to be a physiological luminal stimulus regulating colonic motility in rats (Yajima 1985). Therefore, the increase in production and absorption of VFA, especially butyrate, in CE feeding may also be expected in the lower digestive tract, and may contribute to the improvement of the growth performance observed here.
VFAs, especially propionate and butyrate, absorbed from the intestinal tract increase plasma insulin concentrations in ruminants (Kobayashi & Itabashi 1987; Itoh et al. 1998; Matsunaga et al. 1999). Kobayashi and Itabashi (1987) have reported an increase in plasma insulin concentrations by intraruminal and intraduodenal infusion of propionate and butyrate in weaned calves. In our study, plasma insulin concentrations were higher in the CE feeding group than in the control group at 90 days of age. This may be due to increased absorption of propionate and butyrate from the digestive tract. However, it is unlikely that propionate and butyrate produced from CE itself, which was administrated at 09.00 hour, affected the plasma insulin level on the following day. If anything, it is likely due to the change in the number and/or composition of ruminal microbes, which resulted in the increase in ruminal digestibility and VFA production, especially propionate. Lila et al. (2006) reported that CE increased the number of cellulolytic bacteria and fiber digestibility with the increase in propionate and total VFA production.
Positive correlations exist between serum IGF-I concentrations and post-weaning feed efficiency in calves (Stick et al. 1998). Body weight positively correlates with IGF-I levels in weaned calves (Suda et al. 2003). In the present study, we also observed higher growth performance with a tendency for higher IGF-I concentration in CE feeding compared to that in teh control group during the post-weaning period. The increase in IGF-I in CE feeding may be caused by CE inducing an increase of nutrient absorption in the gastrointestinal tract, inclusive of ruminal VFA, in post-weaning calves. Indeed, the high protein and energy intake diet increased DG and plasma IGF-I concentration in calves (Brown et al. 2005).
Oral administration of probiotics containing Bifidobacterium pseudolongum or Lactobacillus acidophilus to calves at 7–56 days of age improves their DG, feed conversion and fecal condition (Abe et al. 1995). L. acidophilus supplementation for calves during the first 2 weeks of life showed a similar effect (Cruywagen et al. 1996). On the other hand, the use of mixed microbial concentrate containing L. acidophilus, L. lactis and Bacillus subtilis did not cause any significant difference in DG and feed efficiency in calves (Jenny et al. 1991). Our study used L. casei specifically to utilize dextran, but improved DG and feed efficiency were not observed during the test period. SB feeding during the test period did not affect fecal E. coli counts, but produced a slight decrease in the total fecal score. This may have been because of changes in intestinal flora. Further studies are needed to examine the factors in order to improve the intestinal environment of calves fed L. casei.
In conclusion, our results indicate that CE feeding in calves induced improvement in DG and feed efficiency during the post-weaning period. Although SB feeding showed a slight decrease in total fecal score, there was less effect on the growth performance and fecal E. coli counts. Further studies are required to improve growth performance via modification of the intestinal environment in calves.
ACKNOWLEDGMENTS
This study was funded by the Research Project for Utilizing Advanced Technologies in Agriculture, Forestry, and Fisheries (No. 18068) from the Ministry of Agriculture, Forestry, and Fisheries of Japan.




