Neuroendocrine mechanism of seasonal reproduction in birds and mammals
ABSTRACT
In temperate zones, animals use changes in day length as a calendar to time their breeding season. However, the photoreceptive and neuroendocrine mechanisms of seasonal reproduction are considered to differ markedly between birds and mammals. This can be understood from the fact that the eye is the only photoreceptive organ, and melatonin mediates the photoperiodic information in mammals, whereas in birds, photoperiodic information is directly received by the deep brain photoreceptors and melatonin is not involved in seasonal reproduction. Recent molecular and functional genomics analysis uncovered the gene cascade regulating seasonal reproduction in birds and mammals. Long day-induced thyroid stimulating hormone in the pars tuberalis of the pituitary gland regulates thyroid hormone catabolism within the mediobasal hypothalamus. Further, this local thyroid hormone catabolism appears to regulate seasonal gonadotropin-releasing hormone secretion. These findings suggest that although the light input pathway is different between birds and mammals (i.e. light or melatonin), the core mechanisms are conserved in these vertebrates.
INTRODUCTION
In the majority of birds and mammals that inhabit temperate regions, it is found that the member of the species ensures that the birth of the offspring occurs in spring or summer, which is appropriate for their survival. Small birds and mammals that have short incubation or gestation periods, or members of species that have a gestation period of approximately 1 year mate during the spring; they are referred to as ‘long-day breeders.’ In contrast, species with a gestation period of around 6 months mate during the fall; they are referred to as ‘short-day breeders.’ All of these animals use changing day length (photoperiod) as a time standard for them to accurately time their breeding seasons (Rowan 1925; Reiter 1980). However, the photoreceptive and neuroendocrine mechanisms underlying seasonal reproduction are considered to be markedly different between birds and mammals (Dawson et al. 2001; Goldman 2001). In mammals, light information is received by the eyes and transmitted to the pineal gland through the master circadian pacemaker – the suprachiasmatic nucleus (SCN) – and translated into melatonin secretion patterns (Inouye & Kawamura 1979; Reiter 1980; Ralph et al. 1990; Arendt 1995). In contrast, in birds, eyes are not essential for the regulation of seasonal reproduction and light information is received by deep brain photoreceptors that are believed to lie outside of the retina and pineal gland (Homma et al. 1979; Oliver & Bayle 1982); further, master circadian pacemakers are located not only in the SCN but also in the eyes and the pineal gland (Deguchi 1979; Kasal et al. 1979; Ebihara & Kawamura 1981; Takahashi & Menaker 1982; Yoshimura et al. 2001; Ikegami et al. 2009). Melatonin is generally secreted during the night, and the duration of melatonin secreted reflects the length of night in both birds and mammals. Although melatonin plays a critical role in the regulation of seasonal reproduction in mammals, it has little or no importance in the regulation of seasonal reproduction in birds (Gwinner et al. 1997).
Japanese quail: the best model organism for the study of seasonal reproduction
Although mice and fruit flies are used in a wide variety of biological studies, they are generally considered to be non-seasonal organisms. Therefore, it was important that an appropriate model organism be selected to understand the neuroendocrine mechanisms regulating seasonal reproduction. Among vertebrates, birds possess highly sophisticated photoperiodic mechanisms and show robust responses to photoperiodic changes (Rowan 1925; Dawson et al. 2001). This could be one of their adaptations to flight; further, the duration of breeding seasons of birds tends to be more restricted than those of mammals. Among avian species, Japanese quail (Coturnix japonica) has been proven to be the best model organism for the study of seasonal reproduction mechanisms (Follett & Sharp 1969; Follett et al. 1998; Yoshimura et al. 2003).
Identification of key genes regulating seasonal reproduction
In birds, the mediobasal hypothalamus (MBH) is considered to be the center for seasonal reproduction (Sharp & Follett 1969; Juss 1993; Saldanha et al. 1994; Meddle & Follett 1997). Follett and Sharp (1969) demonstrated that when short-day quail were subjected to light pulses between 12 and 16 h after dawn, the secretion of luteinizing hormone (LH) and testicular growth are stimulated. This observation has been exploited to elucidate the key molecular events that occur in the MBH. Differential subtractive hybridization analysis of gene expression performed in the quail MBH demonstrated an increase in the expression of the gene encoding type 2 iodothyronine deiodinase (DIO2) in response to exposure to a light pulse (Yoshimura et al. 2003). In addition, further analysis of MBH demonstrates that expression of the gene encoding type 3 iodothyronine deiodinase (DIO3) is up-regulated under short-day conditions and down-regulated under long-day conditions (Yasuo et al. 2005). When the temporal gene expression profiles of DIO2 and DIO3 within the MBH were examined in quail that were subjected to long-day stimulus, reciprocal switching between DIO2 and DIO3 expression was observed approximately 18 h after dawn of the first long day (Yasuo et al. 2005). Importantly, this two-gene switching preceded the increase in plasma LH concentration that occurs at approximately 22 h after dawn of the first long day. Electrolytic lesions in the expression sites of DIO2 and DIO3 within the quail MBH have been demonstrated to block the photoperiodic response of gonads and LH secretion without destroying the GnRH terminals in the median eminence (Sharp & Follett 1969; Juss 1993), suggesting that DIO2 and DIO3 expressions play critical roles within the MBH in seasonal reproduction.
Thyroid hormones and seasonal reproduction
It has been well known for several decades that thyroid hormones are involved in the regulation of the seasonal reproduction in birds and mammals in some manner (Follett & Nicholls 1985; Nicholls et al. 1988; Dawson et al. 2001). Thyroxine (T4) is a prohormone, and triiodothyronine (T3) is a biologically active thyroid hormone (Fig. 1). DIO2 is a thyroid hormone-activating enzyme that converts T4 to T3 whereas DIO3 is a thyroid hormone-inactivating enzyme that converts T4 and T3 to the inactive metabolites, rT3 and T2, respectively (Chopra et al. 1978). The T3 and T4 content in the quail MBH increases approximately 10-fold under long-day conditions as compared with short-day conditions (Yoshimura et al. 2003). However, parallel changes are not observed in the plasma or other brain regions such as the cerebellum and optic tectum. These results suggest that photoperiodically regulated gene switching between DIO2 and DIO3 within the MBH results in the local activation of thyroid hormones within this region. The functional significance of such local thyroid hormone conversion in the MBH from the perspective of photoperiodic signaling has been proven using intracerebroventricular (ICV) infusion of thyroid hormones or DIO2 inhibitor. ICV infusion of T3 induced testicular growth under short-day conditions, whereas ICV infusion of DIO2 inhibitor under long-day conditions attenuated testicular growth (Yoshimura et al. 2003). It has been confirmed that the local activation of thyroid hormone catabolism within the MBH is central to the regulation of photoperiodic response in various mammalian species, including long-day breeders (Djungarian hamsters: Watanabe et al. 2004, 2007; Barrett et al. 2007; Freeman et al. 2007, Syrian hamsters: Revel et al. 2006; Yasuo et al. 2007a), rats (Yasuo et al. 2007b) and short-day breeding goats (Yasuo et al. 2006).
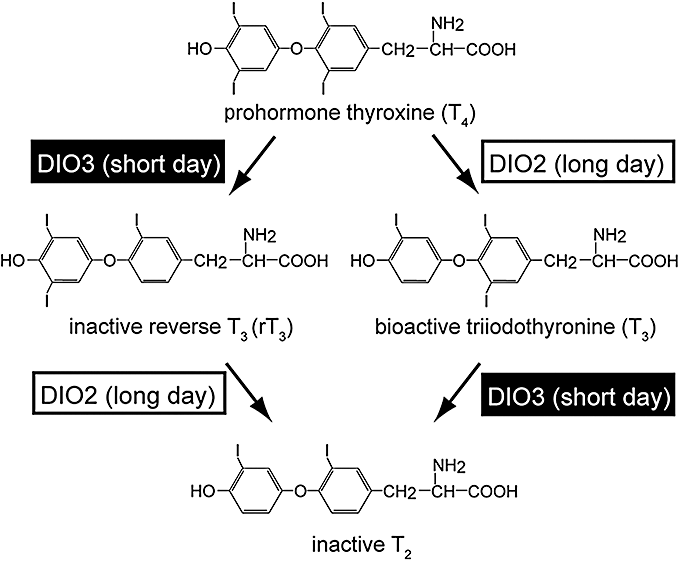
Type 2 iodothyronine deiodinase (DIO2) converts bioactive T3 from prohormone T4 by outer ring deiodination under long-day conditions, whereas type 3 iodothyronine deiodinase (DIO3) metabolizes both T4 and T3 to a inactive form by inner ring deiodination under short-day conditions (modified from Nakao et al. 2008b).
The target of thyroid hormone action
Since thyroid hormone receptors (TRα, TRβ and RXRα) were observed to be expressed in the median eminence, the target site of MBH T3 induced under long-day conditions appeared to be the median eminence (Yoshimura et al. 2003). It is well established that thyroid hormones are important regulators of development and the plasticity of the central nervous system (Bernal 2002). Examination of the ultrastructure of the median eminence of quail were perfomed by electron microscopy under short- and long-day conditions in order to understand the functional significance of this local thyroid hormone conversion (Yamamura et al. 2004). In this study, we demonstrated dynamic morphological changes in gonadotropin-releasing hormone (GnRH) nerve terminals and glial cell processes. GnRH nerve terminals are in close proximity to the basal lamina under long-day conditions, which enables GnRH secretion; however, this is not the case under short-day conditions when the GnRH terminals are extensively encased by the end-feet of glial cell processes. Since the retraction of glial cell end-feet around nerve terminals in the median eminence was mimicked in quail treated with T3 under short-day conditions to stimulate testicular growth (Yamamura et al. 2006), this morphological change in neuron–glial interaction appears to be involved in the regulation of seasonal GnRH secretion.
Thyroid hormone uptake in the MBH
Although thyroid hormones have long been believed to traverse plasma membranes by passive diffusion due to their hydrophobic nature, the existence of a membrane transport system for thyroid hormone has been recently pointed out. Expression of thyroid hormone transporters such as transthyretin, thyroxine-binding globulin, and albumin have been reported to be altered by changing day length in the case of Siberian hamsters (Prendergast et al. 2002); however, the expression of these genes is not observed in the MBH of quail. Recently, certain members of the organic anion-transporting polypeptide (Oatp) family have been shown to transport thyroid hormones in mammals (Abe et al. 2002; Hagenbuch & Meier 2004); further, the expression of a member of this family in the quail brain has been investigated (Nakao et al. 2006). Among 10 Oatp family genes, the gene encoding Oatp1c1 was highly expressed within the MBH in which DIO2 and DIO3 are expressed and also in the choroid plexus. Studies with stably expressed functional proteins of chicken Oatp1c1 in Chinese hamster ovary cells revealed that Oatp1c1 is a transporter that has high specificity for T4. These observations suggest that Oatp1c1 could be involved in the transportation of T4 from the general circulation to cerebrospinal fluid (CSF) and from there to the ependymal cells lining ventro-lateral walls of the third ventricle in the MBH, in which DIO2 and DIO3 genes are expressed.
Pars tuberalis thyroid-stimulating hormone (TSH) is the master factor regulating seasonal reproduction
The availability of the chicken genome sequence allowed the performance of a genome-scale expression analysis of seasonal reproduction. Yoshimura and colleagues performed genome-scale expression analysis by using a high-density chicken oligonucleotide microarray and clarified the gene cascade regulating seasonal reproduction (Nakao et al. 2008a) (Fig. 2). When quail were transferred from short- to long-day conditions, two waves of gene expression were induced during the first day of photostimulation. The first wave comprised two genes and occurred in the pars tuberalis of the pituitary gland approximately 14 h after dawn of the first long day; the second wave comprised 11 genes and occurred in the ependymal cells lining the ventrolateral walls of the third ventricle 4 h after the first wave (Nakao et al. 2008a). DIO2 and DIO3 were associated with the genes of the second wave. Although one of the genes associated with the first wave –EYA3 (eyes absent 3) – was a transcriptional co-activator, distinct localization of the EYA3 (pars tuberalis) and DIO2/DIO3 (ependymal cells) excluded the possibility of EYA3 regulation of DIO2 and DIO3 genes. Therefore, the other gene associated with the first wave, namely, TSHB (thyroid stimulating hormone β subunit), appeared to be involved in the regulation of the genes associated with the second wave, including DIO2 and DIO3. It is important to note that the expression of TSH receptor (TSHR) mRNA and the positive binding of 125I-TSH were observed in the ependymal cells in which DIO2 and DIO3 are expressed. It is interesting to note that median eminence is one of the circumventricular organs that lies outside the blood–brain barrier (Ganong 2000). Therefore, TSH derived from pars tuberalis of the pituitary gland can act on the ependymal cells within the MBH. Upon ICV injection of TSH in quail subjected to short-day conditions, DIO2 expression was induced within the MBH in a dose-dependent manner; in contrast, upon ICV injection of TSHβ antibody (immunoneutralization) in quail subjected to long-day conditions, DIO2 expression was suppressed (Nakao et al. 2008a). The expression of DIO2 in the human thyroid gland is regulated through a TSHR-Gsα-cAMP regulatory cascade (Murakami et al. 2001a,b); further, there are several putative cAMP-responsive elements (CREs) in the 1.5-kb 5′-upstream region of quail DIO2. By performing the luciferase assay, it was demonstrated that the promoter activity of the quail DIO2 gene by TSH is regulated by the Gsα -cAMP signaling pathway.
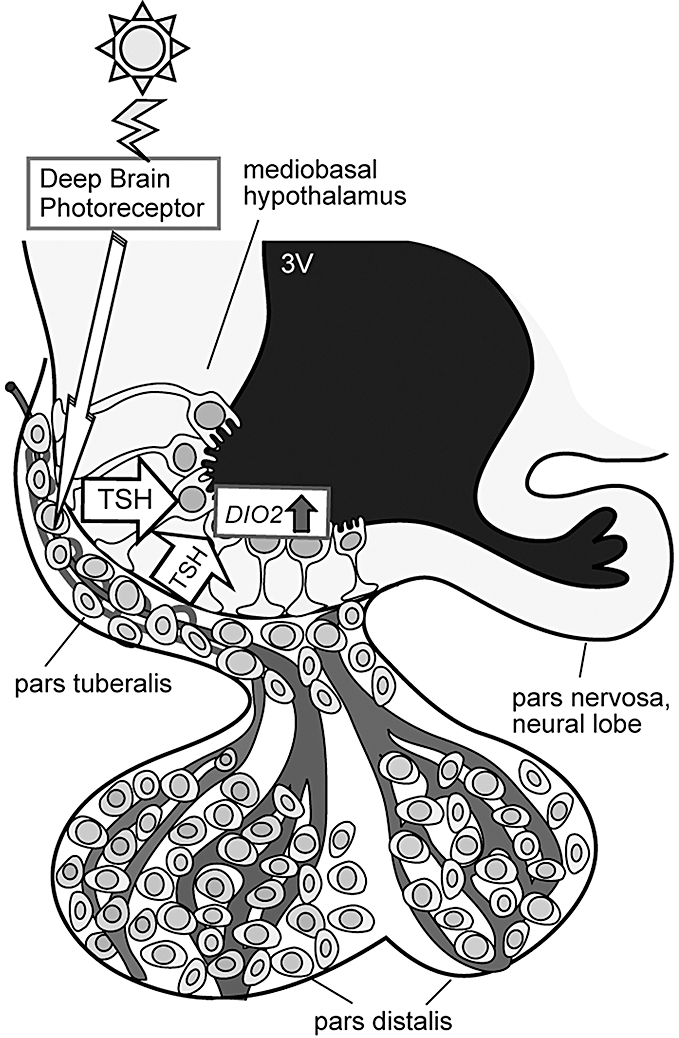
Light information received by unidentified deep brain photoreceptors induces expression of thyroid-stimulating hormone (TSH) in the pars tuberalis of the pituitary gland. The pars tuberalis TSH acts on the TSH receptor localized in ependymal cells lining ventrolateral walls of the third ventricle and induces expression of DIO2 (modified from Nakao et al. 2008a).
A further microarray analysis performed between quail subjected to short-day and long-day conditions for 2 weeks identified 124 up-regulated and 59 down-regulated genes (Nakao et al. 2008a). The up-regulated genes include DIO2 and the down-regulated genes include DIO3. In addition, the expression of TSHB was observed to be up-regulated. Chronic ICV administration of TSH by using osmotic mini-pump induced full testicular growth, indicating that the photo-induced increase in pars tuberalis TSH not only plays a role in initiating photo-induced LH secretion, but is also necessary to maintain reproductive activity under continuous long-day conditions.
The pars tuberalis TSH in mammals
It has been suggested for several decades that the pars tuberalis relays photoperiodic information (Morgan & Williams 1996). Although the photoperiodic regulation of TSH in the pars tuberalis of the hamster has also been known for several decades (Wittkowski et al. 1988; Bockmann et al. 1996), its physiological function remains unclear. Features of the thyrotrophs in the pars tuberalis are markedly different from those in the pars distalis. First, in the pars distalis thyrotrophs, thyrotropin-releasing hormone (TRH) receptors and T3 receptors are expressed, while these receptors are absent in the pars tuberalis (i.e. the pars tuberalis TSH is independent from TRH regulation and T3 negative feedback regulation) (Bockmann et al. 1997). Second, MT1 melatonin receptor mRNA is expressed in the thyrotroph of the pars tuberalis (Klosen et al. 2002), whereas melatonin receptors are absent in the pars distalis (i.e. photoperiodic/melatonin-regulation of TSH is only observed in the pars tuberalis but not in the pars distalis) (Wittkowski et al. 1988). In addition to these lines of evidence, it was also reported that melatonin receptors are not expressed in the ependymal cells of MBH in which the DIO2 and DIO3 genes are expressed (Schuster et al. 2000; Song & Bartness 2001). Therefore, pars tuberalis TSH appeared to mediate the melatonin regulation of DIO2 and DIO3 in mammals. Laboratory mice are generally considered to be insensitive to photoperiod. However, since transgenic and knockout techniques are available, mice are excellent animal models for elucidating the molecular mechanisms underlying various physiological functions. Therefore, the effect of changing day length on TSHB, DIO2 and DIO3 was examined in mice. Although no significant difference in testicular size between mice kept under short-day and long-day conditions was observed, TSHB, DIO2 and DIO3 were observed to be photoperiodically regulated in the CBA/N mice as in the case of quail (Ono et al. 2008). This result suggested that CBA/N mice are potentially capable of transforming photoperiodic information into neuroendocrine responses at the level of the MBH. In contrast to CBA/N mice, most of the inbred strains of mice (i.e. C57BL/6J, 129/Sv) are known to not produce detectable levels of melatonin due to the deficiency of enzyme activity that controls melatonin synthesis (Ebihara et al. 1986; Goto et al. 1989; Roseboom et al. 1998). As expected, melatonin-deficient C57BL/6J mice were blind to photoperiodic changes, but melatonin administration to this strain of mice mimicked short-day effects on the abovementioned gene expression (Ono et al. 2008). This study has established that mice could function as suitable models for investigating molecular mechanisms of seasonal reproduction at the hypothalamus–hypophysial level. In addition to these analyses, the effect of melatonin on TSHR-null mice was examined. In TSHR-knockout mice fed with a diet supplemented with thyroid powder in concentrations that produce serum T3 and T4 in the normal range levels (Marians et al. 2002), the effects of melatonin administration on TSHB, DIO2 and DIO3 expression were abolished (Ono et al. 2008). These results clearly demonstrated that TSH signaling is also crucial for the regulation of seasonal signal transduction in mice. It is also important to note that the involvement of TSH in the regulation of seasonal reproduction has also been reported in sheep (Hanon et al. 2008).
Conclusion
The neuroendocrine mechanisms regulating seasonal reproduction have long been believed to be markedly different between birds and mammals. However, recent molecular and functional genomics analyses uncovered that TSH is a common master factor regulating seasonal reproduction in both birds and mammals (Fig. 3). Genetic manipulation of pars tuberalis TSH may lead to changes in seasonal breeders to non-seasonal breeders and may contribute to an improvement in animal production in the future.
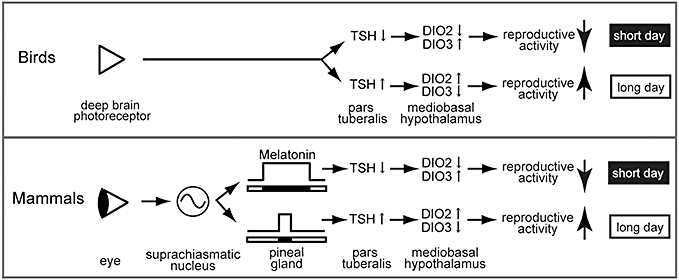
The neuroendocrine mechanisms involved in the control of seasonal reproduction in long-day breeding birds and mammals. In birds, long-day stimulus is perceived by deep brain photoreceptors and regulates the expression of thyroid-stimulating hormone (TSH) in the pars tuberalis. In mammals, light information is received by the eye and is transmitted to the pineal gland through the suprachiasmatic nucleus and controls the production and secretion of melatonin. The melatonin secretion pattern encodes the duration of night. TSH is a master factor regulating seasonal reproduction in both birds and mammals. (Modified from Ono et al. 2008).
ACKNOWLEDGMENTS
This work was supported by Grant-in-Aid for Young Scientists (S) (19678002) from the Japanese Society for the Promotion of Science, the Mitsubishi Foundation and the Toyoaki Scholarship Foundation.




