Summary of the US FDA Approval of Belatacept
Abstract
Elements of the Food and Drug Administration (FDA) review of the clinical data that supported the approval of the Biologics License Application (BLA) for belatacept for prophylaxis of organ rejection in adult patients receiving a kidney transplant are summarized. The article is not intended as a comprehensive summary of the entire belatacept data submission. Rather, the discussion is meant to illustrate aspects of the FDA's process for evaluating efficacy and safety, using belatacept as an example.
Abbreviations:
-
- AR
-
- acute rejection
-
- BLA
-
- Biologics License Application
-
- BMS
-
- Bristol-Myers Squibb Company
-
- BPAR
-
- biopsy-proven acute rejection
-
- CMV
-
- cytomegalovirus
-
- CNI
-
- calcineurin inhibitor
-
- CNS
-
- central nervous system
-
- CsA
-
- cyclosporine
-
- EBV
-
- Epstein–Barr virus
-
- ECD
-
- extended criteria donor
-
- FDA
-
- Food and Drug Administration
-
- FDAAA
-
- Food and Drug Administration Amendments Act
-
- GFR
-
- glomerular filtration rate
-
- DHCP
-
- Dear Healthcare Professional
-
- LD
-
- living donor
-
- LI
-
- less intense
-
- MDRD
-
- modification of diet in renal disease
-
- MI
-
- more intense
-
- MMF
-
- mycophenolate mofetil
-
- NI
-
- noninferiority
-
- NODAT
-
- new onset diabetes after transplantation
-
- PTLD
-
- posttransplant lymphoproliferative disorder
-
- PML
-
- progressive multifocal leukoencephalopathy
-
- PMR
-
- Postmarketing Requirement
-
- REMS
-
- Risk Evaluation and Mitigation Strategy
-
- SCD
-
- standard criteria donor
-
- UNOS
-
- United Network for Organ Sharing
Introduction
Belatacept (Nulojix®) is a recombinant soluble fusion protein developed by Bristol-Myers Squibb Company (BMS) and approved by the US Food and Drug Administration (FDA) in June 2011 for the indication of prophylaxis of organ rejection in adult kidney transplant recipients. It is to be used in combination with basiliximab induction, mycophenolate mofetil (MMF), and corticosteroids (1). Efficacy and safety for this indication was demonstrated in two phase 3 clinical trials conducted in de novo kidney transplant patients. The package insert lists two limitations of use (1) belatacept is to be used only in Epstein–Barr virus (EBV) seropositive patients due to the increased risk of posttransplant lymphoproliferative disorder (PTLD) in EBV seronegative patients, predominantly PTLD of the central nervous system (CNS), and (2) belatacept has only been found to be safe and effective in kidney transplant patients (2). The importance of this second limitation is that increased graft loss and mortality was seen in a clinical trial of liver transplant recipients (3). These results became available during the FDA review of belatacept and this trial is referred to below as study 4.
The approved belatacept dosing regimen for kidney transplantation (Table 1) corresponds to the “less intense” (LI) regimen studied in the two phase 3 clinical trials. The package insert includes a warning against using higher than the recommended doses and/or more frequent dosing because a “more intense” (MI) regimen, also studied in the phase 3 trials, resulted in more cases of PTLD involving the CNS, progressive multifocal leukoencephalopathy (PML) and other CNS infections, and more efficacy failures compared to the approved regimen. BMS did not seek approval of the MI regimen.
| Dosing for initial phase | Dose |
|---|---|
| Day 1 (day of transplantation, prior to implantation) and day 5 (approximately 96 h after day 1 dose) | 10 mg/kg |
| End of week 2 and week 4 after transplantation | 10 mg/kg |
| End of week 8 and week 12 after transplantation | 10 mg/kg |
| Dosing for maintenance phase | Dose |
| End of week 16 after transplantation and every 4 weeks (plus or minus 3 days) thereafter | 5 mg/kg |
- *Use only with silicone-free disposable syringe provided in the container. The dose prescribed for the patient must be evenly divisible by 12.5 mg (e.g. evenly divisible increments are 0, 12.5, 25, 37.5, 50, 62.5, 75, 87.5 and 100) in order for the drug to be accurately prepared for administration.
Background on Belatacept Biologic License Application (BLA)
The applicant, BMS, submitted the Biologics License Application (BLA) for belatacept to FDA on June 30, 2009, following multiple years of preclinical and clinical testing. Based on a preliminary assessment of the data contained in the BLA and internal classification policy, the FDA review division determined that the BLA for belatacept would receive a standard 10-month review (4). During this 10-month period, the FDA staff performed a thorough evaluation of information related to product manufacturing, preclinical testing, pharmacokinetics, pharmacodynamics, as well as efficacy and safety.
The results of the phase 3 clinical trials for belatacept were presented and discussed during an open, public FDA Advisory Committee meeting on March 1, 2010. FDA Advisory Committee meetings are held to discuss specific applications in order to obtain independent opinions and recommendations from outside scientific experts and also provide transparency to the drug review process. After hearing presentations by both BMS and the FDA of complete clinical trial data through 12 months and additional safety information through 24 months, and discussing the findings, the committee voted 13 (yes) to 5 (no) for approval of belatacept (5).
Belatacept was not approved at the end of the first review cycle due to several issues, primarily related to product manufacturing, which were identified during the review process. BMS was able to provide additional information during the latter part of 2010. In that submission, the product quality issues were addressed and 36-month data from the phase 3 trials on outcomes of mortality, graft loss, glomerular filtration rate (GFR), PTLD and other serious adverse reactions were provided. Upon review of the additional information, FDA determined BMS had adequately addressed all outstanding issues and belatacept was approved on June 15, 2011.
FDA's Overall Approach to the Efficacy and Safety Assessment of Belatacept
When reviewing clinical data submitted as part of a BLA, the FDA evaluates two important characteristics: (1) the effectiveness of the product and (2) the safety profile, or adverse reactions, associated with use of the product. To make this determination, FDA is required to rely on “evidence consisting of adequate and well controlled investigations, including clinical investigations” (6,7).
As stated earlier, the efficacy of belatacept in de novo kidney transplantation was primarily assessed in two open-label, randomized, multicenter, active-controlled phase 3 clinical trials. The results of these adequate and well-controlled trials were previously summarized in this journal (8,9). Briefly, these trials evaluated two dose regimens of belatacept: the approved (recommended) dosage regimen (LI regimen), as described in Table 1, and a regimen with higher cumulative doses and more frequent dosing than the recommended dosage regimen (MI regimen). These belatacept regimens were compared to a cyclosporine (CsA) control regimen. All three treatment groups also received basiliximab induction MMF and corticosteroids. Study 1 (Protocol IM103008) enrolled recipients of living donor (LD) and standard criteria deceased (SCD) donor organs and study 2 (Protocol IM103027) enrolled recipients of extended criteria donor (ECD) organs. Additional details regarding the design of the clinical trials may be found in the original publications and also in the belatacept package insert (2,8,9).
The safety data in the belatacept BLA were primarily derived from studies 1 and 2 in a total of 401 patients who received the LI regimen compared to the 405 who received the CsA control regimen. An additional 403 patients were treated with the belatacept MI regimen. Safety data from other trials using belatacept were also considered, particularly a phase 2 trial in kidney transplant (Protocol IM103100; study 3) and a phase 2 trial in liver transplant (Protocol IM103045; study 4) which utilized belatacept regimens which were similar, but not identical, to the LI and MI regimens in studies 1 and 2.
The FDA's evaluation of efficacy is based on quantitative analyses of prespecified hypotheses with respect to the endpoints, as discussed below. The evaluation of safety includes assessment of all treatment emergent adverse events of a product and includes additional considerations beyond those provided by formal statistical testing. A final determination of whether a product should be approved includes consideration of the benefits and risks of the product, including the target patient population, unmet medical needs (if any), demonstration of efficacy and the nature and severity of adverse reactions. Consideration is also given as to whether or not a Risk Evaluation and Mitigation Strategy (REMS) is needed to ensure that the benefits outweigh the risks.
Efficacy Endpoints and Statistical Analyses in the Phase 3 Trials
Both study 1 and study 2 were designed to be 3 years in length, with the primary analysis of efficacy at 1 year (month 12).
The primary efficacy endpoints, as prespecified in the protocols, were
- •
patient and graft survival at 12 months,
- •
renal impairment as assessed by measured glomerular filtration rate (GFR) < 60 mL/min/1.73 m2 at 12 months or a decrease in measured GFR ≥10 mL/min/1.73 m2 from Month 3 to Month 12, as measured by the cold-iothalamate method and
- •
acute rejection (AR), defined as biopsy confirmed histological evidence of rejection along with clinical evidence, at 12 months (study 1 only)
Secondary efficacy endpoints prespecified in the protocols included:
- •
AR, defined as biopsy confirmed histological evidence of rejection along with clinical evidence, at 12 months (study 2 only)
- •
Mean GFR
In both trials, the endpoints of patient and graft survival and AR were to be analyzed at 12 months for noninferiority (NI). BMS's proposed margin was 10% for patient and graft survival and 20% for AR. Renal impairment, as defined above, was to be assessed for superiority (10). Based on the protocol, the primary efficacy endpoints were to be tested in order for significance. Once one endpoint failed, testing would stop. AR was defined by BMS as biopsy-proven acute rejection (BPAR) “either clinically suspected by protocol defined reasons or clinically suspected by other reasons and treated”. The FDA has traditionally assessed BPAR as the primary endpoint in clinical trials for the evaluation of efficacy for various immunosuppressants for the indication of prophylaxis of rejection in kidney transplant recipients. According to FDA's definition, all BPAR events, whether or not clinically suspected, are included in the analysis. Patients who have not developed BPAR, but experience death, graft loss, or loss to follow-up are also included in the analysis and imputed as failures (i.e. as having experienced BPAR), as they do not have complete data at the time point of analysis. This endpoint has also been referred to as “efficacy failure”.
Other prespecified endpoints included the incidence of new onset diabetes after transplantation (NODAT), hypertension and hyperlipidemia.
Discussion of Efficacy Endpoints and Statistical Analyses
BMS and FDA discussed the design of studies 1 and 2, including the efficacy endpoints before the trials were conducted. However, during the review of the BLA submission, FDA noted that the prespecified endpoints used to assess efficacy were not sufficient to provide the requisite substantial evidence of efficacy required to support approval, as discussed below. Therefore, in addition to evaluating the protocol-defined endpoints, the FDA conducted additional analyses for each trial and based the assessment of efficacy on a BPAR endpoint, as has been used to support approval of other transplant immunosuppressants (10). The following discussion summarizes FDA's approach to the primary endpoints used for the analysis of the 12-month results in the belatacept clinical trials.
Defining a noninferiority (NI) margin
In order to be able to conclude that a drug has been shown to have efficacy (i.e. that it has an effect greater than zero) based on an assessment of NI, a scientific justification of the NI margin is needed (11). Selection of a NI margin is a two-step process. The first step involves determining the treatment effect of the active control, in this case CsA, using available literature data. Without this step, the test drug (belatacept) could be shown to be similar to the active control (CsA) in the trial, but it would be unclear if both drugs were similarly effective or ineffective. By understanding how efficacious the active control is in a setting similar to that in the NI trial (i.e. with similar background regimens, similar patient population, etc.), an assessment of how effective the test drug is, in a trial where the test drug is compared to the active control, can be made. The second step in selecting an NI margin involves determining the largest loss of the treatment effect that would be clinically acceptable, and this determination is based on clinical judgment. It is very important that a NI trial be of high quality, since a trial of poor quality can make the test and control arms look more similar and bias the results toward the conclusion of NI when the two treatment groups are not actually noninferior (11).
Patient and graft survival
To justify an NI margin for patient and graft survival, it was necessary to determine the contribution of CsA to the immunosuppressant regimen using available historical data, i.e. a comparison of rates of patient and graft survival for the “putative placebo” regimen of basiliximab induction, corticosteroids and MMF compared to the control regimen of basiliximab induction, CsA, corticosteroids and MMF. No literature data were available to make this comparison; therefore, it was not possible to define an evidence-based NI margin for the endpoint of patient and graft survival in studies 1 and 2 and an assessment of the efficacy of belatacept could not be based on this endpoint. However, given that patient and graft survival is an important clinical outcome, overall patient and graft survival rates were examined and taken into consideration when assessing the overall risk benefit of the belatacept regimen.
Composite renal impairment assessed by glomerular filtration rate (GFR)
Attributing differences in GFR for belatacept compared to CsA for an efficacy assessment of belatacept was also challenging, despite the fact that this endpoint was assessed for superiority and not NI.
CsA causes vasoconstriction of the afferent arterioles resulting in a physiologic decrease in GFR (12) while belatacept is not known to cause vasoconstriction. Given the presence of CsA only in the control group, any analysis of GFR was considered to be confounded. Therefore, a comparison between the belatacept and CsA groups could not serve as the basis for FDA's determination of the efficacy of belatacept. However, because GFR is an important marker of renal function and higher GFRs may translate into clinical benefits, mean GFR in the belatacept group over time was evaluated for safety. Although data on measured GFR were collected at prespecified time points over 2 years, not all patients had measured GFR results available. Because more complete data through 3 years were available for calculated GFR, this information was used by the FDA for a safety assessment of renal function.
Acute rejection (AR)
The endpoint of BPAR, as per the FDA's definition, was also assessed for NI and a NI margin was justified. Ideally, a comparison of rates of BPAR for the “putative placebo” regimen without CsA of basiliximab induction, MMF and corticosteroids would be compared to the active control regimen with CsA of basiliximab induction, CsA, MMF and corticosteroids in multiple comparative studies. However, as there are no published studies available which allowed for a direct comparison of regimens with and without CsA, outcome data for these regimens were obtained from separate studies. Data from six published trials of CsA-based regimens, similar to the control regimen in studies 1 and 2, were compared to data from a single published trial of an IL-2 receptor antagonist plus MMF plus corticosteroids without CsA (13). A conservatively large estimate of the rate of BPAR was obtained by taking the upper bound of the 95% confidence interval for the rate of BPAR at 12 months (including graft loss, death and loss-to-follow-up as failures) for the pooled trials containing the regimen with CsA and a conservatively small estimate was obtained by taking the lower bound of the 95% confidence interval for the one trial containing a regimen without CsA. The difference in these estimates was 22% which indicates a treatment effect of CsA over placebo. Taking into account the limitations in the historical data, including the lack of directly comparative trials, a 20% margin was selected (10). This NI margin allowed for the conclusion that belatacept has efficacy greater than zero. In order to preserve some fraction of the treatment effect of CsA, the margin should be smaller than 20% (see discussion below). Therefore, as an NI margin was found to be justified for the endpoint of BPAR, as defined by FDA, this endpoint was considered as the primary endpoint for the assessment of the efficacy of belatacept in both trials.
Efficacy Results—Studies 1 and 2
Biopsy-proven acute rejection (BPAR) endpoint
Tables 2 and 3 summarize the results of studies 1 and 2, respectively, following 1 and 3 years of treatment with the approved belatacept regimen and the CsA control regimen. The results for the BPAR endpoint, as defined by FDA and denoted as efficacy failure in Tables 2 and 3, in both studies were within a 20% margin at year 1, the time point at which the NI margin is applicable. These results provided assurance that belatacept has an effect greater than zero. In addition, the upper bound of the 97.3% confidence interval (adjusted for multiple treatment comparisons) was 13.2% in study 1 (Table 2) and 11.5% in study 2 (Table 3), indicating that belatacept preserved more than 25% of the treatment effect of CsA. There were no significant differences detected at the end of year 3 for the BPAR endpoint or at the end of year 1 and year 3 for the endpoint of patient and graft survival. Subgroup analyses of the EBV seropositive subpopulation, for which belatacept is labeled, showed similar results for these endpoints.
| Parameter | Belatacept recommended regimen N = 226 n (%) | Cyclosporine (CsA) N = 221 n (%) | Belatacept- CsA (97.3% CI)* |
|---|---|---|---|
| Efficacy failure** by year 1 | 49 (21.7) | 37 (16.7) | 4.9 (−3.3, 13.2) |
| Components of efficacy failure† | |||
| Biopsy-proven acute rejection | 45 (19.9) | 23 (10.4) | |
| Graft loss | 5 (2.2) | 8 (3.6) | |
| Death | 4 (1.8) | 7 (3.2) | |
| Lost to follow-up | 0 | 1 (0.5) | |
| Efficacy failure** by year 3 | 58 (25.7) | 57 (25.8) | −0.1 (−9.3, 9) |
| Components of efficacy failure† | |||
| Biopsy-proven acute rejection | 50 (22.1) | 31 (14.0) | |
| Graft loss | 9 (4.0) | 10 (4.5) | |
| Death | 10 (4.4) | 15 (6.8) | |
| Lost to follow-up | 2 (0.9) | 5 (2.3) | |
| Patient and graft survival § | |||
| year 1 | 218 (96.5) | 206 (93.2) | 3.2 (−1.5, 8.4) |
| year 3 | 206 (91.2) | 192 (86.9) | 4.3 (−2.2, 10.8) |
- *Confidence intervals adjusted for multiple treatment comparisons.
- **All BPAR events and patients who have not developed BPAR, but experience death, graft loss or loss-to follow-up are included as failures.
- †Patients may have experienced more than one event.
- §Patients known to be alive with a functioning graft.
| Parameter | Belatacept recommended regimen N = 175 n (%) | Cyclosporine (CSA) N = 184 n (%) | Belatacept- CSA (97.3% CI)* |
|---|---|---|---|
| Efficacy failure** by year 1 | 51 (29.1) | 52 (28.3) | 0.9 (−9.7, 11.5) |
| Components of efficacy failure† | |||
| Biopsy-proven acute rejection | 37 (21.1) | 34 (18.5) | |
| Graft loss | 16 (9.1) | 20 (10.9) | |
| Death | 5 (2.9) | 8 (4.3) | |
| Lost to follow-up | 0 | 2 (1.1) | |
| Efficacy failure** by year 3 | 63 (36.0) | 68 (37.0) | −1.0 (−12.1, 10.3) |
| Components of efficacy failure† | |||
| Biopsy-proven acute rejection | 42 (24.0) | 42 (22.8) | |
| Graft loss | 21 (12.0) | 23 (12.5) | |
| Death | 15 (8.6) | 17 (9.2) | |
| Lost to follow-up | 1 (0.6) | 5 (2.7) | |
| Patient and graft survival§ | |||
| year 1 | 155 (88.6) | 157 (85.3) | 3.2 (−4.8, 11.3) |
| year 3 | 143 (81.7) | 143 (77.7) | 4.0 (−5.4, 13.4) |
- *Confidence intervals adjusted for multiple treatment comparisons.
- **All BPAR events and patients who have not developed BPAR, but experience death, graft loss or loss-to follow-up are included as failures.
- †Patients may have experienced more than one event.
- §Patients known to be alive with a functioning graft.
The results from studies 1 and 2 support the conclusion that belatacept is effective for the prevention of BPAR. However in study 1 which enrolled LD and SCD recipients, the overall rates of BPAR were higher at 1 and 3 years in the belatacept group (19.9% and 22.1%) compared to the CsA group (10.4% and 14.0%) (Table 2) (2). In addition, 6.2% of belatacept-treated patients at 1 year and 6.6% at 3 years experienced episodes of BPAR classified as Banff grade IIb or higher compared to 1.8% of CsA-treated patients at 1 year and 2.3% at 3 years (14). T-cell depleting therapy to treat episodes of BPAR was used in 10% of belatacept-treated patients compared to 2% of CsA-treated patients during the study. Among those who experienced BPAR, 22% (11/50) of belatacept-treated and 10% (3/31) of CsA-treated patients experienced graft loss and/or death by the end of year 3. The difference in mean calculated GFR at 1 year between patients with and without history of BPAR was 19 mL/min/1.73 m2 for those treated with belatacept compared to 7 mL/min/1.73 m2 for those treated with CsA (2).
In study 2, which enrolled ECD recipients, the differences between the treatment groups were smaller. The overall rates of BPAR at 1 and 3 years were 21.1% and 24.0% in the belatacept group compared to 18.5% and 22.8% in the CsA group (Table 3) (2). The proportion of patients who experienced BPAR classified as Banff grade IIb or higher was 5.1% at 1 year and 5.7% at 3 years in the belatacept group compared to 3.8% at 1 year and 4.9% at 3 years in the CsA group. T-cell depleting therapy to treat BPAR was used in 5.1% of belatacept-treated patients compared to 3.8% of CsA-treated patients during the study. Among those who experienced BPAR, 23.8% (10/42) of the belatacept-treated and 31% (13/42) of the CsA-treated patients experienced graft loss and/or death by the end of year 3 (14). The difference in mean calculated GFR between patients with and without a history of BPAR was 10 mL/min/1.73 m2 for those treated with belatacept compared to 14 mL/min/1.73 m2 for those treated with CsA (2).
Glomerular filtration rate (GFR)
As shown in Figures 1 and 2, the differences in calculated GFR between the groups were apparent in the first month after transplant and were maintained up to 3 years (36 months) posttransplant. Given the hemodynamic effect of CsA, it is unknown if the higher GFR in the belatacept group was attributed to the presence of belatacept or rather the lack of CsA in the regimen. A higher mean GFR may translate into long-term clinical benefits, although the trials did not show a statistical difference in death and/or graft loss between the treatment groups.
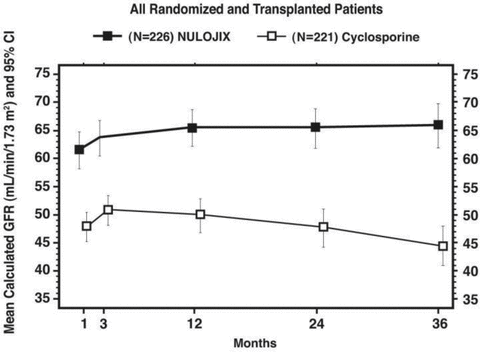
Calculated (MDRD) GFR through month 36; study 1: Recipients of living and standard criteria deceased donor kidneys.
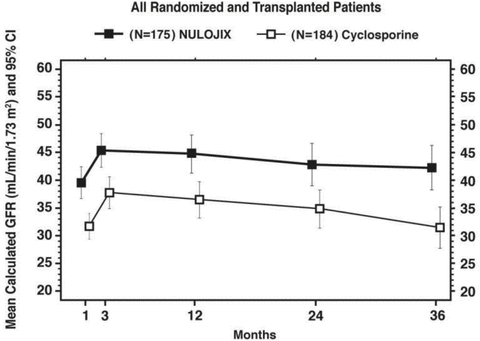
Calculated (MDRD) GFR through month 36; study 2: Recipients of extended criteria donor kidneys.
Patient and graft survival
The results for the patient and graft survival endpoint in studies 1 and 2 at 1 and 3 years are shown in Tables 2 and 3. At 1 year, the lower bound of the 97.3% confidence interval (adjusted for multiple treatment comparisons) was −1.5% in study 1 and −4.8% in study 2. Therefore, the results of both trials were within a 10% margin and, in addition, study 1 was able to exclude a margin of 2% and study 2 was able to exclude a margin of 5%. However, as discussed above, due to the limitations in the available information on the efficacy of CsA in patient and graft survival in a regimen containing basiliximab, MMF and corticosteroids, this information, though supportive, could not be used as proof of efficacy of belatacept.
Selected Safety Results—Studies 1, 2, 3 and 4
The package insert provides complete information on serious, common and less common adverse reactions reported in the trials and includes rates pertinent to belatacept. The following discussion will focus on PTLD, PML and tuberculosis, which were serious adverse events reported disproportionately in patients who received belatacept compared to CsA (2).
Posttransplant lymphoproliferative disorder (PTLD)
Cases of PTLD reported in the three kidney transplantation trials (studies 1, 2, and 3) up to 36 months post-transplant were identified. The cases of PTLD seen in the 949 patients treated with either the approved (recommended) belatacept LI regimen or the nonrecommended MI regimen, and were compared to 476 CsA patients (Table 4). All reported cases presented within 18 months of transplantation.
| Trial | Belatacept nonrecommended regimen* (N = 477) | Belatacept recommended regimen† (N = 472) | Cyclosporine (N = 476) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| EBV positive (n = 406) | EBV negative (n = 43) | EBV unknown (n = 28) | EBV positive (n = 404) | EBV negative (n = 48) | EBV unknown (n = 20) | EBV positive (n = 399) | EBV negative (n = 57) | EBV unknown (n = 20) | |
| Study 1 | |||||||||
| CNS PTLD | 1 | 1 | |||||||
| Non-CNS PTLD | 1 | 2 | 1 | ||||||
| Study 2 | |||||||||
| CNS PTLD | 1 | 1 | 1 | 1 | |||||
| Non-CNS PTLD | 1 | ||||||||
| Study 3 | |||||||||
| CNS PTLD | 2 | ||||||||
| Non-CNS PTLD | 1 | ||||||||
| Total (%) | 2 (0.5) | 5 (11.6) | 1 (3.6) | 3 (0.7) | 2 (4.1) | 0 | 0 | 1 (1.8) | 0 |
- *Regimen with higher cumulative dose and more frequent dosing than the recommended belatacept regimen.
- †In studies 1 and 2 the belatacept regimen is identical to the recommended regimen, but is slightly different in study 3.
Among 472 patients in studies 1, 2 and 3 treated with the belatacept LI regimen,1 there were 5 cases of PTLD: 3 in EBV seropositive patients and 2 in EBV seronegative patients. Two of the 5 cases presented with CNS involvement.
Among the 477 patients in studies 1, 2 and 3 treated with the nonrecommended belatacept MI regimen,2 there were eight cases of PTLD: 2 in EBV seropositive patients and 6 in EBV seronegative or serostatus unknown patients. Six of the 8 cases presented with CNS involvement.
One of the 476 patients in studies 1, 2 and 3 treated with CsA developed PTLD, without CNS involvement.
In studies 1 and 2 in the EBV seropositive subset of patients, the incidence of PTLD was 3/358 (0.8%) in patients treated with the approved belatacept regimen and 0/352 (0%) in patients treated with CsA. In EBV seronegative or EBV serostatus unknown patients in studies 1 and 2, the incidence of PTLD was 3/43 (7.0%) in patients treated with the approved belatacept regimen and 1/53 (1.9%) in patients treated with CsA. Therefore, rate of PTLD was ninefold higher in EBV seronegative or EBV serostatus unknown patients (3/43) compared to EBV seropositive patients (3/358 patients), among the patients in studies 1 and 2 treated with the approved belatacept regimen.
In study 4, a liver transplant trial, there were also 2 additional cases of PTLD, one of which was fatal, reported among the 140 EBV seropositive patients (1.4%). Both cases involved the liver allograft.
Progressive multifocal leukoencephalopathy (PML)
Two fatal cases of PML were reported among 1096 patients treated with a belatacept-containing regimen in studies 1, 2, 3 and 4 one occurred in a kidney and the other in a liver transplant patient. Both patients were treated with an MI regimen. No cases of PML were reported in patients treated with a belatacept LI regimen or the control regimen in these trials.
Tuberculosis
Tuberculosis (TB) was reported in 1.5% (6/401) of patients receiving the approved belatacept regimen compared to 0.2% (1/405) of patients receiving CsA following 3 years of treatment. Two of the patients with tuberculosis in the belatacept group died during the 3-year follow-up period. One of the two patients who died had disseminated TB at the time of death. Studies 1 and 2 were multinational studies and included areas with a high prevalence of TB.
Overall Risk/Benefit
A determination regarding the overall benefits and risks of a particular product takes into consideration both the efficacy findings and also the safety profile. The benefit of belatacept included the demonstration of efficacy for the prevention of BPAR and the lack of a negative effect on GFR in studies 1 and 2. Additional evidence of benefit consisted of lower mean blood pressure, serum triglycerides and incidence of NODAT, which were prespecified endpoints. A detailed discussion of these analyses is beyond the scope of this article (see belatacept package insert for details) (2).
Belatacept was associated with risks, notably the higher rates and grades of BPAR observed in study 1. In addition, other serious adverse events were reported disproportionately higher with belatacept compared to CsA, including PTLD (predominantly CNS PTLD), PML and tuberculosis. There was no significant difference in patient and graft survival. Overall FDA determined that the risk benefit of belatacept supported approval, but a REMS was necessary to ensure that the benefits of belatacept outweighed the risks of PTLD and PML.
Risk Evaluation and Mitigation Strategy (REMS)
Once a product is approved, additional safety information can be obtained from postmarketing studies and routine postmarketing safety surveillance. This information can also be added to the product's package insert. New safety findings are communicated to health care providers via listserv and dear health care professional (DHCP) letters, and can also be found on the FDA's website: Postmarket drug safety information for patients and providers http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/default.htm
Since 2007, FDA has the authority under the Food and Drug Administration Amendments Act (FDAAA) to require risk evaluation and mitigation strategies (REMS) for products, at the time of initial approval or after approval, when deemed necessary to ensure that the benefits of the drug outweigh the risks (15). A REMS can consist of one or more of the following components: a Medication Guide, Communication Plan and Elements to Assure Safe Use.
The FDA determined that a REMS for belatacept was needed to assure that healthcare providers and patients were aware of the serious risks of PTLD, predominantly in the CNS, and the increased risk of PML, and how to mitigate those risks. The belatacept REMS consists of a Medication Guide and a Communication Plan (1). The medication guide is a document that must be provided to patients which explains these risks in clear and easily understood language (16). It is appended to the package insert and is intended to be distributed with each infusion and be used in counseling patients. The belatacept Medication Guide is available on the FDA (Drugs@ FDA),3 NIH (DailyMed),4 and BMS's product (Nulojix)5 web sites. The communication plan consists of several documents that have been approved by FDA and includes letters to all potential prescribers (DCHP letter) and to infusion nurses, pharmacists and infusion center directors (Dear Infusion Specialist letter); a HCP fact sheet with information on PTLD and PML; a preinfusion checklist for use by infusion specialists before each belatacept infusion to ask patients about new, changed, or worsened neurologic signs and symptoms which could be consistent with CNS PLTD or PML (17). These documents will be mailed periodically, and will be available at transplant centers, as well as on the BMS REMS “landing page” at http://www.NULOJIX.com/REMS.aspx. A journal information piece will be published periodically in selected transplant-related medical and pharmacy journals. Finally, a tutorial (educational training) for healthcare professionals and a live webinar are also available via the REMS landing page at preset times and slides with voiceover are available on demand.
Postmarketing Requirements (PMRs)
Under FDAAA, FDA is also authorized to require postmarketing studies or clinical trials at the time of approval, or after approval. These studies or trials can assess the risk of a serious adverse event or toxicity, in order to better characterize the risk (18,19).
For belatacept, the FDA determined three postmarketing clinical studies in adult kidney transplant recipients would be required to further assess the risks of PTLD and PML. One study is a belatacept registry designed to give a more precise estimate of the incidence of PTLD and PML in clinical practice. The registry was recommended by the 2010 advisory committee panel, developed by BMS under the name ENLiST and reviewed by FDA. It will collect data on PTLD and PML beyond which is currently captured on United Network for Organ Sharing (UNOS) forms. All US adult kidney transplant centers dispensing belatacept will be asked to participate, and the goal is that all investigators and kidney transplant patients will provide postmarketing data.6 The second required postmarketing study will analyze the pattern of use of belatacept in routine clinical practice, using the UNOS database. The third study will evaluate the rates of PTLD reported in belatacept regimens and rates in relation to calcineurin inhibitor (CNI)-based regimens, also using the UNOS database. In all studies, information on EBV serostatus, cytomegalovirus (CMV) serostatus, belatacept use, diagnosis and location of the PTLD, PML and outcome (survival or mortality) are to be collected. The data from these studies will be used by FDA to evaluate whether the risks are managed and whether updates to product labeling or the REMS are necessary to adequately reflect risk information.
Summary
With the FDA approval of belatacept, clinicians now have an additional treatment option for the prophylaxis of organ rejection in kidney transplant patients. Scientific and regulatory issues regarding the choice of endpoints used to assess efficacy in studies 1 and 2 were addressed during the FDA's review process. The overall risk benefit assessment of belatacept took into consideration the relative safety and efficacy of approved alternative treatments for the indication, effects on BPAR and renal function, and the nature and severity of reported adverse events. In order to ensure postapproval that the benefits of belatacept outweigh the risks, a REMS was implemented with the goals of informing healthcare providers and patients of the increased risks of PTLD and PML. Ongoing postmarketing studies will collect data on belatacept use and provide a more precise estimate of the incidence of PTLD and PML in the treated population. Based on the information BMS collects from the REMS assessments and postmarketing studies, FDA may modify the REMS and/or revise the product labeling in order to adequately reflect the new findings.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Disclaimer
This article contains the professional views of the authors and does not necessarily represent the official position of the US FDA.




