Diabetes Mellitus Following Liver Transplantation in Patients With Hepatitis C Virus: Risks and Consequences
Abstract
Recurrent hepatitis C virus (HCV) infection of the allograft occurs universally following liver transplantation. Longitudinal natural history studies have identified several pre- and posttransplant factors associated with more rapid fibrosis progression, including baseline host and viral factors, donor factors and posttransplant immunosuppression effects, such as metabolic syndrome. Evidence accumulated over the past two decades indicates that HCV has metabolic associations, in particular insulin resistance and diabetes mellitus. Approximately half of HCV-positive liver transplant recipients develop posttransplant diabetes mellitus (PTDM), which is associated with accelerated fibrosis progression and poorer graft and patient survival outcomes. This review summarizes the risks and consequences of insulin resistance and PTDM in HCV-positive liver transplant recipients. Risk for developing PTDM is one factor that should be considered when choosing the primary immunosuppressive regimen following liver transplantation. Comparative studies suggest that cyclosporine A-based immunosuppression may provide improved responses to antiviral therapy and reduced incidence of PTDM compared with tacrolimus-based immunosuppression. Addressing insulin resistance and PTDM in HCV-positive liver transplant recipients may have the potential to slow HCV complications and improve survival outcomes.
Abbreviations:
-
- CI
-
- confidence interval
-
- CNI
-
- calcineurin inhibitor
-
- CsA
-
- cyclosporine A
-
- DM
-
- diabetes mellitus
-
- HBV
-
- hepatitis B virus
-
- HCC
-
- hepatocellular carcinoma
-
- HCV
-
- hepatitis C virus
-
- NASH
-
- nonalcoholic steatohepatitis
-
- NODM
-
- new-onset diabetes mellitus
-
- OR
-
- odds ratio
-
- Peg-IFN
-
- pegylated interferon
-
- PTDM
-
- posttransplant diabetes mellitus
-
- SVR
-
- sustained virological response
Introduction
Hepatitis C virus (HCV) infection is the leading indication for liver transplantation in the Western world. HCV has become a global epidemic, with an estimated 180 million people infected worldwide (1). Although the rate of new HCV infections has peaked, the proportion of infected people with HCV complications continues to increase (2). The proportion with cirrhosis was expected to reach 25% in 2010 and 45% by 2030, and the number with hepatic decompensation and/or hepatocellular carcinoma (HCC) is expected to double over the next decade. This escalation in HCV complications is leading to increasing numbers of patients awaiting liver transplantation.
Re-infection with HCV after liver transplantation for HCV is immediate and virtually universal following reperfusion of the allograft, and is associated with accelerated fibrosis progression leading to cirrhosis in 10–30% of cases within 5 years and >40% within 10 years (1). The median interval from transplant to cirrhosis is 9.5 years (range 7–12), compared with a median of 30 years (range 20–50) from infection to cirrhosis in immunocompetent patients. Many predictors for rapid fibrosis progression in HCV-positive liver transplant recipients have been identified, including pretransplant factors (e.g. high HCV–RNA level, reduced multispecific CD4+/CD8+ responses), donor factors (e.g. older age) and posttransplant factors (e.g. immunosuppression, diabetes mellitus [DM], cytomegalovirus). The natural history of HCV cirrhosis is accelerated after liver transplantation: Hepatic decompensation rates are >40% at 1 year and >70% at 3 years in recipients with established cirrhosis, compared with <5% and <10%, respectively, in immunocompetent patients. Graft and patient survival outcomes are poorer in liver transplant recipients with HCV recurrence than in HCV-negative recipients.
HCV infection is further complicated in nontransplant and transplant settings by association with the extrahepatic effects of insulin resistance and DM. This is a two-way effect: not only is chronic HCV infection linked with the onset of insulin resistance, but insulin resistance may also contribute to the morbidity and mortality associated with chronic HCV infection. Following liver transplantation for HCV, approximately half of recipients develop posttransplant DM (PTDM; Ref. 3), which contributes to the poorer long-term graft and patient survival rates associated with HCV recurrence (4,5).
This review focuses on the development of insulin resistance and PTDM in liver transplant recipients with HCV recurrence, and the consequences in terms of fibrosis progression and other HCV complications, response to antiviral therapy and choice of calcineurin inhibitor (CNI) in the primary immunosuppressive regimen.
Is HCV a Metabolic Disease?
Metabolic syndrome is common in liver transplant recipients and is defined as the presence of ≥3 of the following metabolic manifestations: impaired glucose tolerance, abdominal obesity, hypertriglyceridemia, low high-density lipoprotein (HDL) levels and high blood pressure. Metabolic syndrome increases the risk for cardiovascular disease and diabetes, and leads to the development of nonalcoholic fatty liver disease, including steatosis and nonalcoholic steatohepatitis (NASH). NASH is associated with progressive hepatic fibrosis leading to cirrhosis and complications such as liver failure and HCC. NASH is becoming an increasingly frequent indication for liver transplantation.
Evidence accumulated over the past two decades indicates that HCV has metabolic associations, in particular insulin resistance and DM, in nontransplant and transplant settings.
HCV and insulin resistance/DM in the nontransplant setting
A connection between HCV and DM was first noted in the mid-1990s, when the prevalence of DM was observed to be higher in patients with HCV-related cirrhoses than in those with cirrhoses due to other etiologies (multivariate odds ratio [OR] 10.0; p < 0.0001; Ref. 6). In a subsequent general population study involving >5000 people aged ≥40 years, those with HCV were more than three times as likely to have type 2 diabetes as those without HCV (7). A recent meta-analysis found a significantly increased risk for DM in patients with HCV compared with either noninfected control individuals (OR 1.7) or patients with hepatitis B virus (HBV; OR 1.8; Ref. 8).
In a matched-control study of 121 patients with HCV and 137 healthy volunteers, chronic HCV infection was associated with insulin resistance (p = 0.002; Ref. 9). In the same investigation, a cross-sectional study of 260 patients with HCV demonstrated that insulin resistance was a risk factor for the severity of fibrosis (multivariate OR 1.3; p < 0.001). In a sub-analysis of 117 patients with known duration of infection, insulin resistance was a risk factor for rate of fibrosis progression (p = 0.03). Insulin resistance was further identified as a risk factor for fibrosis severity in a study of 346 patients with HCV (multivariate OR 3.2; p = 0.001); in subgroup analyses, insulin resistance remained independently associated with worse fibrosis stage irrespective of HCV genotype (10).
Pathogenesis of insulin resistance by HCV
The pathophysiologic mechanisms of insulin resistance, and ultimately DM, in chronic HCV infection are incompletely understood but are likely to be multifactorial (Figure 1). Of note, DM in chronic HCV infection is not associated with pancreatic β-cell failure, and reflects both hepatic and peripheral insulin resistance (Figure 2; Ref. 11). Experimental models suggest that HCV directly induces hepatocyte insulin resistance via upregulation of the suppressor of cytokine signaling three pathways (12). In addition, recent studies employing hyperinsulinemic clamps have identified peripheral insulin resistance in patients with chronic HCV infection, predominantly in skeletal muscle. This may reflect the systemic effects of tumor necrosis factor-α and other inflammatory cytokines on lipid oxidation (13,14). In addition to these direct effects on liver and skeletal muscle, chronic HCV infection may have indirect effects on insulin utilization once cirrhosis has developed. These include reduced clearance of insulin through portosystemic shunting, decreasing hepatic extraction secondary to liver synthetic failure and hyperinsulinemia due to increased serum glucagon.
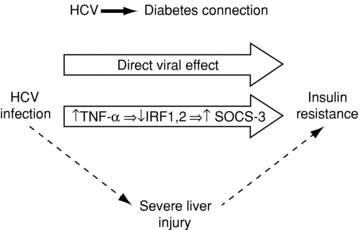
The connection between HCV and diabetes. HCV = hepatitis C virus; TNF = tumor necrosis factor; IRF = interferon regulatory factor; SOCS = suppressor of cytokine signaling.
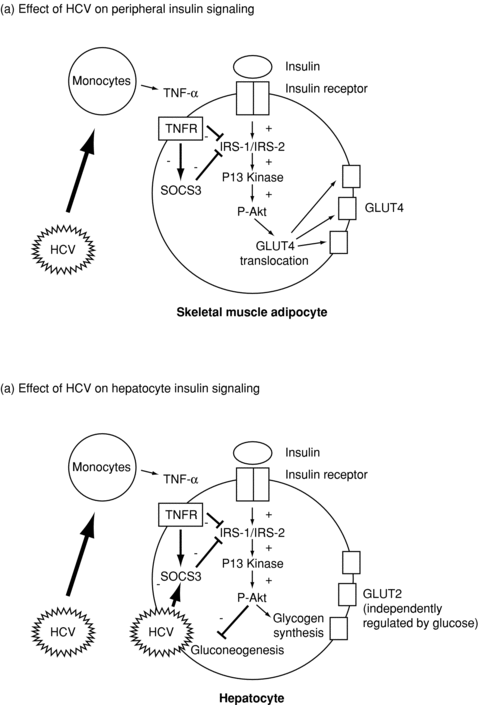
Diagrammatic representation of mechanisms of insulin resistance in recurrent HCV infection (17). Reprinted with kind permission from John Wiley & Sons, Inc. via Rightslink. HCV = hepatitis C virus; TNF = tumor necrosis factor; TNFR = tumor necrosis factor receptor; IRF = interferon regulatory factor; SOCS = suppressor of cytokine signaling; P-Akt = phosphorylated Akt; GLUT = glucose transporter.
Insulin Resistance and PTDM in HCV-Positive Liver Transplant Recipients
HCV recurrence after liver transplantation is also associated with insulin resistance and PTDM. In a review of 278 liver transplant recipients, prevalence of PTDM at 1 year posttransplant was 37% in those transplanted for HCV compared with 10% and 5% in those transplanted for HBV and cholestatic liver disease, respectively (p < 0.001; Ref. 15). A meta-analysis of studies up to January 2008, including 1889 liver transplant recipients from seven retrospective studies, revealed that HCV-positive recipients had a significantly higher rate of new-onset PTDM than HCV-negative recipients (54% vs. 38%; OR 2.5; Figure 3; Ref. 3).
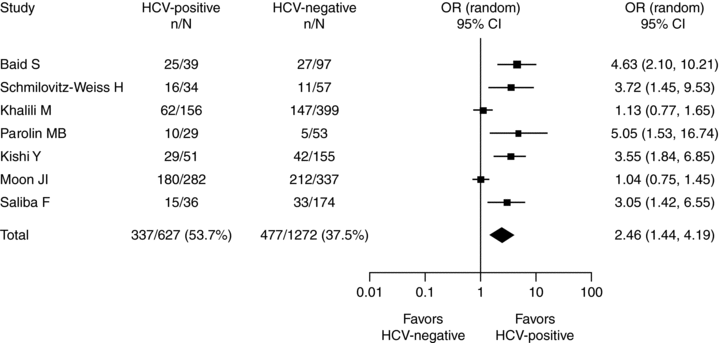
Meta-analysis of the effect of HCV on the rate of PTDM in liver transplant recipients with no history of diabetes pretransplant; data from 1889 patients from seven studies (3). Reprinted with kind permission from John Wiley & Sons, Inc. HCV = hepatitis C virus; OR = odds ratio; CI = confidence interval.
In a prospective study, the incidence of new-onset insulin resistance during the first year postliver transplant was higher in HCV-positive recipients than in HCV-negative recipients (when controlled for BMI; p = 0.035; Ref. 16). HCV-positive recipients were four times more likely to develop PTDM than HCV-negative recipients (from the second month onward; p < 0.01). In the HCV-positive cohort, higher HCV–RNA levels correlated with more rapid development of insulin resistance (p = 0.03).
Of note, pretransplant HCV infection is also associated with an increased incidence of PTDM among renal transplant recipients (17).
Consequences of Insulin Resistance and PTDM for HCV-Related Complications Following Liver Transplantation
As in the nontransplant HCV setting, insulin resistance and PTDM are risk factors for fibrosis progression in HCV-positive liver transplant recipients. A study in 160 HCV-positive liver transplant recipients found that those with insulin resistance were twice as likely to develop fibrosis stage ≥3 at 5 years posttransplant as those with normal insulin sensitivity (43% vs. 21%; p = 0.016; Ref. 18). In a study of 163 HCV-positive liver transplant recipients, 35% had PTDM at median follow up of 49 months and 33% progressed to severe fibrosis (stage ≥4), and PTDM was a significant predictor for severe fibrosis (multivariate hazard ratio 3.3; p = 0.004; Ref. 19). Metabolic syndrome has also shown an association with fibrosis progression: In a study of 82 liver transplant recipients with HCV recurrence, metabolic syndrome was present in half of patients at 1 year posttransplant and was associated with fibrosis progression beyond 1 year (multivariate OR 6.3; p = 0.017; Ref. 20).
In the overall liver transplant population, the development of insulin resistance and PTDM are associated with reduced graft and patient survival. There are fewer studies on the consequences of insulin resistance and PTDM in the HCV-positive liver transplant population, but available evidence suggests a similar adverse impact on long-term outcomes. In a small study of 39 HCV-positive liver transplant recipients, cumulative mortality at a mean of 50 months posttransplant was 56% versus 14% in those with and without PTDM, respectively (p = 0.01; Ref. 5). In a large study of 778 HCV-positive liver transplant recipients followed up for a median of 6 years, recipients with PTDM (including those with DM before transplant) had increased HCV-related mortality (4.5% vs. 1.8%; p = 0.036) and HCV-related graft loss (4.7% vs. 1.8%; p = 0.026) compared with recipients with no or transient PTDM (4).
Chronic HCV infection and metabolic syndrome (obesity or DM) are independent risk factors for hepatocarcinogenesis (21). In a study of 541 patients with HCV and advanced fibrosis, the presence of DM was associated with an increased cumulative incidence of HCC (11.4% vs. 5.0% after 5 years; p = 0.013; Ref. 22). Such data are not available in HCV-positive liver transplant recipients with PTDM because de novo, HCC is extremely rare, reflecting the accelerated progression to graft failure and death or retransplantation once cirrhosis has developed. However, HCC incidence is likely to increase in the future following the introduction of more effective antiviral therapies that will rescue decompensated HCV, analogous to current HBV oral nucleoside therapy. Statin use may be an approach to reduce the risk for HCC in patients with PTDM (23).
Successful antiviral therapy is the only factor proven to improve graft and patient survival following liver transplantation for HCV (24). Nonetheless, the current standard-of-care antiviral therapy (pegylated interferon [Peg-IFN]+ ribavirin) has suboptimal efficacy and tolerability in HCV-positive liver transplant recipients, with sustained virological response (SVR) rates of only ∼30% (25), reflecting direct effects of immunosuppression. Over recent years, this efficacy has deteriorated further because of worsening donor quality, delay in starting antiviral therapy and changes in immunosuppression strategies (1). New direct-acting antiviral agents will improve the efficacy and tolerability of antiviral therapy before and after transplant. A recent study suggested that the rapid and profound viral decline produced by protease inhibitors may improve insulin sensitivity in patients with HCV genotype 1 infection (26). However, caution will be required when treating recurrent HCV with protease inhibitors due to the existence of important direct drug interactions. Direct-acting antiviral agents are potent inhibitors of CYP3a4 drug metabolism and will, therefore, significantly increase tacrolimus and cyclosporine A (CsA; Neoral, Novartis AG, Basel, Switzerland) exposure, requiring dose reduction and careful drug monitoring.
Baseline insulin resistance and DM are additional predictors of inadequate treatment response. In a study of 1059 nontransplanted chronic HCV patients receiving antiviral therapy (IFN/Peg-IFN + ribavirin), impaired fasting glucose and/or type 2 diabetes at baseline was associated with a lower SVR rate (44% vs. 59%; p = 0.002; Ref. 27). In a case-control study, baseline DM was associated with increased nonresponse to antiviral therapy (multivariate OR 4.6; p = 0.003; Ref. 28). Conversely, in patients with normal baseline glucose metabolism, lack of SVR was associated with the development of impaired fasting glucose and/or type 2 diabetes (multivariate OR 0.44; p = 0.04).
Impact on Choice of CNI
CNIs remain the cornerstone of all maintenance immunosuppressive regimens in liver transplantation; the two options are CsA and tacrolimus (Prograf, Astellas Pharma Inc., Tokyo, Japan). Early studies using the original galenic formulation of emulsified CsA—Sandimmune (29)—or emulsified CsA with C0 monitoring (30), demonstrated reduced rejection rates with tacrolimus, whereas later trials using emulsified CsA with C2 monitoring observed equivalent acute rejection rates (31). Recently, a retrospective analysis of 8809 patients, in the United Network for Organ Sharing/Organ Procurement and Transplantation Network database, who were transplanted for HCV, observed an increased risk of primary graft failure and biopsy-proven acute rejection with emulsified CsA- versus tacrolimus-based immunosuppression at time of discharge (32). The adverse-event profiles of CsA and tacrolimus vary considerably, and the two agents have differing effects in terms of direct anti-HCV activity and indirect diabetogenicity.
Although most risk factors for insulin resistance and PTDM are not adjustable (age, gender, etiology of liver disease), the choice of posttransplant CNI is potentially modifiable.
CsA versus tacrolimus: Anti-HCV activity
In vitro experiments in cultured hepatocytes found that CsA, but not tacrolimus, suppressed HCV replication and HCV protein production in a dose-dependent manner (33). CsA and tacrolimus act through binding to immunophilins, with CsA binding to cyclophilins and tacrolimus to FK-binding protein. Cyclophilins play a role in HCV replication by promoting RNA interaction with viral nonstructural proteins that form the HCV replication complex, including NS5A and NS5B. There is some debate about the roles of specific cyclophilin interactions in HCV replication; however, recent evidence suggests the cyclophilin A/NS5A interaction may be the most important (34). As a result of the observations with CsA, several cyclophilin inhibitors are now in development as specific direct-acting antiviral agents against HCV, including alisporivir (Debio-025) and SCY635.
The clinical relevance of the observed in vitro anti-HCV activity of CsA is still to be fully determined. A meta-analysis of studies up to 2005 found no significant differences between CsA and tacrolimus in terms of HCV recurrence or survival outcomes in patients undergoing liver transplantation for HCV (35). A recent, nonrandomized, single-center study in which 253 HCV-positive liver transplant recipients were prospectively treated with CsA or tacrolimus found no relationship between posttransplant outcomes and the use of CsA versus tacrolimus (36).
Several studies have suggested that SVR to antiviral therapy (IFN/Peg-IFN + ribavirin) is more likely to be achieved in patients administered CsA than in those administered tacrolimus (Table 1). A recent prospective study observed similar SVR rates with CsA (37.8%) as with tacrolimus (39.3%; p = 0.9; Ref. 36).
| Study design | SVR, n (%) | ||
|---|---|---|---|
| CsA | Tacrolimus | p-Value | |
| Randomized, pilot, single center, controlled (37)1 | 7/18 (38.9) | 7/20 (35) | 0.8 |
| Nonrandomized, prospective (36) | 14/37 (37.8) | 11/28 (39.3) | 0.9 |
| Nonrandomized, prospective (38) | 59/123 (48.0) | 106/287 (37.0) | 0.037 |
| Nonrandomized, multicenter (39) | 5/18 (27.8) | 19/34 (55.9) | Univariate 0.053; Multivariate 0.12 |
| Nonrandomized, single center (40) | 10/33 (30.3) | 7/28 (25.0) | Not reported |
| Nonrandomized, single center (41) | 10/22 (45.5) | 8/29 (27.6) | NS |
| Nonrandomized, single center (42) | 46/80 (57.5) | 40/92 (43.5) | 0.05 |
| Nonrandomized (43) | 16/37 (43.2) | 9/62 (14.5) | 0.001 |
- CsA = cyclosporine A; NS = nonsignificant; SVR = sustained virological response.
- 1Patients were randomized to continue treatment with tacrolimus or switch to CsA.
However, in a recent Spanish, multicenter study including more than 400 patients treated for recurrent HCV infection, SVR rates were higher in those on CsA than on tacrolimus (48% vs. 37%, p = 0.037; Ref. 38). On treatment, virological response rates (rapid virological response, early virological response and end of treatment response) were similar in the CsA and tacrolimus groups, but patients on CsA had a significantly lower rate of virological relapse after completion of antiviral therapy (18% vs. 36%, p = 0.008).
Few studies have prospectively evaluated the effects of CNI choice on fibrosis progression in HCV-positive liver transplant recipients (Table 2). A retrospective analysis of HCV-positive liver transplant recipients identified an association between tacrolimus therapy and risk of progression to bridging fibrosis/cirrhosis (19). In contrast, a recent study observed no significant difference in fibrosis progression between CsA and tacrolimus (36). Final results are awaited from the REFINE (Randomized Evaluation of Fibrosis due to Hepatitis C after De Novo Liver Tranpslant) study, a multicenter, randomized comparison of CsA and tacrolimus in 450 HCV-positive liver transplant recipients, where the primary outcome was rate of fibrosis stage ≥2 at 1-year posttransplant.
| Study design | Outcome | Sample size | Outcome, n (%) | |||
|---|---|---|---|---|---|---|
| CsA | Tacrolimus | CsA | Tacrolimus | p-Value | ||
| Prospective, randomized (44) | F = 3–4 in first year biopsy | 44 | 46 | 12 (27.3%) | 14 (30.4%) | NS |
| Prospective, nonrandomized (36)1 | F = 3–4 in first year biopsy | 90 | 91 | 27 (30%) | 22 (24.2%) | 0.37 |
| Retrospective, single center (19) | Progression to bridging fibrosis/cirrhosis | 59 | 104 | Tacrolimus versus CsA: multivariate HR = 2.017 (95% CI 1.096–3.713); p = 0.024 | ||
- CI = confidence interval; CsA = cyclosporine A; HCV = hepatitis C virus; HR = hazard ratio; NS = nonsignificant.
- 1Study enrolled 253 patients, of whom 181 provided liver biopsies within 1 year.
CsA versus tacrolimus: Diabetogenic effects
Tacrolimus appears to be associated with a higher incidence of PTDM than CsA; this association has been noted in liver transplant recipients and in other transplantation settings, notably renal (45). One explanation for the greater diabetogenic potential of tacrolimus may lie in a direct effect on insulin-producing β cells of the pancreas (46).
In the three large, prospective, randomized, controlled studies of CsA versus tacrolimus for primary immunosuppression following liver transplantation, a significantly lower proportion of patients in the CsA arm developed new-onset DM (NODM) compared with those in the tacrolimus arm (30,47,48). In the European FK506 registration study, the incidence of NODM was 15% in tacrolimus-treated recipients compared with 9% in those receiving nonemulsified CsA (47,48). The UK TMC study, using emulsified CsA without C2 monitoring, observed the same incidences of NODM as the European FK506 study (30). Finally, in the LIS2T study, in which C2 monitoring was employed, a significantly lower proportion of patients in the CsA arm were treated for DM at 12 months than in the tacrolimus arm (5% vs. 13%; p < 0.01; Ref. 49). By contrast, other studies have demonstrated similar rates of NODM between CsA- and tacrolimus-based regimens, such as a prospective study of 60 de novo liver transplant recipients, which observed 12-month NODM rates of 19% with tacrolimus and 17% with CsA (50).
A lower rate of PTDM was observed with CsA versus tacrolimus in an HCV-positive cohort of liver transplant recipients analyzed retrospectively (17% vs. 47%; Ref. 51). In the tacrolimus group, the rate of PTDM was significantly higher in the HCV-positive versus the HCV-negative cohort (47% vs. 19%; p = 0.0014), whereas in the CsA group the rate was 17% irrespective of HCV status.
For liver transplant recipients who developed PTDM on tacrolimus, evidence from two small studies suggests that conversion to CsA may improve glycemic control. In one study in 25 patients, switching to CsA significantly decreased hemoglobin A1c (p < 0.05) and fasting blood glucose levels (p < 0.05; Ref. 46). The other study in 46 patients found that conversion to CsA allowed insulin discontinuation in 48% of patients within 6 months and significantly (p < 0.05) reduced fasting blood glucose and pre- and postprandial blood glucose levels (52). Given the small sample size in both studies, validation in larger patient populations is required.
Conclusions
The interactions between HCV infection, metabolic syndrome and choice of immunosuppression are complex. Evidence presented in this review demonstrates that HCV infection exacerbates the metabolic syndrome and is a risk factor for PTDM by inducing the development of insulin resistance, in both the hepatocyte and the skeletal muscle. HCV and insulin resistance/PTDM are independent predictors for poorer long-term outcomes in liver transplant recipients. Conversely, the development of insulin resistance or PTDM is associated with worse outcomes in patients transplanted for HCV, with more rapid fibrosis progression and reduced patient and graft survival. Successful eradication of HCV would be expected to improve insulin resistance and reduce long-term cardiovascular and liver-related complications.
Risk for developing severe recurrent HCV and/or PTDM should be considered when choosing the CNI in the primary immunosuppressive regimen following liver transplantation, with evidence suggesting CsA is associated with a lower incidence of PTDM than tacrolimus. PTDM accelerates fibrosis progression in recurrent HCV and may reduce the response rates to antiviral therapy. A possible additional benefit of CsA compared with tacrolimus may be an improved SVR to antiviral therapy for recurrent HCV. However, it is difficult to draw firm conclusions about the relative benefits of CsA or tacrolimus with regard to PTDM, fibrosis progression or SVR because the majority of studies performed to date have been underpowered and/or retrospective in nature. Additional, large and prospectively designed studies are required to provide definitive answers.
Severe recurrent HCV infection remains the most important unmet medical need in adult liver transplantation. Addressing insulin resistance and PTDM in HCV-positive liver transplant recipients may have the potential to slow fibrosis progression to cirrhosis and other HCV complications, and improve graft and patient survival outcomes and is clearly an important area for future research.
Acknowledgments
The author would like to thank Cath Carsberg, PhD, from Complete HealthVizion for editorial assistance; this assistance was funded by Novartis. The author would also like to thank Dr. Roberto Orsenigo for reviewing the manuscript.
Disclosure
The author of this manuscript has conflicts of interest to disclose as described by the American Journal of Transplantation. During the past 5 years, Professor Gane has participated in international advisory boards or appeared on speaker bureau for the following pharmaceutical companies: Abbott, Gilead, GSK, F. Hoffmann La-Roche, Janssen, Merck, Pharmasset and Roche Diagnostics.




