Improved Survival with Recent Post-Transplant Lymphoproliferative Disorder (PTLD) in Children with Kidney Transplants
Abstract
Post-transplant lymphoproliferative disorder (PTLD) has been associated with high mortality, but recent anecdotal survival appeared better. From 1988 to 2010, the NAPRTCS registry had 235 registered PTLD cases. We sent a special 25-point questionnaire study to the NAPRTCS centers with the most recent 150 cases to obtain additional follow-up data not collected in the master registry, our objective being to determine the recent outcomes after PTLD and determine prognostic factors. We received 92 completed responses, in which only 12 (13%) deaths were reported, 2 from nonmedical causes, 10 with a functioning graft. Kaplan–Meier-calculated patient survival was 90.6% at 1 year and 87.4% at 3, 4 and 5 years post-PTLD. Graft survival post-PTLD was 81.8% at 1 year, 68.0% at 3 years and 65.0% at 5 years. Seven patients received a retransplant after PTLD, with no PTLD recurrence reported. Using all 235 PTLD cases, the covariates associated with better patient survival were more recent year of PTLD diagnosis (adjusted hazard ratio AHR 0.86, p < 0.001), and with worse survival were late PTLD (AHR 1.98, p = 0.0176) and patient age above 13 at PTLD (AHR 3.43, p value 0.022). In children with kidney transplants, patient survival has improved with more recent PTLDs.
Abbreviations:
-
- PTLD
-
- post-transplant lymphoproliferative disorder
-
- NAPRTCS
-
- North American Pediatric Kidney Trials and Cooperative Studies
-
- USRDS
-
- United States Renal Data System
-
- K–M
-
- Kaplan–Meier
-
- CNS
-
- central nervous system
-
- LDH
-
- lactate dehydrogenase
-
- AHR
-
- adjusted hazard ratio
Introduction
Post-transplant lymphoproliferative disorder (PTLD) has been a major complication of organ transplantation (1). Since its emergence in 1968 (2) and rise to prominence two decades ago (1), this condition has been historically associated with a high morbidity and mortality. A retrospective questionnaire study of pediatric kidney transplant centers found a 48% mortality rate among PTLD cases in the 1990s (3). However, more recent single center series and prospective trial data suggested a better prognosis (4,5). Srivastava et al. reported no deaths in their single center series of 7 PTLD cases (5). McDonald et al., though they found an unacceptably high PTLD rate in their multicenter trial, reported only 1 death in 19 cases (4).
Prognosticating the outcome of PTLD has also changed over the years. Prior prognostic factors were similar to traditional Hodgkin lymphoma: elevated lactate dehydrogenase, advanced disease stage, multifocal or CNS disease (6–8). The advent of anti-CD20 antibody treatment and CD20 tumor staining has added to the list, such that CD20 negative tumors carry a worse prognosis (9). However, EBV positivity has been controversial, with some studies suggesting that EBV negative PTLD had a worse prognosis and other studies suggesting no difference in prognosis (8,10).
We therefore wished to determine recent outcomes and prognostic factors for PTLD cases in recent years in the pediatric kidney transplant population.
Methods
We undertook this retrospective special questionnaire study of centers that participate in the NAPRTCS database. Details of the data collected by NAPRTCS have been covered in detail previously. Briefly, the transplant database has been active continuously since 1988 and has collected data on more than 11 000 transplants performed in children under 21 years of age across more than 130 centers within the United States, Canada, Mexico and Costa Rica. One of the questions in the transplant follow-up form of the master database asks whether PTLD has occurred. The definition of PTLD, similar to other transplant registries such as OPTN, is by center report. In this form, the centers are not asked about additional PTLD diagnosis or confirmation criteria intervention or outcome details related to the PTLD. The main transplant database of NAPRTCS, at the time of the study, had 235 PTLD cases reported.
PTLD survey
In March 2008, we identified the most recent 150 PTLD cases and sent those centers a detailed 25 question questionnaire that covered aspects of tumor pathology, location, interventions performed and responses after interventions, EBV serology and immunosupression therapy after PTLD. Each center received a separate incentive payment for completion of the questionnaire forms. Subsequently, NAPRTCS added this form to their regular data form set. Data received were analyzed for completeness and inconsistencies; centers were queried back for clarifications. Survival rates were calculated by the Kaplan–Meier (K–M) method and significance was tested by the log-rank test. We also compared the patient survival of the survey study PTLD population to the survival of all PTLD cases reported in the master NAPRTCS database and to survey nonresponders cases that were in year 1997 or after. Graft and patient survival were also compared to a cohort of patients without PTLD using a case control methodology, selecting two non-PTLD patients from within the NAPRTCS database for each PTLD patient, matched by age at transplant, donor source and transplant year. The survival between groups was compared using a stratified proportional hazards regression model.
Results
Study population
We received 90 questionnaire responses back (60% response rate) to the paid survey, with an additional 13 forms filled after the form was added to the regular database. Of these 103 filled forms, 11 were censored for being non-PTLD other malignancies upon review or for incomplete information. The final study group consisted of 92 completed questionnaires from 35 different centers (75 from the original survey responders, 9 new PTLD cases identified during the survey process, 8 new PTLD cases entered after the survey was closed). The 35 centers entered a median of two PTLD cases per center with a range of 1–9. Of the 92 patients with PTLD, 12 have died (13%), 2 from nonmedical causes and 1 from fungal sepsis. Nine deaths were directly attributed by center investigators to PTLD, of which one patient was diagnosed with PTLD directly at death, hence survival was 98.9% at time of diagnosis (Figure 1).
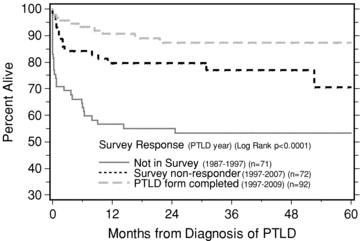
Kaplan–Meier calculated patient survival following PTLD diagnosis in survey patients, survey nonresponders and earlier nonsurvey patients.
The demographic characteristics of the 92 cases are presented in Table 1. As previously shown by us in analyses of the entire PTLD database, the patients with PTLD tend to be male and of Caucasian race and with no specific predilection toward any primary diagnosis (11–13). Table 2 shows that the demographic distribution of age at PTLD, sex and race were virtually identical between the survey cohort and the master database cases, suggesting no bias due to the questionnaire type design. Table 3 shows the median follow-up times of these 92 survey study subjects compared to the 235 cases in the master database. The follow-up times from transplant and after PTLD were very similar. Unexpectedly, the median time to PTLD was 24.7 months in the more recent survey cases, while all reported NAPRTCS PTLDs had a median of 13.9 months. With greater awareness and improved diagnosis of PTLD, we expected the more recent cases to show a shorter time to PTLD, similar to what was seen in a recent prospective trial in the pediatric kidney transplant population (4). Early PTLD (defined as within 1 year post-transplant) was reported in 40 of the 92 survey cases and late PTLD (after 1 year post-transplant) in 52 cases.
| Demographic characteristics | N | % |
|---|---|---|
| Total PTLD cases | 92 | 100.0 |
| Age at transplant | 5 | 5.4 |
| 0–1 years | ||
| 2–5 years | 29 | 31.5 |
| 6–12 years | 36 | 39.1 |
| 13+ years | 22 | 23.9 |
| Race | 69 | 75.0 |
| White | ||
| Black | 9 | 9.8 |
| Hispanic | 7 | 7.6 |
| Other | 7 | 7.6 |
| Sex | 62 | 67.4 |
| Male | ||
| Female | 30 | 32.6 |
| Primary disease | 7 | 7.6 |
| Missing | ||
| Obstructive uropathy | 13 | 14.1 |
| Focal segmental glomerulosclerosis | 9 | 9.8 |
| Renal dyplasia | 16 | 17.4 |
| Reflux nephropathy | 4 | 4.3 |
| Other | 43 | 46.7 |
| Transplant year | 13 | 14.2 |
| 1987–1994 | ||
| 1995–1998 | 24 | 26.1 |
| 1999–2002 | 32 | 34.8 |
| 2003–2009 | 23 | 25.0 |
| Induction antibodies | 37 | 40.2 |
| None | ||
| OKT3 | 7 | 7.6 |
| Basiliximab | 16 | 17.4 |
| Daclizumab | 13 | 14.1 |
| Other | 2 | 2.2 |
| Antithymocyte globulin/antilymphocyte globulin | 17 | 18.5 |
| All NAPRTCS PTLDs | Cases with PTLD form | |||
|---|---|---|---|---|
| N | % | N | % | |
| All | 235 | 100.0 | 92 | 100.0 |
| Age at PTLD | ||||
| <6 years | 38 | 16.2 | 11 | 12.0 |
| 6–12 years | 89 | 37.9 | 42 | 45.7 |
| 13+ years | 108 | 46.0 | 39 | 42.4 |
| Race | ||||
| White | 176 | 74.9 | 69 | 75.0 |
| Black | 23 | 9.8 | 9 | 9.8 |
| Hispanic | 26 | 11.1 | 7 | 7.6 |
| Other | 10 | 4.3 | 7 | 7.6 |
| Sex | ||||
| Male | 147 | 62.6 | 62 | 67.4 |
| Female | 88 | 37.4 | 30 | 32.6 |
| Follow-up times in months | Median | Range | Median | Range |
| Follow-up time from PTLD | 24.5 | 0–161.9 | 28.1 | 0–139 |
| Follow-up time from transplant | 64.4 | 2.3–218.6 | 69.2 | 5.1–218.6 |
| Time from transplant to PTLD | 13.9 | 0.9–161.8 | 24.7 | 0.9–161.8 |
| Factor | Comparison group | Reference group | Hazard ratio | 95% CI | p-Value |
|---|---|---|---|---|---|
| PTLD 1987–1996 (as time-varying covariate) | PTLD | No PTLD | 6.14 | 4.18–9.02 | <0.0001 |
| PTLD (1997–2009) (as time-varying covariate) | PTLD | No PTLD | 3.07 | 2.12–4.45 | <0.0001 |
| Age at transplant (in years) | 0–1 | 1.88 | 1.40–2.53 | <0.0001 | |
| 2–5 | 13+ | 1.27 | 1.01–1.60 | 0.0440 | |
| 6–12 | 0.97 | 0.79–1.19 | 0.7690 | ||
| Donor Source | Deceased | Living | 1.59 | 1.34–1.88 | <0.0001 |
| donor | donor | ||||
| Transplant era | 1987–1997 | 11998–2009 | 2.33 | 1.89–2.86 | <0.0001 |
PTLD characteristics
PTLD location was distributed as follows: (a) lymph node 59.8%; (b) allograft 9.8%; (c) central nervous system 7.6%; (d) other 53.3% (not mutually exclusive). Free text data on location distribution revealed that liver/GI tract locations were seen in nine cases, lung in five cases, bone marrow only in three cases. Where known, PTLD was of B-cell lineage in 74/87 cases (85.1%), T-cell lineage in 3 cases (3.4%), other in 10 cases (11.5%). Most cases were polymorphic (43 cases; 46.7% of total but 67% of known cases), some were monomorphic (21 cases; 22.8%), but a sizeable number were reported as unknown or missing (28 cases; 30.4%). Clonality was reported as unknown or missing in 41 cases (44.6%), as polyclonal in only 22 cases (23.9%) and as monoclonal in 29 cases (31.5%). Pretransplant EBV serology in the recipient was negative in 49 cases (53.3%), unknown in 23 cases (25.0%), positive in just 20 cases (21.7%). Pretransplant EBV serology in the donor was unknown or not performed in the majority (58 cases; 63%), followed by positive (29 cases; 31.5%) and negative in just five cases (5.4%). Tumor EBV positivity was reported in 54/90 cases and CD20 positivity reported in 60/90 cases. The high proportion of B-cell lineage, polymorphic PTLD cases in our series is in accord with other pediatric PTLD series by Allen et al. (14) and Webber et al. (15).
Interventions for PTLD
Interventions performed after PTLD diagnosis included (a) reduction of immunosuppression 83 cases (90.2%), of whom 8 cases had complete stoppage of immunosuppression; (b) anti-CD20 antibody use (26 cases; 28.3%), (c) alpha-interferon use (only 4 cases; 4.3%), (d) anti-viral therapy use (52 cases; 56.5%); (e) surgical reduction (28 cases; 30.4%); (f) chemotherapy use (47 cases; 51.1%). Only four patients were reported to have only reduction of immunosuppression. Many diverse forms of immunosuppression reduction were reported. When the responses were aggregated, CNI stoppage or reduction were both common (41 cases and 21 cases). In contrast, antimetabolite drug was stopped much more commonly than reduced (45 cases and 5 cases). Steroids were stopped in five cases, reduced in only two cases. Sirolimus was stopped in nine cases and reduced in three.
Outcomes after PTLD among survey responders
Overall, the patient survival was quite good among survey responders after a diagnosis of PTLD (Figure 1). All reported mortality was within the first 2 years, with subsequent K–M survival steady at 87.4% out to 5 years. We then analyzed for differences in survival after PTLD based on different covariates. From our 92 survey cases with more detailed information, among other covariates, K–M analysis suggested that early PTLD, defined as PTLD within 1 year post-transplant, was associated with significantly better patient survival than PTLD presenting after 1 year (Figure 2A; p = 0.032).
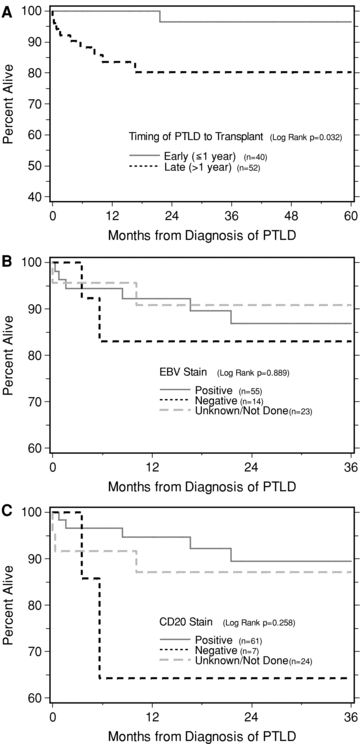
Kaplan–Meier calculated patient survival following PTLD diagnosis, stratified by early or late time to PTLD (panel A), EBV positivity (panel B) or tumor CD20 positivity (panel C).
Other covariates reported to be poor prognostic factors in other series, such as monomorphic, monoclonal, central nervous system involvement, absence of EBV within tumor/blood, absence of CD20 positivity, did not associate with poor outcome, likely due to low frequency of death events. Based on EBV markers, EBV positivity versus negativity showed no significant differences in survival (p value = 0.769, Figure 2B). Tumor CD20 positivity was associated with 89% survival at 3 years, compared to 64% if tumor CD20 marker was negative (p value 0.105, Figure 2C, only seven cases in the CD20 negative group). Based on morphology, 3-year survival was 90% for both polymorphic and polyclonal tumors (p value = 0.956 Figure 3A). Based on clonality, 3-year survival was 93% for polyclonal 85% for monoclonal tumors (p value 0.307; Figure 3B). Patient survival after CNS PTLD (n = 7 cases only) was surprisingly high at 85.7% at 1, 3 and 5 years postdiagnosis (log rank p value = 0.876). Similarly, no form of treatment was significantly better or worse than the others (data not shown). Since a large number of centers (35) entered the 92 PTLD survey cases with a median number of 2 cases per center, we could not meaningfully analyze for center effects.
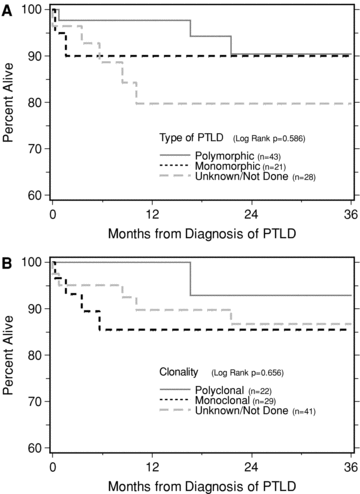
Kaplan–Meier calculated patient survival following PTLD diagnosis, stratified by tumor morphology (panel A) or clonality (panel B).
Concomitant rejection treatment while still receiving PTLD treatment was reported in 19 cases (20.7%). Graft loss was reported in 30 cases, overall K–M graft survival was 81.8% at 1 year, 68% at 3 years and 65.0% at 5 years post-PTLD diagnosis. Allograft nephrectomy was performed in only two cases, though PTLD had been reported within the allograft in 11 cases and one of the nephrectomies was in a patient reported as lymph node and tonsil only PTLD. Seven patients were reported to receive a retransplant after PTLD, with no PTLD recurrence reported in the retransplant.
Tumor CD20 positivity was reported in 61 cases, but anti-CD20 antibody usage was reported in only 26 cases. Median doses used were four (range 1–16), median dose was 1650 mg (range 3.5–9999 mg). Where used, antiviral agents were prescribed as PTLD treatment for a median of 19 months, ganciclovir or valganciclovir being the most common agent used. Chemotherapy usage was reported as a heterogenous group of different drug combinations. The median cycle length was six, range 1–17, over median 5 months (range 0–14).
Once PTLD had resolved, immunosuppressive agents used included prednisone (68 cases), cyclosporine (19 cases), tacrolimus (25 cases) sirolimus (16 cases), mycophenolate (11 cases), azathioprine (5 cases).
Peripheral blood viral load data were reported for 56 subjects at time of PTLD diagnosis. The data were reported by centers in several different units, based on the laboratory that the assay was performed. Only six cases had viral loads reported as per 100 000 lymphocytes. For data reported as genomes or copies per mL (n = 15), the median load at diagnosis was 2768.
At the time of PTLD diagnosis, estimated GFR by the Schwartz formula was median 68.9 mL/min/1.73 m2, the median serum creatinine at diagnosis being 1.2 mg/dL, last stable creatinine was 1.1. The median serum creatinine after treatment was complete was not worse at 1.0, net change of 0.1 mg. Graft loss occurred in 30 cases, of which 10 were death with a functioning graft, 9 attributed to chronic rejection, 5 attributed to malignancy, 2 from other cause and only 1 each from acute rejection/thrombosis/primary disease recurrence.
To ascertain if our results were because centers entered cases with less mortality into our survey, we compared the patient survival between our 92 PTLD survey cases, the 72 nonsurvey responders and the remaining 71 cases from the total 235 cases in the master database. As shown in Figure 1, the survey nonresponders showed no significant difference in survival from the responders, p = 0.0944, 1997 being the earliest PTLD reporting year in both sets. However, the 71 earlier cases in the master database that we did not send the survey to (earliest PTLD reporting year 1988) had a significantly worse survival (p = 0.0033) compared to the survey nonresponders. We then analyzed the era effect in more detail. Using all 235 cases and dividing era of PTLD into four year segments, the patient survival after PTLD was significantly better in the two more recent eras starting in 1997–2009 (p < 0.001, Figure 4).
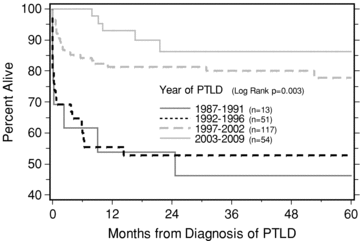
Kaplan–Meier calculated patient survival following PTLD diagnosis, stratified by era of PTLD diagnosis, in 4 year subsets (all 235 NAPRTCS cases).
We then fitted a Cox proportional hazards model looking at survival after transplant (Table 3) that incorporated PTLD as two time-varying covariates, categorized by the era (1987–1996 or 1997–2009). There is a significantly higher risk of dying after developing a PTLD, especially if the PTLD occurred from 1987–1996, adjusted hazard ratio (AHR) = 6.1. This risk drops 50% if the PTLD occurs in the later years (1997–2009), AHR = 3.1; however developing a PTLD still has a significantly higher rate of death when compared to the non-PTLD population. In another Cox model that looked at survival after PTLD (Table 4), patient age at PTLD above 13 and late PTLD were significant for worse patient survival, while more recent year of PTLD predicted a better patient survival. Recipient race and gender did not impact survival. Combined, the above sets of data confirm a significantly improved patient survival after recent PTLD.
| Factor | Comparison group | Reference group | Hazard ratio | 95% CI | p-Value |
|---|---|---|---|---|---|
| Year of PTLD | 1987–2009 | 0.86 | 0.81–0.91 | <0.0001 | |
| PTLD timing | Late (after 1st year) | Early (within 1st year) | 1.98 | 1.13–3.46 | 0.0176 |
| Age at PTLD (in years) | 6–12 | 2.28 | 0.77–6.77 | 0.1377 | |
| 13+ | 2–5 | 3.43 | 1.19–9.85 | 0.0223 | |
Finally, we compared the patient and graft survival of patients who had developed PTLD to matched patients without PTLD in a 1:2 ratio. The patients without PTLD had significantly better survival than the PTLD patients: 94 ± 1.2% versus 85 ± 2.5% at 5 years. The hazard ratio was 3.57 for patients with PTLD, p < 0.0001. Graft survival results were also significantly better in patients without PTLD, being 78±2.2% in the no PTLD group and 70 ± 3.2% graft survival in the PTLD group at 5 years; HR = 1.59 (p = 0.0042) for patients with PTLD.
Discussion
In the present study, the calculated patient survival of 87.4% at 5 years and actual cross-sectional survival of 86% appears to be much better than the 48% actual cross- sectional survival reported in a prior questionnaire study of a similar pediatric kidney transplant population with PTLD (3). A detailed comparison of all the 235 cases in the master database confirmed an improvement in survival in the more recent eras. This result is in accord with more recent data from this population of transplant recipients (4,5). Many different reasons for the improvement in survival are possible, including differences in disease severity, better therapies, earlier diagnosis or as yet unknown reasons. Since this study was based on a voluntary survey, it is also possible that those centers with higher mortality rates may have been less likely to respond to the survey. However, the survival analysis of the 235 PTLD cases in the master database or the 78 nonsurvey responders suggest that such a reporting bias was not present. If the survey cases had included more early PTLD than present in the master registry, that may have contributed to a finding of better survival. However, the opposite finding was seen, yet survival was still improved in the recent era.
Our high patient survival in children contrasts with comparatively worse survival in adult kidney transplant recipients with PTLD. Caillard et al. reported 73% 1-year survival and 61% 5-year survival in adult kidney transplant recipients in France (6). In a separate study of the USRDS database in the United States, Caillard et al. reported 64% survival at 5 years (16). Opelz et al. reported a 60% 1-year survival and 40% 5-year survival among kidney transplant recipients from the multinational CTS registry (17). In adults, PTLD often presents later, with median time to PTLD being 25–72 months (6,18,19), compared to 5.5–25 months in children (16,20–22).
Also, PTLD presenting in children with other organ transplants tend to have worse patient survival. Boyle et al. reported a 43% survival among pediatric heart transplant recipients (23) and Newell et al. reported 60% survival among pediatric liver transplant recipients (24), both studies published in the 1990s. Allen et al. found a 2-year mortality of 36% in children with PTLD in a group of diverse organ transplants (14). More recent studies suggest improved survival after PTLD in this decade even in nonkidney organ transplants (80% in the series by Younes et al., only 18% early mortality in series of Fernandez et al.; [22,25]). Webber's series of 56 PTLD cases from the Pediatric Heart Transplant Study showed that probability of survival was 75% at 1 year, 68% at 3 years and 67% at 5 years after diagnosis (15). It is believed that kidney transplant recipients do better since greater degrees of immunosuppression reduction are possible and the fear of allograft loss is less with the fall back option of dialysis. Such an option is not available for liver or thoracic organ transplant recipients. In fact, in the series by Webber et al., death from graft loss was as frequent as death from PTLD (15).
Poor prognostic factors that have been found in other PTLD studies include high LDH levels (8), multifocal disease (6), CNS disease (26) and negative tumor staining for CD20 (9). Early PTLD presentation in adults was associated with worse patient survival in one series (7), while late onset PTLD was a poor prognostic factor in another series (6). In contrast, children tend to have better survival but also tend to have earlier PTLD. In our study, evidence of EBV involvement, either systemically or within tumor, did not make a difference in patient survival. Similarly, tumor CD20 positivity, which would allow for the rational use of rituximab and potentially avoid chemotherapy, also did not impact patient survival. This may be related to the shorter follow-up in the cases that received this relatively recent drug. Our data also suggest that many CD20 positive PTLD cases did not receive rituximab, perhaps because staining techniques preceded familiarity with the drug. Another possible confounder could be a relative preponderance of early lesion PTLD cases in this survey. Our questionnaire asked targeted questions related to clonality (monoclonal, polyclonal or unknown), morphology (polymorphic, monomorphic or unknown) and location (allograft, lymph node, central nervous system or other, not mutually exclusive). Specific WHO histological classification was not requested, and in fact may not be used at all centers since data on morphology or clonality were reported as unknown or missing in 30/90 cases and 43/90 cases, respectively. We cannot conclusively exclude that early tonsillar lesions may have been over-represented in the survey population.
In the present study population, 51% of children received chemotherapy, yet treatment-related mortality was not high. Gross has previously shown that children with PTLD have much lower treatment-related mortality than adults (Thomas Gross, personal communication), the exact reasons for this still being unclear. Our questionnaire did not ask the sequence of treatment, so we do not know how many subjects received rituximab or chemotherapy as first line versus salvage therapy. Treatment toxicity data were not collected. No form of treatment seemed better than the other. However, because of the retrospective survey study design and low mortality overall, we cannot provide much information on relative treatment effects.
PTLD recurrence in a retransplant, to our knowledge, has not been reported before and was not seen in our study either. The few series looking at results of retransplantation after PTLD in a prior allograft did not report any recurrence of PTLD (27–29).
In summary, our questionnaire study of recent PTLD cases within a national pediatric kidney population revealed a low mortality and improved prognosis if PTLD presentation is in the more recent era.
Acknowledgments
Part of this study was presented as a platform presentation at the International Pediatric Transplant Association Congress in Istanbul, Turkey in April 2009. The North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) is a voluntary collaborative effort comprising over 130 pediatric renal disease treatment centers in the United States, Canada, Mexico and Costa Rica. It is supported by major, unrestricted educational grants from Novartis, AMGEN and Genentech. Participating NAPRTCS centers are listed in the most recent NAPRTCS Annual Reports in Pediatric Transplantation 2003; 7:321-335.
Disclosure
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation.
Disclosure—commercial organization: This study was funded by an American Society of Transplantation achievement award for clinical scientists at Assistant Professor level to VRD in 2006.
Disclosure—conflict of interest: VRD has received consulting fees from Bristol-Myers-Squibb and honoraria from Genzyme for work unrelated to this study.




