The Economic Implications of Broader Sharing of Liver Allografts
Abstract
Liver transplantation has evolved over the past four decades into the most effective method to treat end-stage liver failure and one of the most expensive medical technologies available. Accurate understanding of the financial implication of recipient severity of illness is crucial to assessing the economic impact of allocation policies. A novel database of linked clinical data from the Organ Procurement and Transplantation Network with cost accounting data from the University HealthSystem Consortium was used to analyze liver transplant costs for 15 813 liver transplants. This data was then utilized to consider the economic impact of alternative allocation systems designed to increase sharing of liver allografts using simulation results. Transplant costs were strongly associated with recipient severity of illness as assessed by the MELD score (p < 0.0001); however, this relationship was not linear. Simulation analysis of the reallocation of livers from low MELD patients to high MELD using a two-tiered regional sharing approach (MELD 15/25) resulted in 88 fewer deaths annually at estimated cost of $17 056 per quality-adjusted life-year saved. The results suggest that broader sharing of liver allografts offers a cost-effective strategy to reduce the mortality from end stage liver disease.
Abbreviations:
-
- DRI
-
- donor risk index
-
- HCC
-
- hepatocellular carcinoma
-
- LSAM
-
- liver simulated allocation system model
-
- MELD
-
- model of end stage liver disease
-
- OPTN
-
- organ procurement and transplant network
-
- UHC
-
- university healthsystem consortium
-
- UNOS
-
- united network for organ sharing
-
- SRTR
-
- scientific registry of transplant research
-
- QALYS
-
- quality-adjusted life-years
Introduction
As liver transplantation enters its fourth decade as a widespread clinical treatment for end-stage organ failure, the liver transplant community is facing unprecedented challenges both clinically and economically (1). Broader application of this life-saving technology has led to waiting lists that increasingly outpace organ supplies, resulting in higher average severity of illness at the time of transplantation. Adoption of the severity of illness-based allocation system in 2002 using the Model of End Stage Liver Disease score (MELD) has further increased the acuity of patients reaching transplantation nationally (2). Finally, dramatic differences in the severity of illness at transplant and corresponding waitlist mortality exist across allocation regions due to geographic disparities in supply–demand ratios (3,4).
Simultaneously, there is a broad recognition that the current rate of increase in the cost of health care is unsustainable (5,6). Liver transplantation offers a life-saving treatment to many patients certain to die without transplant, but also ranks among the highest cost Medicare-approved procedures (7,8). Restrictions on insurance premiums, lower Medicare spending and an increasing number of patients with Medicaid only insurance are likely to further increase the financial risk of liver transplantation. Given the geographic variation in access to liver transplant, areas with a relative donor shortage are forced to transplant patients with higher MELD scores using more marginal organs. Consequently, these transplant centers face disproportionate financial and clinical risk (9,10). Broader organ-sharing proposals have been considered by the United Network for Organ Sharing (UNOS) Liver and Intestine Transplant Committee. These proposals are designed to increase the clinical benefit of available liver allografts by raising the MELD thresholds for local use and thereby increasingly sharing within a geographic region. While simulation models have demonstrated that these systems are likely to save lives, the economic implications of alternative sharing schemes are not defined.
Prior assessment of the relationship between MELD and cost has been limited by a lack of nationally representative cost data (1,9,11–14). Using a novel database that links clinical data from the Organ Procurement and Transplantation Network (OPTN) with cost accounting data from a national sample of liver transplant centers, we developed a detailed analysis of the relationship between severity of illness and cost. We use resulting estimates to assess the economic implications of novel allocation proposals, and to determine the incremental costs incurred through the shift in organs from less expensive low-MELD to more costly high-MELD patients, the expected benefit in terms of life-years gained, and the corresponding cost per year of life saved if these proposals were adopted given current simulation modeling results.
Methods
Clinical and economic data
Donor and recipient data for liver transplant recipients were extracted from the OPTN Standard Analytic Research Files. MELD at the time of transplant was calculated from laboratory data and did not include upgrades awarded for hepatocellular carcinoma (HCC) or other conditions. Status 1 was used as the illness severity measure in Status 1 patients. The donor risk index (DRI), a measure of organ quality that predicts the relative likelihood of graft failure, was calculated by the eight-parameter formula developed by Feng et al. (15).
OPTN clinical data were linked to University HealthSystem Consortium (UHC) cost accounting data. (Appendix A) Data on the cost of care during the initial transplant hospitalization were available for liver transplants performed in 2002–2008 at 56 centers in 29 states. Cost estimates are derived from charges reported at the individual charge level which are converted to cost using hospital-specific Medicare ratios of cost to charge for the entirety of the hospitalization that included the transplant procedure. UHC adjusts for the labor component costs using federally reported area wage indexes. Costs were adjusted for medical inflation using the medical component of the Consumer Price Index to 2008 levels for this analysis (16). This analysis does not include physician charges or posttransplant costs incurred in this population. Data linkage was accomplished through a multiple-step procedure to ensure fidelity of the match (Appendix A). The final analytic sample comprised 15 813 adult liver transplant recipients, capturing approximately 50% of all similarly defined liver transplants performed throughout the United States during this time.
All data collection and linkage was conducted in accordance with the restrictions of the Health Insurance Portability and Accountability Act of 1996. The project was approved by the Institutional Review Board of St. Louis University.
Cost analysis
We utilized multivariate regression analysis to quantify the relationship between MELD score at transplant and resource utilization for the population. Initial graphical analysis suggested a nonlinear, accelerating relationship between MELD score and cost of the liver transplant hospitalization.
Therefore, smoothed natural cubic splines were used to optimize fit of a curve describing costs by MELD. Estimates were adjusted for other recipient, donor and transplant characteristics reported to the OPTN (Table 1). To account for similarity in costs among patients transplanted at the same center, the regression analyses were clustered at the center level.
| Characteristic | Study sample % | Liver transplant recipients in OPTN, 2006 % |
|---|---|---|
| Illness severity at transplant MELD score at transplant | ||
| <10 | 9.9% | 10.0% |
| 10–14 | 18.3% | 17.9% |
| 15–19 | 22.0% | 23.5% |
| 20–24 | 17.8% | 19.3% |
| 25–29 | 11.1% | 11.1% |
| 30–34 | 7.6% | 7.9% |
| ≥35 | 8.4% | 4.6% |
| Status 1 | 4.9% | 5.6% |
| Recipient age | ||
| 12–17 | 1.4% | 1.8% |
| 18–29 | 3.7% | 3.8% |
| 30–44 | 12.1% | 11.1% |
| 45–59 | 59.7% | 59.1% |
| 60+ | 23.1% | 24.0% |
| Female gender | 31.5% | 33.8% |
| Race | ||
| White | 70.1% | 71.2% |
| Black | 10.2% | 10.2% |
| Other | 19.7% | 18.6% |
| Hispanic ethnicity | 12.2% | 13.3% |
| Blood type | ||
| AB | 5.9% | 5.5% |
| A | 37.2% | 37.1% |
| B | 14.1% | 12.9% |
| O | 42.8% | 44.3% |
| Cause of liver failure | ||
| Hepatitis C | 36.2% | 32.5% |
| Hepatocellular carcinoma | 12.8% | 15.6% |
| Autoimmune | 14.8% | 16.5% |
| Fulminant Hepatic Failure | 6.2% | 5.7% |
| Primary sclerosing cholangitis | 6.2% | 5.4% |
| Alcoholic cirrhosis | 12.1% | 12.5% |
| Primary biliary cirrhosis | 3.7% | 3.5% |
| Hepatitis B | 3.6% | 2.3% |
| Nonalcoholic steatohepatosis | 0.3% | 0.2% |
| Cancer | 0.4% | 0.5% |
| Other | 3.7% | 5.3% |
| BMI category (kg/m2) | ||
| <18.5 | 2.0% | 2.4% |
| 18.5 to <25 | 23.9% | 24.6% |
| 25 to <30 | 32.6% | 32.4% |
| 30 to <35 | 19.3% | 21.2% |
| 35+ | 11.8% | 12.2% |
| Missing | 10.1% | 6.9% |
| Donor risk index | ||
| <1.17 | 17.7% | 16.2% |
| 1.17 –1.36 | 18.5% | 18.9% |
| 1.37–1.61 | 18.3% | 18.7% |
| 1.62–1.92 | 17.5% | 19.0% |
| >1.93 | 14.8% | 17.9% |
| Missing | 13.2% | 9.1% |
| Region | ||
| 1 | 2.3% | 2.9% |
| 2 | 12.3% | 11.9% |
| 3 | 7.9% | 14.9% |
| 4 | 4.0% | 8.8% |
| 5 | 17.4% | 14.2% |
| 6 | 5.4% | 3.2% |
| 7 | 15.3% | 8.9% |
| 8 | 6.8% | 6.6% |
| 9 | 9.8% | 10.5% |
| 10 | 6.3% | 9.0% |
| 11 | 12.4% | 9.2% |
| Year of transplant | ||
| 2002 | 11.6% | 0.0% |
| 2003 | 13.1% | 0.0% |
| 2004 | 13.7% | 0.0% |
| 2005 | 14.9% | 0.0% |
| 2006 | 16.4% | 100.0% |
| 2007 | 15.5% | 0.0% |
| 2008 | 14.8% | 0.0% |
To allow integration with simulation outputs, transplant costs were determined using the MELD and Status thresholds considered in the broader sharing proposals of the OPTN Liver and Intestine Transplant Oversight Committee. Estimates were similarly adjusted for other recipient, donor and transplant factors. We did not include pretransplant dialysis and liver–kidney transplant adjustment variables as it is not possible to determine the exact distribution of liver–kidney transplants that would be performed under a new allocation system. The cost per liver transplant at each MELD score will reflect the average cost of transplant including the proportion of liver–kidney transplants currently observed at that MELD score.
Organ allocation reform analysis
Data prepared for the OPTN Liver and Intestine Transplant Oversight committee by the Scientific Registry of Transplant Research (SRTR) was used to evaluate the cost-effectiveness of alternative organ sharing proposals. On March 4, 2010, the SRTR presented a series of analysis of the clinical impact of revising the current liver allocation system to provide a two-tiered system of liver allocation (Figure 1) (17). As summarized in Table 3, five alternative allocation systems using OPTN 2006 data were considered in which there were varying MELD thresholds for local and regional utilization of available allografts. The results of these proposed allocation systems were analyzed using the Liver Simulated Allocation System model (LSAM), a simulation program that estimates the clinical impact of alternative allocation systems on key transplant outcomes (18).
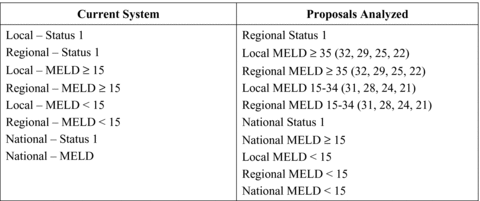
Alternative allocation proposals analyzed two-tiered liver allocation schemes assess by the SRTR for the OPTN Liver and Intestine Transplant Oversight committee in March 2010.
| Current | 15/35 | 15/32 | 15/29 | 15/25 | 15/22 | |
|---|---|---|---|---|---|---|
| Transplants performed | ||||||
| Status 1 | 440 | 453 | 454 | 455 | 450 | 455 |
| MELD 35+ | 692 | 769 | 744 | 733 | 719 | 703 |
| MELD 30–34 | 595 | 600 | 652 | 667 | 654 | 626 |
| MELD 25–29 | 1000 | 1047 | 1045 | 1062 | 1197 | 1081 |
| MELD 20–24 | 2134 | 2215 | 2194 | 2179 | 2124 | 2383 |
| MELD 15–19 | 1153 | 1205 | 1196 | 1182 | 1125 | 1011 |
| MELD 10–14 | 255 | 25 | 25 | 28 | 27 | 25 |
| MELD <10 | 55 | 12 | 12 | 12 | 12 | 12 |
| Total | 6324 | 6324 | 6320 | 6319 | 6309 | 6296 |
| Deaths | ||||||
| Waitlist | 1659 | 1592 | 1583 | 1574 | 1572 | 1585 |
| Posttransplant | 602 | 606 | 624 | 618 | 621 | 616 |
| Postremoval | 406 | 392 | 387 | 393 | 386 | 389 |
| Total | 2667 | 2589 | 2594 | 2585 | 2579 | 2589 |
| Lives saved | 0 | 78 | 73 | 82 | 88 | 78 |
We utilized the number of transplants done at each MELD score and Status category under the current allocation system and each of the proposed allocation systems modeled through LSAM. The expected cost of transplant for patients in each illness severity category was estimated using each MELD and status-specific cost estimate from the categorical regression analysis; the distribution of other clinical characteristics in the cost regression was drawn according to illness category distributions for patients in the OPTN in 2006 (Appendix B) and multiplied by the corresponding categorical cost regression parameters. Finally, the total cost of a sharing proposal was computed as product of expected costs for patients transplanted in each illness severity category with the count of transplants performed at each severity stratum.
To compute the benefit of an alternative-sharing proposal, we quantified the posttransplant survival expectations (quality-adjusted life-years, QALYS) according to the expected counts of net lives saved in each illness severity category at transplant produced by the LSAM simulations. Long-term survival was estimated using a two-part method in which a Kaplan–Meier curve was fit to observed survival data over the first year after transplant, and a parametric survival function with a Weibull form is fit to observed survival data in years 1–5 (19,20). The Weibull form is then applied to project survival to 20 years posttransplant and QALYs saved are computed as the area under the 20-year projection, adjusted for a transplant utility of 0.8 and discount rate of 5% (21). Posttransplant QALYs were computed for each illness severity category at transplant using historical OPTN data for patients transplanted in 2002–2008. The benefit of a sharing proposal was computed as the product of net lives saved per illness severity category with stratum-specific QALY expectations, and used to calculate the costs per QALY gained.
Results
Patient characteristics
Cost data were analyzed for 15 813 liver transplants performed in academic medical centers. Not all academic centers participate in the UHC; however, compared to the national cohort of patients examined in the LSAM simulations, the study cohort was generally representative (Table 1). Patients in regions 3 and 4 were under-represented, while 6, 7 and 11 were over represented. There were a greater number of patients with a MELD > 35 in the UHC cohort compared to the overall population while less patients were categorized as MELD 15–19 or Status 1.
Economic analysis
The mean cost of the transplant hospitalization in the study sample was $122 862 (standard deviation $96 669; interquartile range $65 744). Multivariate regression confirmed a strong association between total hospital cost and key clinical variables (Table 2). Recipient factors associated with significantly increased cost in this analysis included recipient age ≥60 years, female gender, other race, low body mass index and hepatitis B (p < 0.05 for all). While DRI > 1.17 was associated with an increased cost of transplant, there was not a completely linear increase in costs associated with donor risk in this population. There was a gradual increase in the overall cost of transplant care in more recent years despite adjustment for medical inflation. Thus, mean cost of transplant increased by more than $60 000 between 2002 and 2008 in this cohort (p < 0.0001).
| Characteristic Regression intercept | Parameter estimate, (Standard error) $ 129 519 (6338) | p-Value <0.0001 |
|---|---|---|
| Illness severity at transplant MELD score at transplant | ||
| <10 | Reference | |
| 10–14 | 6876 (2853) | 0.02 |
| 15–19 | 7876 (2826) | 0.005 |
| 20–24 | 21 782 (2939) | <0.0001 |
| 25–29 | 42 171 (3214) | <0.0001 |
| 30–34 | 79 136 (3507) | <0.0001 |
| ≥35 | 87 625 (3402) | <0.0001 |
| Status 1 | 60 012 (4758) | <0.0001 |
| Recipient age | ||
| 12–17 | –6999 (6552) | 0.28 |
| 18–29 | –5642 (4346) | 0.19 |
| 30–45 | Reference | |
| 45–59 | 3560 (2308) | 0.12 |
| 60+ | 8547 (2588) | 0.001 |
| Female gender | 8613 (1597) | <0.0001 |
| Race | ||
| White | Reference | |
| Black | 510 (2431) | 0.83 |
| Other | 16 882 (3074) | <0.0001 |
| Hispanic ethnicity | –5197 (3586) | 0.15 |
| Blood type | ||
| AB | 1900 (3155) | 0.55 |
| A | Reference | |
| B | 5493 (2238) | 0.01 |
| O | 3687 (1592) | 0.02 |
| Cause of liver failure | ||
| Hepatitis C | Reference | |
| Hepatocellular carcinoma | 1900 (244) | 0.44 |
| Autoimmune | 3678 (2281) | 0.11 |
| Fulminate Hepatic Failure | 5479 (3803) | 0.15 |
| Alcoholic cirrhosis | 1221 (2377) | 0.61 |
| Primary sclerosing cholangitis | –4807 (3180) | 0.13 |
| Primary biliary cirrhosis | 6960 (4013) | 0.08 |
| Hepatitis B | 9311 (4142) | 0.02 |
| Nonalcoholic steatohepatosis | –2990 (11 863) | 0.80 |
| Cancer | 13 339 (11 219) | 0.23 |
| Other | 6341 (3927) | 0.11 |
| BMI category (kg/m2) | ||
| <18.5 | Reference | |
| 18.5 to <25 | –21 554 (4949) | <0.0001 |
| 25 to <30 | –24 485 (4933) | <0.0001 |
| 30 to <35 | –23 971 (5051) | <0.0001 |
| 35+ | –16 302 (5215) | 0.002 |
| Missing | –34 282 (5259) | <0.0001 |
| Donor risk index | ||
| <1.17 | Reference | |
| 1.17–1.36 | 2883 (2351) | 0.22 |
| 1.37–1.61 | 7735 (2356) | 0.001 |
| 1.62–1.92 | 5241 (2392) | 0.03 |
| >1.93 | 11 013 (2516) | <0.0001 |
| Missing | 11 306 (2598) | <0.0001 |
| Year of transplant | ||
| 2002 | –61 183 (2827) | <0.0001 |
| 2003 | –51 603 (2723) | <0.0001 |
| 2004 | –47 946 (2682) | <0.0001 |
| 2005 | –34 244 (2618) | <0.0001 |
| 2006 | –23 411 (2544) | <0.0001 |
| 2007 | –13 312 (2570) | <0.0001 |
| 2008 | Reference | |
Increasing calculated MELD score was strongly associated with overall cost of liver transplantation (p < 0.0001). However, the increase in cost associated with higher MELD score was nonlinear (Figure 2). Above MELD of 25, the cost of liver transplant increased exponentially in the continuous analysis. For example, incremental costs for patients with MELD >30 exceed those with MELD <10 by approximately $80 000 (p < 0.0001). The analysis of costs according to illness severity category (categorical MELD strata and status 1), which was employed to integrate with the LSAM models, shows similar patterns albeit with less granularity across MELD values.

Independent associations of MELD score at transplant with transplant hospitalizations costs by multivariate spline regression. Adjusted transplant hospitalization costs at each MELD score. Adjusted for donor and recipient factors including status 1 transplants.
Impact of re-allocation systems on cost of liver transplant
Broader sharing of available allograft using a two-tiered allocation system of priority resulted in a substantial increase in lives saved (Table 3). Compared to current practice, a two-tiered sharing system at 15 and 25 MELD points would reduce deaths by 88 per year (87 fewer deaths among waitlisted patients, 20 fewer deaths after removal from the list, 19 more deaths among posttransplant patients). This resulted from a redistribution of organs among MELD groups. The transplants among patients with a MELD > 30 were estimated to increase from 1287 to 1373 while transplants with a MELD < 15 were reduced by 273.
The economic impact of these policy shifts was estimated by calculating the estimated incremental cost of transplant and expected outcomes. Compared with the current allocation system, the shift to the system with the greatest reduction in death (MELD 15/22) would increase mean cost by $1164 per transplant (Table 3). This would increase the overall annual cost for transplants performed by $7 359 371 resulting in an average cost per life saved of $94 351.
The shift in transplants to higher MELD score patients is likely to a have an effect on posttransplant survival. We calculated the net increase in the total expected QALYs for patients who would undergo transplantation in each simulation output. For example, the 88 lives saved through implementation of the 15/25 allocation policy would result in an estimated 551 years of additional QALYs using a 20-year time frame across the MELD strata. Thus, this allocation policy would be expected to increase the cost of inpatient liver transplant care by $17 056 per QALY saved. This cost could be reduced through the adoption of the 15/35 policy. Under this policy, only 78 additional lives would be saved; however, the cost per QALY saved would be only $9449. Comparing all of the options available, the 15/25 policy results in the greatest QALY gain while the 15/22 offers lowest cost per incremental QALY gained (Table 4).
| Allocation system | # Transplants | Marginal cost per transplant | Lives saved | Cost per life saved | Quality-adjusted life years (QALYs) saved | Cost per QALY saved |
|---|---|---|---|---|---|---|
| 15/35 | 6323 | $ 1164 | 78 | $ 94 351 | 494 | $ 14 893 |
| 15/32 | 6320 | $ 1323 | 73 | $ 114 530 | 460 | $ 18 193 |
| 15/29 | 6319 | $ 1431 | 82 | $ 110 248 | 519 | $ 17 421 |
| 15/25 | 6309 | $ 1490 | 88 | $ 106 819 | 551 | $ 17 056 |
| 15/22 | 6296 | $ 728 | 78 | $ 58 802 | 485 | $ 9449 |
Discussion
Liver transplantation has matured into a well-accepted treatment for patients with end-stage liver failure, but application of this therapy is constrained by access to acceptable donor allografts. This analysis of a novel database containing linked clinical and cost-accounting data from a large, national sample of liver transplant centers confirms prior single center-based observations that severity of illness, patient age, diagnosis and body mass index affect the cost of liver transplantation. Among these factors, severity of illness is the most dramatic cost driver, affecting costs according to a nonlinear functional form. Thus increases in MELD from 4 to 25 have a minimal impact on cost while MELD scores above this level are associated with rapidly escalating costs. The analysis also demonstrates marked differences in center-reported costs of transplant as a function of OPTN region, even after local wage-price adjustment to allow comparability across geography. Using this unique database, we have also assessed the economic impact of novel allocation systems on the incremental cost of transplant care expected with broader sharing of available donor allografts. Our findings suggest that broader sharing of organs may be highly cost effective, with a transplant hospitalization-related cost per year of life saved of less than $20 000.
Prior analyses of factors influencing the cost of liver transplantation using single center data have demonstrated that severity of illness is associated primarily with longer length of stay (LOS). Thus, high MELD patients use more pharmaceuticals, laboratory tests, radiographs and dialysis services (1). There was no significant association with operating room costs, blood bank or anesthesia. These findings are consistent with the MELD-cost curve identified in the current study. At low MELD scores, the ‘fixed’ costs of liver transplant are dominant and consistent. Thus, all liver transplants require operating room time, anesthesia and, in general, a period of ICU care. This corresponds with the generally flat lower part of the cost curve. Above a MELD of 25, the ‘variable’ costs dominate which reflect the cost of delivery care to the individual patient as a function of degree of illness. We have previously demonstrated that the shift to a MELD-based system of organ allocation was associated with increased costs and reduced profits for liver transplant centers as a result of an increase in high-MELD, costly patients (11). Washburn et al. recently assessed factors, which influence LOS and resource utilization in two large, geographically disparate centers (12). In this experience, the relationship between MELD and cost was consistent across the institutions. Interestingly, however, adjusted LOS was actually reduced at the hospital with the higher MELD score, suggesting, perhaps, some learning curve in managing high MELD recipients.
The desire to expand access to liver transplant for patients dying on the waiting list has given rise to multiple strategies to broaden the pool of potential organs. We have previously explored the impact of the DRI on LOS and estimated cost at a registry level (9). In that analysis, it was principally the highest DRI livers, which drove costs. In the current analysis, increasing DRI was associated with cost, though the effect was small in comparison to recipient factors. This may reflect our emphasis on inpatient cost or a learning curve associated with the use of marginal donors (22). MELD and DRI do interact to increase costs of care in both the inpatient stay and over the life of the transplant, with variation according to MELD score dominating the cost during the initial transplant hospitalization and DRI predominantly affecting long-term costs (10). Other analyses have demonstrated that using organs from Donors following Cardiac Death (DCD), dramatically increases the cost of transplant within the first posttransplant year while decrease graft and patient survival (23–25). Jay et al. demonstrated that compared with livers from donors after brain death, the cost of DCD liver transplant was over 25% higher driven principally by the high incidence of biliary complications (13). Finally, centers have turned to living donors to attempt to bridge the gap between supply and demand. Data from the NIH sponsored Adult to Adult Living Donor Liver Transplant (A2ALL) registry were used to construct a Markov model the estimated the cost-effectiveness of living donor liver programs. Northrup et al. estimated the incremental cost-effectiveness ratio of adding a living donor program to a deceased donor liver transplant (direct evaluation costs, organ recovery and cost of the transplant) to be >$100 000 per quality adjusted life year (26).
Unlike the use of marginal donor organs, this analysis suggests that a re-allocation of available liver allografts has the potential to increase the benefit of transplantation far more cost effectively. Through the implementation of a 15/25 tiered allocation system, we could increase the life-years benefit from transplant by 551 at a cost of less than $20 000 per life year saved. This compares favorably with the incremental costs effectiveness of procedures accepted by CMS for Medicare coverage, which range from <$50 000 to more than $100 000 per life year saved (27). In general, a ratio less than $50 000–$100 000 per QALY is considered cost effective in the US (28,29). This estimate is likely a significant under estimate of the true benefit of this re-allocation, because we did not include the differential in cost of caring for patients on the waiting list. We have shown through previous analyses that pretransplant costs and hospitalization are highly correlated with MELD score (30). Thus, transplanting a high MELD patient rather than maintaining that patient on the waiting list results in more savings than that achieved through the transplantation of a low MELD patient. Posttransplant costs, however, are minimally associated with MELD score. Thus, after their transplant hospitalization, we would not expect a marked difference in the cost of posttransplant care if more high MELD patients were transplanted. We also did not capture the costs incurred by patients who are forced to travel long distances in search of organs under the current system. For these patients, the direct medical costs are likely to be similar, but the costs of travel, lost work and the need to find housing are likely significant.
Although this is a robust database including OPTN clinical data and hospital reported cost data, several limitations must be acknowledged. First, there are likely to be some clinical factors, which are poorly captured in UNOS and affect the cost of transplant (e.g. poor social support). However, these factors are unlikely to diminish the association between MELD and transplant cost. They may, however, increase the cost of transplant disproportionately in areas with long waiting times, which would benefit from re-allocation of available livers. Second, these analyses include only the cost of the initial transplant hospitalization. Thus, physician fees and posttransplant costs are not captured in this database. This limitation is mitigated by the fact that the vast majority of the costs of liver transplant are the hospital costs incurred during the initial hospitalization (10). In addition, MELD score prior to transplant has a limited impact on posttransplant costs (30). Third, we have included the cost of liver–kidney transplantation within the overall cost of liver transplant at each MELD score. Thus, a portion of the increased cost of transplanting higher MELD patients reflects the greater need for liver–kidney transplants. Given current reimbursement under Medicare, which does not include additional payment for liver–kidney transplant, it is appropriate to include these cases in the cost of liver transplantation. Fourth, we did not directly factor in the increase in transportation costs incurred through broader sharing. To address this concern, we considered two additional factors. The SRTR model estimates that implementation of the 15/25 sharing policy would increase the average distance from donor hospital to transplant center by only 32 miles. We also examined the proportion of shared allografts. Using of the proposed national sharing system of 15/25 would increase the proportion of allografts used out of the DSA by approximately 10% or 630 transplants. We estimated that even if this sharing these organs resulted in a $10 000 per organ incremental transportation cost, this would increase the total cost of liver transplantation by < 1%.
It is also very important to acknowledge the fundamental limits of the simulation models (LSAM) to completely predict what will happen under alternative allocation systems. First, although the LSAM models consider the use of exception points in the allocation assignment, the models reported in this simulation use laboratory MELD score and status 1 only. We also use the laboratory MELD/status 1-stratified costs to calculate costs as this score is more closely tied to costs than exception MELD. Because the exception patients are likely to be lower cost than predicted by considering MELD in terms of allocation MELD score at transplant, it is possible our cost-effectiveness models over-estimate the cost consequences of the shifting of organ to higher MELD patients, as some of these patients may, in fact, be tumor patients who will cost less than predicted here. This would further reduce the cost-per-QALY incurred in reallocation of available allografts. Second, the simulation models are based upon current listing and acceptance practices. Changing allocation may lead to increased acceptance of marginal livers in regions where the average MELD at transplant begins to increase. These changes may increase the cost of transplantation in these regions. However, there will likely be an offsetting reduction in the use of marginal organs in what are currently the most under-supplied regions, and corresponding savings by reducing the need to use high DRI organs in the highest MELD recipients (9,10). Third, we could not directly account for changes in the proportion of livers that would be used for liver–kidney transplants. However, it is unlikely that broader sharing would impact the use of liver–kidney transplant as a proportion of all transplants performed within a given MELD strata. Thus, the average costs we used to estimate cost effectiveness are marginally higher than costs incurred for liver transplants alone, as our cost data includes patients who receive liver–kidney transplants at a given MELD score.
In conclusion, use of this detailed source of clinical and economic data has provided an improved method to estimate the economic implications of changes in transplant practice. Detailed understanding of transplant costs allows a better understanding of the impact of recipient and donor factors on cost. Among the factors identified, perhaps MELD at transplant has the greatest potential to be affected by local factors. This analysis is reassuring in that it provides support that broader organ sharing offers an opportunity to reduce the death from end-stage liver disease in a durable, highly cost-effective manner that can be implemented without risk to living donors. Further implementation of this policy should be strongly considered in light of the ongoing disparity in access to transplant organs and the need to maximize the benefit of each available organ.
Acknowledgments
This work was supported by an American Recovery and Reinvestment Act grant from the National Institute of Diabetes Digestive and Kidney Diseases, RC1 1RC1DK086450–01. Dr. Lentine also received career development support from an NIDDK grant, K08DK073036. Data reported here have been supplied by the United Network for Organ Sharing (UNOS) as the contractor for the Organ Procurement and Transplantation Network (OPTN) and by the University HealthSystem Consortium (UHC). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the OPTN, UHC, the US Government, the NIDDK or the National Institutes of Health.
Funding Sources
This work was supported by an American Recovery and Reinvestment Act grant from the National Institute of Diabetes Digestive and Kidney Diseases, RC1 1RC1DK086450–01. Dr. Axelrod was supported by a grant from the Hitchcock Foundation. Dr. Lentine also received career development support from an NIDDK grant, K08DK073036.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Appendix
Appendix A. Detailed Description of Cost Data and Matching Algorithm
UHC is an alliance of 107 academic medical centers and 232 affiliated hospitals, which represents over 90% of the nation's nonprofit academic centers. UHC members contribute de-identified patient data using the UB-04 billing form including demographic data and ICD-9 diagnosis and procedure codes. Data on the costs of care during the initial transplant hospitalization were available for liver transplants performed in 2002–2008 at 56 centers in 29 states. Cost estimates are derived from charges reported at the individual charge level. Using Medicare cost reporting categories, the charges are converted to cost using hospital specific Medicare ratios of cost to charge, and these costs are aggregated at the patient level. UHC adjusts the labor portion of the estimated costs for differences in area labor costs using federally reported area wage indexes (AWI). Costs for adjusted for medical inflation using the medical component of the Consumer Price Index to 2008 levels for this analysis (16).
Cost data were linked to OPTN clinical data using the following steps, which are designed to promote fidelity in the data. Liver transplant procedures were identified in the UHC data using International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) procedure code 50.59 during the period of study (N= 24 240). After exclusion of re-transplant procedures, 23 030 unique recipients of a primary liver transplant remained, representing 56.6% of the 40 655 primary transplants in the OPTN in this period. Next, the transplants were matched to OPTN records based on the transplant center, date of transplant, recipient age and recipient gender with verification by residential ZIP code, if available. Overall, 20 833 (90.4%) of the UHC transplants were successfully matched to OPTN data. To favor match fidelity over sample size, we excluded potential matches for which the administrative data and the UNOS-supplied dates of transplant did not agree or for which there were other disagreements in the matching elements. For this study, we selected liver transplant recipients aged 12 years or older with valid data in both the OPTN and UHC databases and excluded recipients of multivisceral and living donor transplants, yielding 15 859 recipients. We also excluded low-cost outliers (n= 46) with reported transplant costs less than $25 000 per our previous methods using the UHC data (10), as such low reported costs likely reflect potential mismatching of UNOS data to nontransplant admissions.
We utilized multivariate regression analysis to quantify the relationship between MELD score at transplant and resource utilization for the population. Initial graphical analysis suggested a nonlinear, accelerating relationship between MELD score illness and cost of the liver transplant hospitalization. Therefore, a flexible nonlinear technique known as smoothed natural cubic splines was used to optimize fit of a curve describing costs by MELD. Spline fitting of curves was first described by Schoenberg in 1946 (31). The smoothed natural splines employed here are cubic, or third power, polynomial expansions of an independent variable of interest in a regression equation (32), in this case MELD. Basis knots, which define the form of the polynomial expansion, were set at MELD scores of 6, 15, 21, 28, 41 based on graphical analysis of the best fit of the curves. Estimates were adjusted for other recipient, donor and transplant characteristics reported to the OPTN. This approach was used to develop a granular understands of costs across MELD levels. To account for similarity in costs among patients transplanted at the same center, the regression analyses were analyzed using clustering at the center level. While cost data are characteristically right-skewed, linear regression produces estimates applicable to prediction; thus, we did not transform the cost outcome, as per our prior methods with transplant cost regressions (10,19,30,33).
| Characteristic | Status 1 % | MELD Category | ||||||
|---|---|---|---|---|---|---|---|---|
| <10 % | 10–14 % | 15–19 % | 20–24 % | 25–29 % | 30–34 % | ≥35 % | ||
| Recipient Age | ||||||||
| 12–17 | 8.4% | 1.4% | 1.0% | 1.3% | 1.3% | 1.0% | 0.3% | 0.2% |
| 18–29 | 26.6% | 2.4% | 1.4% | 2.1% | 3.5% | 3.7% | 3.3% | 3.0% |
| 30–45 | 31.6% | 9.0% | 8.2% | 10.0% | 13.2% | 13.0% | 15.5% | 14.8% |
| 45–59 | 25.7% | 57.1% | 62.0% | 62.9% | 60.3% | 60.7% | 60.1% | 63.5% |
| 60+ | 7.7% | 30.2% | 27.4% | 23.8% | 21.7% | 21.6% | 20.8% | 18.5% |
| Female gender | 64.0% | 30.6% | 27.7% | 30.0% | 32.2% | 31.1% | 32.5% | 29.9% |
| Race | ||||||||
| White | 58.4% | 63.0% | 72.6% | 77.9% | 74.1% | 73.0% | 65.7% | 62.3% |
| Black | 19.4% | 8.9% | 7.4% | 7.4% | 11.2% | 10.9% | 11.9% | 12.1% |
| Other | 22.2% | 28.1% | 20.0% | 14.8% | 14.7% | 16.1% | 22.4% | 25.6% |
| Hispanic ethnicity | 12.4% | 10.0% | 12.4% | 10.6% | 11.0% | 12.2% | 16.5% | 17.9% |
| Blood type | ||||||||
| AB | 4.7% | 5.0% | 7.8% | 7.3% | 5.1% | 3.7% | 4.0% | 3.9% |
| A | 34.5% | 35.7% | 37.6% | 37.8% | 39.4% | 38.5% | 36.3% | 33.1% |
| B | 14.5% | 17.7% | 14.3% | 14.9% | 12.7% | 12.3% | 12.1% | 12.3% |
| O | 46.3% | 41.6% | 40.4% | 39.9% | 42.9% | 45.4% | 47.5% | 50.8% |
| Pretransplant dialysis | 20.4% | 0.8% | 0.8% | 1.9% | 9.9% | 14.1% | 21.9% | 37.9% |
| Cause of liver failure | ||||||||
| Hepatitis C | 3.3% | 29.3% | 37.6% | 39.1% | 40.0% | 37.9% | 38.0% | 40.6% |
| Hepatocellular carcinoma | 0.4% | 34.3% | 24.0% | 12.1% | 5.6% | 4.1% | 3.9% | 4.2% |
| Autoimmune | 8.7% | 6.1% | 10.6% | 17.0% | 18.8% | 19.6% | 18.0% | 14.5% |
| Fulminate | ||||||||
| Alcoholic | 2.6% | 5.8% | 3.6% | 2.7% | 2.5% | 2.6% | 4.2% | 6.2% |
| Primary sclerosing cholangitis | 0.9% | 6.2% | 5.6% | 7.7% | 8.0% | 6.8% | 7.3% | 7.5% |
| Primary biliary cirrhosis | 7.9% | 2.9% | 2.5% | 3.2% | 4.5% | 4.2% | 3.8% | 3.7% |
| Nonalcoholic steatohepatosis | 8.9% | 6.1% | 3.6% | 2.6% | 3.3% | 3.5% | 3.4% | 3.3% |
| Cancer | 0.1% | 2.0% | 0.3% | 0.2% | 0.3% | 0.2% | 0.3% | 0.3% |
| Other | 0.1% | 0.1% | 0.3% | 0.4% | 0.5% | 0.7% | 0.3% | 0.1% |
| BMI category (kg/m2) | ||||||||
| <18.5 | 16.2% | 9.0% | 9.5% | 10.6% | 9.6% | 10.6% | 11.4% | 10.9% |
| 18.5 to <25 | 3.2% | 2.7% | 1.8% | 1.4% | 2.3% | 2.7% | 2.0% | 1.6% |
| 25 to <30 | 30.8% | 29.4% | 22.9% | 22.8% | 23.9% | 23.2% | 24.0% | 23.9% |
| 25.1% | 34.1% | 36.3% | 34.3% | 31.9% | 30.1% | 28.9% | 31.5% | |
| 14.0% | 17.1% | 19.9% | 19.6% | 19.7% | 19.4% | 19.5% | 18.6% | |
| Region | ||||||||
| 1 | 1.5% | 2.7% | 2.8% | 1.9% | 2.2% | 3.0% | 3.5% | 3.7% |
| 2 | 10.4% | 10.5% | 10.1% | 12.0% | 14.4% | 15.4% | 11.7% | 10.5% |
| 3 | 7.6% | 5.7% | 6.6% | 8.7% | 11.5% | 7.2% | 5.5% | 3.4% |
| 4 | 2.7% | 6.6% | 3.8% | 3.7% | 4.1% | 4.0% | 3.0% | 2.2% |
| 5 | 25.0% | 21.1% | 17.1% | 12.1% | 11.9% | 15.1% | 25.4% | 31.2% |
| 6 | 3.2% | 4.7% | 6.3% | 7.4% | 5.1% | 3.9% | 2.6% | 2.7% |
| 7 | 13.7% | 14.1% | 15.0% | 15.5% | 15.2% | 18.5% | 18.3% | 17.9% |
| 8 | 4.7% | 4.5% | 7.3% | 8.0% | 7.4% | 7.1% | 7.0% | 5.0% |
| 9 | 16.8% | 14.8% | 10.7% | 8.8% | 7.5% | 8.6% | 11.5% | 12.0% |
| 10 | 5.5% | 7.9% | 7.2% | 6.5% | 6.7% | 4.5% | 3.0% | 3.3% |
| 11 | 8.9% | 7.6% | 13.1% | 15.5% | 14.0% | 12.8% | 8.5% | 8.2% |
| Donor risk index | ||||||||
| <1.17 | 12.5% | 15.5% | 15.3% | 17.5% | 18.6% | 18.0% | 17.8% | 18.9% |
| 1.17 –1.36 | 23.5% | 15.7% | 16.0% | 16.4% | 17.9% | 18.6% | 20.8% | 18.6% |
| 1.37–1.61 | 17.2% | 15.7% | 16.7% | 17.8% | 17.6% | 17.7% | 17.4% | 20.3% |
| 1.62–1.92 | 16.7% | 17.5% | 16.1% | 16.0% | 16.5% | 17.7% | 18.7% | 16.1% |
| >1.93 | 15.8% | 16.6% | 15.6% | 14.0% | 13.5% | 13.3% | 11.3% | 12.1% |
| Missing | 14.2% | 19.0% | 20.4% | 18.4% | 15.9% | 14.8% | 14.0% | 14.0% |




