Accepting Kidneys from Older Living Donors: Impact on Transplant Recipient Outcomes
Abstract
Older living kidney donors are regularly accepted. Better knowledge of recipient outcomes is needed to inform this practice. This retrospective cohort study observed kidney allograft recipients from Ontario, Canada between January 2000 and March 2008. Donors to these recipients were older living (≥60 years), younger living, or standard criteria deceased (SCD). Review of medical records and electronic healthcare data were used to perform survival analysis. Recipients received 73 older living, 1187 younger living and 1400 SCD kidneys. Recipients of older living kidneys were older than recipients of younger living kidneys. Baseline glomerular filtration rate (eGFR) of older kidneys was 13 mL/min per 1.73 m2 lower than younger kidneys. Median follow-up time was 4 years. The primary outcome of total graft loss was not significantly different between older and younger living kidney recipients [adjusted hazard ratio, HR (95%CI): 1.56 (0.98–2.49)]. This hazard ratio was not proportional and increased with time. Associations were not modified by recipient age or donor eGFR. There was no significant difference in total graft loss comparing older living to SCD kidney recipients [HR: 1.29 (0.80–2.08)]. In light of an observed trend towards potential differences beyond 4 years, uncertainty remains, and extended follow-up of this and other cohorts is warranted.
Abbreviations:
-
- CAD
-
- Canadian dollars
-
- CCI
-
- Charlson Comorbidity Index
-
- CI
-
- Confidence interval
-
- CIHI-DAD
-
- Canadian Institute for Health Information Discharge Abstract Database
-
- CKD-EPI
-
- Chronic Kidney Disease Epidemiology Collaboration
-
- DCD
-
- Donation after cardiac death
-
- DONOR
-
- Donor Nephrectomy Outcomes Research Network
-
- ECD
-
- Expanded criteria donor
-
- GFR
-
- Glomerular filtration rate
-
- HR
-
- Hazard ratio
-
- ICES
-
- Institute for Clinical Evaluative Sciences
-
- IKN
-
- ICES Key Number
-
- IQR
-
- Interquartile range
-
- LOESS
-
- Locally weighted scatterplot smooth
-
- MDRD
-
- Modification of diet in renal disease
-
- OHIP
-
- Ontario Health Insurance Plan
-
- PRA
-
- Panel reactive antibody
-
- RPDB
-
- Registered Persons Database
-
- SCD
-
- Standard criteria donor (deceased kidney donor)
-
- SD
-
- Standard deviation
-
- STROBE
-
- Strengthening the Reporting of Observational Studies in Epidemiology Statement
-
- TGLN
-
- Trillium Gift of Life Network
-
- Tx
-
- Transplant
-
- UNOS
-
- United Network for Organ Sharing
Background
Living donor kidney transplantation provides the best and most flexible option for individuals with end stage renal failure. As more individuals develop renal failure, a larger spectrum of potential donors is being considered. Consequently, the definitions of previously unacceptable living kidney donors are changing. For example, close to a quarter of all living kidney transplants performed in the United States in recent years now involve donors with one or more medical complexities (1). Many transplant programs report that they no longer have an upper age limit for living donors (2,3). Older donors are most often defined by an age ≥60 years old (4). In addition to being driven by the demand for kidneys, their increasing prevalence may be a function of the overall aging population structure (5), or the increasing proportion of older transplant recipients with similarly aged potential donors (e.g. spouse or sibling).
Despite growth in the acceptance of older living donors, knowledge of recipient outcomes in this circumstance is limited. Biologically, an age-related decline in renal function may reduce the duration of recipient graft survival, as may an age-related predisposition to ischemia and drug toxicity, a reduced capacity for repair, and a higher degree of immunogenicity (6). In a recent meta-analysis of 12 clinical studies, 5-year survival was worse for recipients of kidneys from older living donors compared to younger donors (unadjusted relative risk of survival: 0.89, 95% CI 0.83 to 0.95) (7). Notably, studies included in this review were typically from single-centers, with limited numbers of patients, and there was a great deal of between-study heterogeneity. The majority of studies failed to account for confounding variables such as predonation donor renal function, and pretransplant duration of dialysis. While analyses of large U.S. data sets exist (8,9), no multicenter Canadian studies have ever been performed, where practice patterns and patient outcomes have been shown to differ (10).
The objectives of this study were to compare recipients of older living kidney donations (≥60 years) to recipients of younger living and deceased standard criteria donor (SCD) kidneys on outcomes of death and/or graft loss.
Methods
Data sources
This was a retrospective cohort study using Ontario-based electronic healthcare data. Transplant recipient data from January 2000 to March 2008 were obtained from the Trillium Gift of Life Network (TGLN), Ontario's central organ and tissue donation agency. The medical records of each living kidney donor across five transplant centers were also manually reviewed to ensure data accuracy, and to supplement TGLN data. Data were then linked to the Canadian Institute for Health Information Discharge Abstract Database (CIHI-DAD) which contains data on in-hospital diagnoses and procedures, the Ontario Health Insurance Plan (OHIP) which records inpatient and outpatient physician and allied health claims, and Ontario's Registered Persons Database (RPDB) which has demographic and vital statistics on all Ontario residents. Personal identifiers were removed from linked data sets. All recipients were followed until March 2009.
This study was conducted and reported according to recommendations from the STROBE Statement (Appendix A) (11). Ethics approval was obtained from the Sunnybrook Health Sciences Centre.
Study participants
The study included adult kidney transplant recipients, whose donors were: (1) living and older (≥60 years of age); (2) living and younger (<60 years of age) and (3) deceased SCD. Recipients of deceased expanded criteria donor (ECD) kidneys, donation after cardiac death kidneys, multiorgan, or dual/en bloc transplants were excluded. Electronic healthcare data codes for criteria meeting the UNOS definition of ECD were used to exclude ECD: previous diagnosis of hypertension, chronic kidney disease (proxy for serum creatinine ≥133 μmol/L), or diagnosis of cerebrovascular accident prior to death (12). The selection criteria for living kidney donors used in the five major transplant programs in Ontario during the period of study were quite conservative. All donors had a glomerular filtration rate ≥80 mL/min per 1.73 m2 (through direct or indirect measurements). Patients accepted with hypertension on a single agent were relatively uncommon during the study period.
Outcomes
Outcomes were defined using electronic health care and TGLN data. The primary outcome, total graft loss, was a composite of time from transplantation to graft loss (i.e. codes for chronic dialysis over three consecutive months or more, or identified in TGLN as having had another kidney transplant), or all-cause mortality (i.e. death with a functioning graft). Secondary outcomes included recipient death due to all causes (not censored for graft loss), and death-censored graft loss. Recipients were censored at study end (March 31, 2009) or earlier if they emigrated from the province during the study period.
Statistical analysis
Sample size for this study was fixed based on the total number of kidney allograft recipients during the study period (all events captured in electronic healthcare databases). 95% CIs were reported to suggest a plausible range where the true point estimate may lie. Baseline characteristics were compared using t-tests, Mann–Whitney U or chi-square tests as appropriate. For missing predonation GFR and peak-PRA (missing <5%), mean values were imputed. For each comparison, univariable and multivariable Cox proportional hazards regression analyses were performed for each outcome. As recommended in the STROBE statement, age was modeled as both a continuous and dichotomous exposure (11). Departures from linearity were assessed by plotting a locally weighted scatterplot smooth (LOESS) curve through martingale residuals as a function of donor age (13). The proportional hazards assumption was assessed by plotting the log-minus-log transformed Kaplan–Meier estimates of the survival. Time-dependent covariates, which allowed for a change in the hazard ratio over time were considered (14). To account for clustered data (i.e. re-transplants; two kidneys from one deceased donor), sandwich estimators of the standard error of the hazard ratio were used (15).
Recipient age, pretransplant duration of dialysis, transplant year and predonation donor renal function were adjusted for in all models. Additional factors [donor and recipient sex, donor–recipient relationship, recipient race, recipient Charlson Comorbidity Index (CCI), open versus laparoscopic surgery, number of renal arteries on the donated kidney and recipient panel reactive antibody (PRA)] were assessed empirically. A 10% change between crude and adjusted estimates was considered important (16). All models were stratified by transplant center to allow for distinct baseline hazard functions across all five sites. Subgroup analyses by recipient age (≥ or <60 years) and donor eGFR (≥ or <90 mL/min per 1.73 m2) at the time of donation were tested using interaction terms (17). A two-sided p-value <0.05 was considered statistically significant. Statistical analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, North Carolina, USA).
Results
Baseline characteristics
From January 1, 2000 to March 31, 2008, TGLN collected information on 3511 kidney transplant recipients. Figure 1 shows a flow diagram of applied exclusions. Analyses included recipients of 73 older living donor kidneys (5.8% of living transplants), 1187 younger living kidneys and 1400 deceased SCD kidneys. Nineteen recipients had two kidney transplants over the study period. Deceased kidney allografts were from 828 donors; 572 donated both of their kidneys.
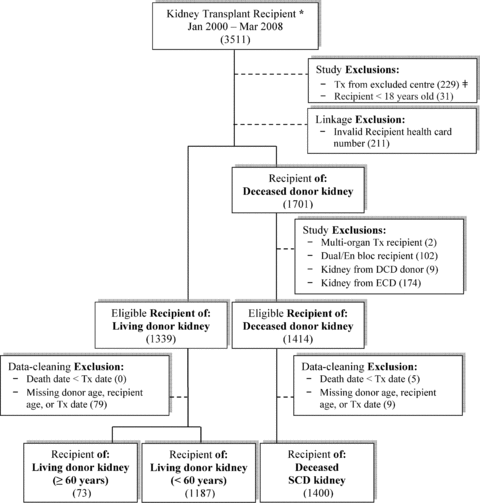
Flow diagram of participation in this study. Note: Number included/excluded indicated in (parentheses). (*) As identified by Trillium Gift of Life Network, Ontario, Canada. (#) Excluded centers were Hospital for Sick Children (exclusively performs pediatric transplants) and Kingston General Hospital (discontinued transplant program during study period). TGLN = Trillium Gift of Life Network; Tx = Transplant; IKN = ICES Key Number; DCD = Donation after cardiac death; ECD = Expanded criteria donor; RPDB = Registered persons database; SCD = Standard criteria donor.
Recipient characteristics at the time of transplant are summarized in Table 1. Recipients of older living kidneys were older than recipients of younger living kidneys [mean age: 49 vs. 45 years (p = 0.03), 33% vs. 14% over 60 years]. Both groups of living kidney recipients spent similar times on dialysis prior to transplant (p = 0.94), and had similar CCI scores (p = 0.55). When comparing recipients of older living kidneys to SCD deceased kidney recipients, their average age did not differ (p = 0.38). Recipients of older living kidneys spent significantly less time on dialysis than deceased kidney recipients (23 vs. 49 months, p < 0.001), but had a similar distribution of CCI scores (p = 0.93).
| Living donor recipients | Deceased donor recipients | ||||
|---|---|---|---|---|---|
| Older Kidney (≥60 years) N = 73 | Younger Kidney (<60 years) N = 1187 | p-Value1 | SCD Kidney (<60 years) N = 1400 | p-Value2 | |
| Age (years), mean (SD) | 49 (14) | 45 (13) | 0.03 | 50 (13) | 0.38 |
| 18–39 years | 26 (36) | 408 (34) | <0.001 | 317 (23) | 0.001 |
| 40–49 years | 11 (15) | 302 (25) | 342 (24) | ||
| 50–59 years | 12 (16) | 315 (27) | 385 (28) | ||
| 60–69 years | 23 (32) | 141 (12) | 274 (20) | ||
| ≥70 years | ≤5 (≤7)3 | 21 (2) | 82 (6) | ||
| Gender (Female) | 24 (33) | 477 (40) | 0.22 | 511 (37) | 0.53 |
| Preemptive transplant | 19 (26) | 228 (19) | 0.15 | ≤5 (≤0.4)3 | <0.001 |
| (no dialysis history) | |||||
| Duration of dialysis history, months4 | |||||
| Mean (SD) | 23 (22) | 23 (24) | 0.94 | 49 (31) | <0.001 |
| Median (IQR) | 16 (8–30) | 16 (8–29) | 0.81 | 44 (23–71) | <0.001 |
| Charlson Score | |||||
| 2–3 | 51 (70) | 887 (75) | 0.55 | 976 (70) | 0.93 |
| 4–5 | 18 (25) | 258 (22) | 360 (26) | ||
| ≥6 | ≤5 (≤7)3 | 42 (4) | 64 (5) | ||
| Peak PRA | |||||
| <20% | 60 (82) | 980 (83) | 0.74 | 1036 (74) | <0.001 |
| 20–50% | ≤5 (≤7)3 | 61 (5) | 128 (9) | ||
| ≥50% | 7 (10) | 88 (7) | 236 (17) | ||
| Missing | ≤5 (≤7)3 | 58 (5) | 0 | ||
- Notes: Values reported as N (%), unless stated otherwise. SCD = Standard criteria donor; PRA = Panel reactive antibody.
- 1Comparing recipients of older living donors to younger living donors.
- 2Comparing recipients of older living donors to standard criteria deceased donors.
- 3Note: Cells with ≤5 observations were suppressed to prevent indirect identification of individuals.
- 4Only applies to patients with a history of dialysis prior to transplant.
Donor characteristics at the time of transplant are shown in Table 2. The mean age of older living donors was 63 years, while younger living donors were 42 years and deceased SCDs were 39 years old. As expected, mean predonation eGFR was lower in older living donors compared to younger living donors (Chronic Kidney Disease Epidemiology Collaboration [CKD-EPI]: 83 vs. 96 mL/min per 1.73 m2, p < 0.0001). However, there was no difference in serum creatinine between the two groups (77 vs. 75 μmol/L, p = 0.27).
| Living kidney donors | Deceased kidney donors | ||||
|---|---|---|---|---|---|
| Older kidney (≥60 years) N = 73 | Younger kidney (<60 years) N = 1187 | p-Value1 | SCD Kidney (<60 years) N = 1400 | p-Value2 | |
| Age (years), mean (SD) | 63 (3) | 42 (10) | – | 39 (14) | – |
| Age (years), median (IQR) | 62 (60–64) | 42 (34–50) | – | 42 (28–50) | – |
| 60–69 years | 70 (96) | ||||
| ≥70 years | ≤5 (≤7)3 | ||||
| Gender [Female, n (%)] | 36 (49) | 731 (62) | 0.04 | 593 (42) | 0.24 |
| Relationship to their Recipient | |||||
| Parent | 24 (33) | 103 (9) | <0.0001 | ||
| Child | ≤5 (≤7)3 | 186 (16) | |||
| Sibling | 13 (18) | 404 (34) | |||
| Spouse | 18 (25) | 232 (20) | |||
| Other related | ≤5 (≤7)3 | 84 (7) | |||
| Unrelated | 11 (15) | 178 (15) | |||
| Serum Creatinine (μmol/L) | |||||
| Mean (SD) | 77 (14) | 75 (14) | 0.27 | ||
| Median (IQR) | 77 (67–86) | 73 (65–85) | 0.23 | ||
| CKD-EPI eGFR (mL/min per 1.73 m2) | |||||
| Mean (SD) | 83 (10) | 96 (15) | <0.0001 | ||
| Median (IQR) | 84 (74–91) | 96 (85–106) | <0.0001 | ||
| MDRD eGFR (mL/min per 1.73 m2) | |||||
| Mean (SD) | 84 (12) | 92 (17) | <0.0001 | ||
| Median (IQR) | 83 (74–92) | 90 (80–101) | <0.0001 | ||
| Laparoscopic | 43 (60) | 587 (50) | 0.12 | ||
- Notes: Values reported as N (%), unless stated otherwise. SCD = Standard criteria donor; CKD-EPI = Chronic Kidney Disease Epidemiology Collaboration; MDRD = Modification of diet in renal disease.
- 1Comparing older living donors to younger living donors.
- 2Comparing older living donors to standard criteria deceased donors.
- 3Cells with ≤5 observations were suppressed to prevent indirect identification of individuals.
Transplant outcomes
Median (IQR) follow-up time for the cohort was 4 (2 to 6) years. For the primary outcome, total graft loss, there were 269, 5199 and 5810 person-years of follow-up for recipients of older living, younger living and SCD deceased kidneys, respectively. Less than 2% were censored due to provincial emigration. Among living kidney transplants, there were 195 events of total graft loss: 17 for recipients of older living kidneys (6.3 per 100 person years) and 178 for recipients of younger living kidneys (3.4 per 100 person years). For SCD deceased recipients, 355 events of total graft loss were observed (6.1 per 100 person years).
Older living kidneys versus younger living kidneys
When donor age was modeled as a continuous predictor, each additional year increase in donor age was not associated with an increase in total graft loss [adjusted hazard ratio (HR): 1.01, 95% confidence interval (CI): 0.98 to 1.03, p = 0.49]. Assessment of the functional form of donor age confirmed no significant departures from linearity.
Donor age was next modeled as a dichotomous exposure using a cut-off of ≥60 years. The results are presented in Table 3. The adjusted hazard of total graft loss was increased by 56% for recipients with older living donors, but this was not statistically significant (95% CI: 0.98 to 2.50, p = 0.06). The hazard of death for recipients of older living donor kidneys was significantly higher (adjusted HR: 2.70, 95% CI: 1.39 to 5.26, p = 0.0004). There was no significant difference for death-censored graft loss (p = 0.72). Visual inspection of the log-minus-log transformed K–M plots revealed possible violations of the proportional hazards assumption. Models with a time-dependent interaction suggested that the HR of total graft loss with older living kidneys compared to younger living kidneys increased with time. Recipient age (≥ or <60 years) and donor eGFR (≥ or <90 mL/min per 1.73 m2) did not significantly modify the effect of living donor age on transplant outcomes (interaction p ranged from 0.10 to 0.58).
| Recipient outcome | Younger donor (<60 years) N = 1187 | Older donor (≥60 years) N = 73 | p-Value |
|---|---|---|---|
| Total graft loss (n) | 178 | 17 | – |
| Rates (Events per 100 person-years) | 3.4 | 6.3 | – |
| Unadjusted HR (95% CI) | 1.0 (reference) | 1.78 (1.11–2.87) | 0.02 |
| Multivariable adjusted HR (95% CI) | 1.0 (reference) | 1.56 (0.98–2.49) | 0.06 |
| Death (alone), n | 74 | 11 | – |
| Rates (Events per 100 person-years) | 1.3 | 3.8 | – |
| Unadjusted HR (95% CI) | 1.0 (reference) | 2.97 (1.58–5.56) | 0.001 |
| Multivariable Adjusted HR (95% CI) | 1.0 (reference) | 2.73 (1.39–5.35) | 0.004 |
| Death-censored graft loss (n) | 114 | 6 | – |
| Rates (Events per 100 person-years) | 2.2 | 2.2 | – |
| Unadjusted HR (95% CI) | 1.0 (reference) | 0.96 (0.43–2.14) | 0.91 |
| Multivariable Adjusted HR (95% CI) | 1.0 (reference) | 0.84 (0.34–2.11) | 0.72 |
- Notes: All multivariable models adjusted for: Recipient age, dialysis duration, donor GFR and year of transplant. Additional adjustment on the basis of operational confounding criteria depends on outcomes: Total graft loss, No additional covariates added to the model; Death, Donor–recipient relationship; Death-censored graft loss, Recipient race, Charlson score, peak PRA, donor gender, donor–recipient relationship, surgical technique and number of renal arteries.
Older living kidneys versus SCD deceased kidneys
A comparison of the outcomes of older living versus SCD deceased kidney recipients is presented in Table 4. The adjusted hazard of total graft loss was not significantly different for recipients of older living kidneys compared to recipients of SCD deceased kidneys (adjusted HR: 1.28, 95% CI: 0.79 to 2.08, p = 0.30). There was also no difference between groups for death or death-censored graft loss. Visual assessment revealed possible violations of the proportional hazards assumption. Time-dependent interaction reached statistical significance for total graft loss (p = 0.01), suggesting that recipients of older donor kidneys had a risk of total graft loss that increased with time. No significant interaction by recipient age was detected for any of the outcomes (p ranged from 0.13 to 0.79).
| Recipient outcome | SCD deceased (<60 years) N = 1400 | Older living (≥60 years) N = 73 | p-Value |
|---|---|---|---|
| Total graft loss (n) | 355 | 17 | – |
| Rates (Events per 100 person-years) | 6.1 | 6.3 | – |
| Unadjusted HR (95% CI) | 1.0 (ref) | 1.03 (0.64–1.64) | 0.91 |
| Multivariable adjusted HR (95% CI) | 1.0 (ref) | 1.29 (0.80–2.08) | 0.30 |
| Death (alone), n | 201 | 11 | – |
| Rates (Events per 100 person-years) | 3.1 | 3.8 | – |
| Unadjusted HR (95% CI) | 1.0 (ref) | 1.27 (0.69–2.32) | 0.44 |
| Multivariable adjusted HR (95% CI) | 1.0 (ref) | 1.83 (0.96–3.48) | 0.07 |
| Death-censored graft loss (n) | 173 | 6 | – |
| Rates (Events per 100 person-years) | 3.0 | 2.2 | – |
| Unadjusted HR (95% CI) | 1.0 (ref) | 0.71 (0.32–1.56) | 0.39 |
| Multivariable adjusted HR (95% CI) | 1.0 (ref) | 0.74 (0.34–1.62) | 0.45 |
- Notes: All multivariable models adjusted for: Recipient age, dialysis duration and year of transplant. Additional adjustment on the basis of operational confounding criteria depends on outcomes: Total graft loss, Recipient race, Charlson score and peak PRA; Death, Peak PRA; Peak PRA, Death-censored graft loss.
Discussion
In Ontario, about 6% of living kidney donors may be considered ‘older’. Trends suggest that this proportion is increasing with time (18). In other countries, older donors are accepted more frequently than ever before. For example, according to the Norwegian Renal Registry, 16% of living kidney donors are now ≥60 years old and 7.7% are ≥65 years of age (19). In the United States, the majority of transplant centers report no upper age limit, which precludes an individual from becoming a living donor (3). Despite these trends in the acceptance of older living kidney donors, knowledge of recipient outcomes from such donors is limited. This disconnect between evidence and practice has been highlighted elsewhere (7).
To better inform current practice, we studied recipient outcomes of all older living kidney donors across five transplant centers in Ontario, Canada. We found that acceptable 4-year recipient outcomes were achieved when using older living donor kidneys. Initial unadjusted analyses suggested an increased hazard ratio for total graft loss when comparing recipients of older and younger living donor kidneys. After accounting for age differences and other confounding factors, this difference was no longer statistically significant. The risk of death with a functioning graft was observed to be significantly higher for recipients of older living donor kidneys. This may partly be mediated by differences in kidney function in the absence of graft failure, or in recipient case mix. There may also be a role for residual confounding by factors related to health status that were difficult to ascertain. However, there was no difference in death-censored graft loss between older and younger living donors, and no relationship with graft loss when age was modeled as a continuous covariate. Recipient outcomes for older living kidney donors were no different than deceased SCDs.
A systematic review summarizing the results of 31 previous studies suggested that total graft survival in older living kidney recipients was significantly worse at 5 years compared to younger living donors (7). Several factors may explain the discrepancy between the current data and previous studies. First, living donor selection criteria in Canada are quite conservative, which may lead to better outcomes for Canadian kidney transplant recipients. This contrasts to practices in the United States, where almost a quarter of living kidney donors have some preexisting, moderate health condition (4). Such conditions would be most prevalent among older individuals. This is best highlighted in a well-conducted study using data of United States Renal Data System, which showed a progressively higher risk of graft loss in recipients of older living kidneys (55 years and older) (9). In both settings, excellent clinical outcomes for older living donors compared to deceased SCDs were observed. Second, this analysis accounted for potential confounding factors that were not considered in most previous studies, such as pretransplant duration of dialysis in months, transplant year, predonation donor renal function, surgical type (open versus laparoscopic) and recipient PRA. Finally, this study focused on the most recent era of transplants, those performed from the years 2000 to 2008. Results were consistent with the trend highlighted in a meta-regression of previous studies suggesting a less prominent ‘period’ effect over time (7).
To our knowledge, this is the first study to assess the effect of donor age on transplant outcome in a Canadian setting. It is one of the largest studies to date; only two other studies of US health administrative data have followed a larger group of donors (8,9). Almost all adult-transplant recipients (first and retransplants) from multiple centers in Ontario were considered. Recipient follow-up during the full study period was excellent (<2% was lost due to provincial emigration). It is also the first study to deterministically link transplant recipients to Canadian electronic healthcare data; previous studies relied on a probabilistic linkage, which linked a maximum of 70% of the eligible cohort (20). Our use of electronic healthcare data was also supplemented by manual chart review to ensure accurate and complete information.
A few limitations of this study merit consideration. Data for several potential confounding factors were not well documented in the data sources used, and were often difficult to ascertain from medical records. Thus, some residual confounding may be present. Such factors included various laboratory measures, donor blood pressure, ischemic time, cytomegalovirus mismatch, induction therapy and baseline maintenance immunosuppression. We studied transplants from all older living donors in Ontario during the study period. However, this number was finite. A greater number of donors would have resulted in more precise estimates, and a greater ability to rule out clinically important differences between our study groups.
Implications for clinical care
Using the results from this study, transplant professionals can inform their patients that graft survival from older living kidney donors is not inferior to receiving a kidney from a deceased SCD. This information may be particularly well received given long waiting times for deceased donation in many jurisdictions.
In practice, more than one eligible potential living donor may come forward for a single potential recipient, and these donors may differ in age (e.g. a parent and child). Based on current data, there remains uncertainty as to the comparability of selecting the younger versus the older donor. Practically speaking, 4-year outcomes when using older living donor kidneys may be considered acceptable in some settings. For example, in cases involving young recipients who may need another transplant in their lifetime, transplant professionals may choose to accept an older donor despite shorter graft survival, in order to save the younger donor for a possible future transplant (immunological sensitization notwithstanding). In cases involving older recipients where projected life expectancy is not as high, transplant professionals may feel comfortable selecting the older donor (i.e. old-for-old). Arguments have been proposed suggesting this may be the safer practice in considering donor health as well (21).
A living donor paired exchange registry was recently established in Canada in 2009 (22). Similar registries have been established in other nations. As part of the exchange process, the transplant team is responsible for assessing the ‘fairness’ of each proposed exchange. When donors involved in an exchange are of markedly different ages, there may be a question of whether the recipient of an older kidney is receiving a kidney of equal quality to another recipient of a younger kidney. These results suggest that a matching algorithm involving similarly aged donors may not be too critical on 4-year recipient outcomes.
Finally, from an economic perspective, every older donor who may have otherwise been precluded from donation contributes a cost savings of about $100 000 Canadian dollars over a 20-year period, compared to the patient who waits on dialysis (23).
In conclusion, this study extends current understanding of the utility of older living kidney donors by observing outcomes among Ontario kidney transplant recipients in the most recent era, with better follow-up and supplementation of electronic health data with more detailed techniques for data ascertainment. Recipients of older living donor kidneys had similar 4-year total graft survival when compared to recipients of SCD deceased donor kidneys. As for outcomes when using older versus younger living donor kidneys, the difference was not statistically significant. In light of an observed trend towards potential differences beyond 4 years, uncertainty remains and extended follow-up of this and other cohorts is warranted.
Acknowledgments
We thank Dr. Ping Li and Mr. Nelson Chong from the Institute for Clinical Evaluative Sciences, and Dr. Frank Markel, Ms. Versha Prakash, Ms. Anjeet Bhogal and Mr. Keith Wong from Trillium Gift of Life Network for their support. We would also like to thank Ms. Laura Agar, Mr. Nishant Fozdar, Ms. Mary Salib, Ms. Sonia Thomas and Ms. Robyn Winterbottom, who abstracted data from medical charts at five transplant centers for this project.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Conflict of Interest
None declared.
Funding Source
This study was funded through an operating grant from the Canadian Institutes of Health Research. Dr. Ann Young was supported by a Doctoral Research Award from the Canadian Institutes of Health Research and a Schulich Graduate Scholarship from the University of Western Ontario. Dr. Amit Garg was supported by a Clinician Scientist Award from the Canadian Institutes of Health Research. This project was conducted at the Institute for Clinical Evaluative Sciences (ICES). ICES is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). The opinions, results and conclusions reported in this paper are those of the authors, and are independent of the funding sources, the MOHLTC and Trillium Gift of Life Network.




