Apoptotic Cell Death Is Initiated During Normothermic Ischemia in Human Kidneys
Abstract
Ischemic damage plays an important role in post-transplant organ failure. Activation of the apoptotic cascade is crucially involved in post-ischemic inflammation resulting in tissue damage and organ dysfunction. Here we investigate the initiation of the apoptotic cascade during normothermic ischemia in human kidneys using a model for normothermic ischemia with kidneys nephrectomized because of renal cell carcinoma. Ex vivo, kidneys were stored at 37°C, and consecutive biopsies were taken from disease-free tissue. Pro- and anti-apoptotic proteins were assessed by Western blotting and immunofluorescence. During normothermic ischemia the pro-apoptotic proteins Bax and activated caspase-9 increased with ischemia time, whereas caspase-8 was not activated. The anti-apoptotic proteins Bcl-2 and cFLIP decreased in time. Data on Bcl-2 and Bax were supported by immunofluorescence for Bcl-2 and activated Bax. However, activation of the central effector caspase-3, essential for execution of the apoptotic process, was not detected. In conclusion, during normothermic ischemia the apoptotic cascade in the human kidney is initiated, but not fulfilled. Our data show that the duration of ischemia significantly correlates with activation of the apoptotic cascade. These findings provide insight in the initiation of apoptotic cell-death during warm ischemia and may be useful in the assessment of ischemic injury.
Introduction
Post-transplant acute renal failure (ARF) is an important determinant of renal transplant survival. The occurrence of ARF is substantial in organs derived from cadaveric donors and in particular non-heart beating (NHB) category 2 donors (1), as compared to living donors (2). The inevitable ischemia of such NHB donor kidneys explains these differences in graft failure to a large extent (2). Understanding the mechanisms of injury initiated by ischemia reperfusion (I/R) is therefore of critical importance for defining strategies to improve post-transplant organ function.
In different experimental models it has been established that renal ischemia followed by reperfusion leads to apoptotic cell death (3,4). Moreover, activation of the apoptotic cascade is crucially involved in the induction of post-ischemic inflammation and subsequent organ failure (3). In line, enhanced apoptotic cell death established by tunel-staining was detected in post-transplant biopsies from cadaveric allografts as compared to transplants derived from living donors, indicating that ischemic injury induces apoptosis in clinical transplantation (5).
Apoptosis is executed by a family of cysteine proteases, called caspases (6). Two major pathways of caspase activation have been described, that is the extrinsic and intrinsic pathway (7). Activation of the extrinsic pathway, triggered by binding of extracellular ligands to cell surface death receptors, results in recruitment of the adaptor molecule and Fas-associated death domain (FADD) and activation of caspase-8 upon recruitment to the death-inducing signaling complex (DISC). The latter are controlled by cFLIP, an intracellular protein that inhibits death receptor signaling by regulating both recruitment and processing of pro-caspase-8 within the DISC (8).
The intrinsic pathway is activated by various intracellular stimuli, such as anoxia, growth factor deprivation and DNA damage, all affecting the mitochondria, where they promote mitochondrial membrane permeabilization and the release of caspase activating factors, among others cytochrome c, into the cytosol (9,10). Cytosolic cytochrome c interacts with apaf-1, leading to its oligomerization and the recruitment of pro-caspase-9, which autoactivates within the so-called apoptosome (11). Members of the Bcl-2 family, among others pro-apoptotic Bax and anti-apoptotic Bcl-2, control activation of the intrinsic pathway. For any given apoptotic stimulus, the balance between death and survival seems to be determined by the ratio of the apoptosis-stimulating and -suppressing Bcl-2 family members (12,13). The activation of either extrinsic or intrinsic pathway participates in a cascade that culminates in the activation of effector caspases. Activation of primarily effector caspases-3, -6 and -7, results in cleavage of a wide range of substrates leading to chromosomal DNA fragmentation and cellular morphologic changes characteristic of apoptosis (6).
Although it appears that apoptotic cell death plays a role in the induction of organ damage due to renal I/R in humans (5,14), it is currently unknown whether the apoptotic cascade is already initiated during ischemia or becomes initiated upon reperfusion. In this context, we developed a human model for renal normothermic ischemia. This model was used to study proteins involved in the initiation of the apoptotic cascade during normothermic ischemia. In particular, we investigated the consumption of endogenous caspase inhibitors as well as the activation of initiator and effector caspases. Our data indicate that during normothermic ischemia the apoptotic cascade is initiated in the human kidney. These data implicate that during normothermic ischemia, the circumstances are created that facilitate execution of apoptosis upon reperfusion. Therefore, our data may be useful in the development of methods to assess ischemic injury. Moreover, renewed insight in early ischemic changes provides important opportunities to prevent clinical manifestations of reperfusion injury in the kidney, and potentially in other organs.
Materials and Methods
Antibodies
The following antibodies (ab) were used: anti-murine Bax ab P19 from Santa Cruz Biotechnology (Santa Cruz, CA); anti-Bcl-2, anti-human caspase-9 and anti-human caspase-3 from Stressgen (Victoria, Canada); monoclonal murine anti-caspase-8 was kindly provided by Dr. Klaus Schulze-Osthoff (Institute of Molecular Medicine, University of Duesseldorf, Germany); anti-human cFLIP (Biocarta, San Diego, CA); Goat anti-rabbit Texas Red and peroxidase conjugated goat anti-rabbit IgG from Jackson Immuno-Research Laboratories (West Grove, PA) and peroxidase conjugated goat anti-mouse IgG from Caltag (Burlingame, CA). Anti-Bcl-2 and anti-Bax from Upstate Biotechnology (Lake Placid, NY) and Lotus Tetragonolobus Lectin from Vector (Burlingame, CA) were used for immunohistochemistry.
Human model for renal normothermic ischemia
In the present study, kidneys used were nephrectomized because of a renal cell carcinoma. These patients did not have a family history of renal cell carcinomas, neither did they have other signs of hereditary diseases like von Hippel-Lindau (VHL). Taking into consideration the pathology, the age of the patients and the absence of a family history/patient anamnesis, the chance that a hereditary effect influences our data, is certainly far below 1%.
Normothermic ischemia started at ligation of the renal artery about 5 min before nephrectomy. Thereafter, kidneys (n = 5 per group) were immediately stored at 37°C and rapidly inspected by the responsible pathologist. Full-thickness biopsies were taken from non-diseased tissue. Subsequently, normothermic renal ischemia was prolonged up to 85 min. Renal temperature was measured during storage and remained between 36 and 37.5°C. Approximately every 15 min, full-thickness biopsies were taken. Control kidneys (n = 4) were cooled at 0°C immediately upon nephrectomy. Biopsies from these control kidneys were taken within 10 min after nephrectomy. All biopsies were divided in several parts and either immediately stored in liquid nitrogen for Western blotting, or embedded in Tissue Tek (EMS, Washington, PA) and snap-frozen in isopentane at −80°C for immunohistochemistry. All biopsies were stored at −80°C until further processing.
Western blotting
Renal tissue samples were homogenized in lysis buffer (200 mM NaCl, 10 mM Tris base, 5 mM EDTA, 10% Glycerin, 1 mM PMSF, 0.1 U/mL Aprotinin and 1 μg/mL Leupeptin). Homogenates were centrifuged at 300 rpm for 10 min; supernatants were collected and centrifuged again at 10 000 rpm for 3 min. Final supernatants were harvested and total protein concentrations were determined using the Bradford method. To confirm equal protein loading, immunoblotting was performed with an anti-actin antibody (Sigma, Chicago, IL). Aliquots with equal amounts of protein were heated at 100°C for 5 min in SDS sample buffer, separated on 15% SDS–polyacrylamide gels and transferred to polyvinylidene fluoride membranes (Immobilon P, Millipore, Bedford, MA). After transfer of proteins, blocking, ab incubation steps and washing of the membrane were performed in Phosphate buffered saline (PBS) supplemented with 3% non-fat dry milk and 0.05% Tween. Except for incubations with ab to cFLIP, which were blocked in 50 mM Tris, 150 mM NaCl, 0.1% Tween 20 (TBST) supplemented with 5% milk and washed in TBST with 0.5% milk.
After the incubation with the primary ab and washing, membranes were incubated with the appropriate horseradish peroxidase–conjugated secondary ab to rabbit or mouse IgG. Positive bands were visualized using chemiluminescence's (Supersignal, Pierce, Rockford, IL).
Immunoreactive bands for all proteins were evaluated densitometrically using a computer-controlled display camera (Imagemaster, Pharmacia, Piscataway, NJ) and image analysis Sigma Gel software (SPSS). For each protein, the optical density of each ischemic sample was compared with the average value of the four control samples.
Immunohistochemistry
Cryostat sections (5 μm) of frozen tissue were cut and stained for Bcl-2 or active Bax. Briefly, slides were dried, fixed in acetone for 10 min and air-dried. Slides were re-hydrated in PBS for 5 min and subsequently incubated in 10% normal goat serum in PBS to block aspecific ab binding. Slides were stained for 2 h at RT with either anti-Bcl-2 or anti-Bax primary ab in PBS with 1% bovine serum albumin. After three washes in PBS for 5 min each, slides were incubated for 30 min with the Texas Red-labeled secondary ab diluted in the same buffer with the addition of FITC-labeled Lotus Lectin for identification of proximal tubular epithelium (15). After three washes in PBS, the slides were mounted using glycerol-PBS with 1,4-diazabicyclo(2,2,2)octane and 4,6-diamidino(2)phenylindole (DAPI) and viewed with an immunofluorescence microscope. No significant staining was detected in slides incubated with control ab instead of the primary ab indicating the absence of significant background staining.
Statistical analysis
We used the Mann–Whitney test to compare Bax protein expression between the control and the ‘early’ ischemia group. The Spearman analysis was used to determine correlation between the duration of normothermic ischemia and initiation of apoptosis. A p-value of less than 0.05 was considered significant.
Results
Initiation of the intrinsic pathway during normothermic renal ischemia
To determine whether apoptogenic processes are initiated during ischemia, we evaluated the effect of normothermic ischemia on proteins involved in activation of both the intrinsic and extrinsic pathway. Since activation of the intrinsic pathway is regulated by members of the Bcl-2 protein family, we first determined the pro-apoptotic Bax protein content during ischemia by Western blotting. As described by others, we observed that monomeric Bax (21 kDa) is constitutively expressed in human renal tissue (16) (Figure 1). A short period of renal ischemia (15 until 35 min) led to a significant increase of Bax protein (p < 0.02, Mann–Whitney test) (Figure 1). Bax levels remained clearly elevated up to 60 min. Further prolongation of ischemia reduced Bax expression back to basal levels. Next the localization of active Bax was studied using immunohistochemistry. For this purpose, an ab directed to the N-terminal region of active Bax that is inaccessible in the inactive conformation was applied. The data show that active Bax protein is nearly absent in control tissue (Figure 2). In concordance with the enhanced Bax protein content, as detected by Western blotting, cytoplasmic active Bax staining increased in the early ischemic phase. After 30 min of ischemia, activated Bax was predominantly localized in distal tubular epithelial cells. At this timepoint, also some focal staining was observed in proximal tubules. Proximal tubules were identified using Lotus Lectin staining. Similarly, after 80 min of ischemia, the majority of active Bax was detected in distal tubules (Figure 2).
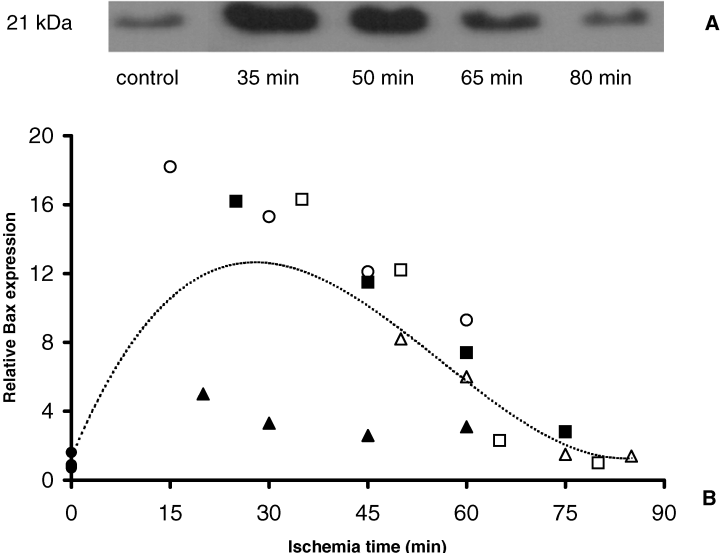
Bax protein shows a rapid increase followed by decrease during normothermic ischemia. (A) Representative immunoblot analysis of Bax. (B) Densitometric analysis of Bax immunoblotting of ischemic (n = 5) and control kidneys (n = 4). Data of ischemic kidneys are expressed as the fraction of mean of control data. Individual ischemic kidneys are displayed by different symbols (,,,,) and control kidneys by a filled circle. Constitutive Bax expression (21 kDa) was detected in control tissue. After a short period of ischemia renal Bax expression levels were significantly increased (p < 0.02, Mann–Whitney test). Bax protein levels remained elevated up to 60 min. Dotted line represents trendline of all data.
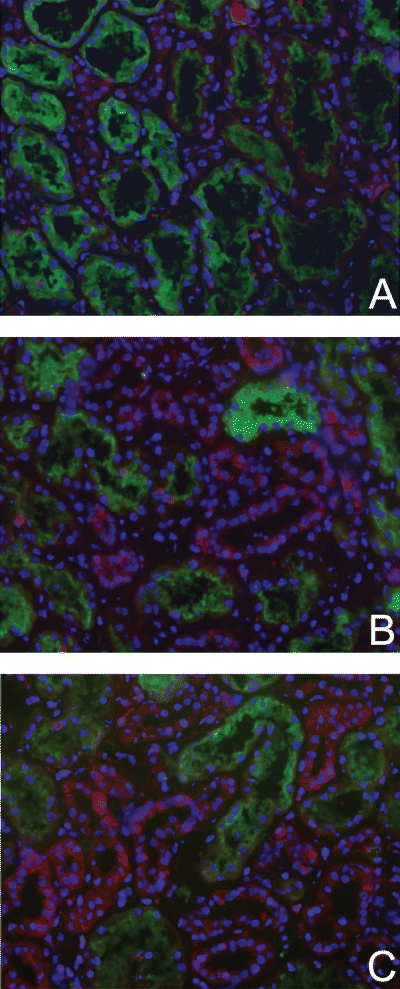
Activated Bax protein is predominantly expressed by distal tubular epithelial cells. While hardly detectable in control kidneys (A), Bax protein was increasingly expressed (red) in distal tubules in kidneys exposed to 30 min (B) or 80 min (C) of ischemia. In the ischemic sections also some focal staining of proximal tubules was observed (B,C). Proximal tubules were identified by FITC Lotus Lectin staining (green). Red staining, active Bax protein (Texas Red); blue staining, nuclei (4,6-diamidino(2)phenylindole). Magnification: x200.
Subsequently, we investigated the effect of ischemia on the regulation of the anti-apoptotic protein Bcl-2. Abundant monomeric Bcl-2 expression (26 kDa) was observed in control tissue (Figure 3). Bcl-2 Western blot analysis revealed that during ischemia Bcl-2 levels were reduced. Moreover, this reduction of Bcl-2 significantly correlated with the duration of the ischemic insult (r = 0.86, p < 0.001, Spearman correlation analysis) (Figure 3). Immunohistology for Bcl-2 resulted in similar data; a decrease of Bcl-2 with time. Bcl-2 was primarily detected at the basolateral side of tubular epithelial cells. The expression was predominantly observed in proximal tubules while a lower expression was detected in distal tubules (Figure 4).
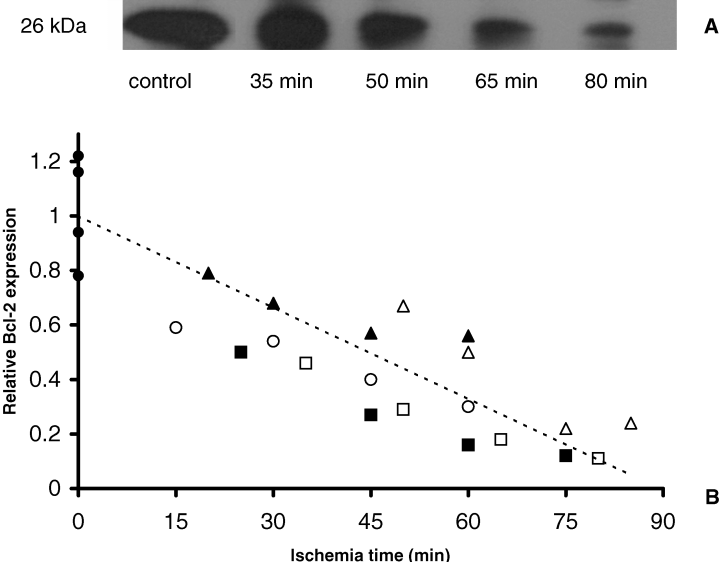
Depletion of renal Bcl-2 during normothermic ischemia. (A) Representative immunoblot analysis of Bcl-2. (B) Densitometric analysis of Bcl-2 immunoblotting with ischemic (n = 5) and control kidneys (n = 4). Data of ischemic kidneys are expressed as fraction of the mean of control data. Individual ischemic kidneys are displayed by different symbols (,,,,) and control kidneys by a filled circle. A significant correlation was detected between renal 26 kDa Bcl-2 levels and the length of normothermic ischemia (r = 0.86, p < 0.001, Spearman correlation analysis). Dotted line gives trendline of all data.
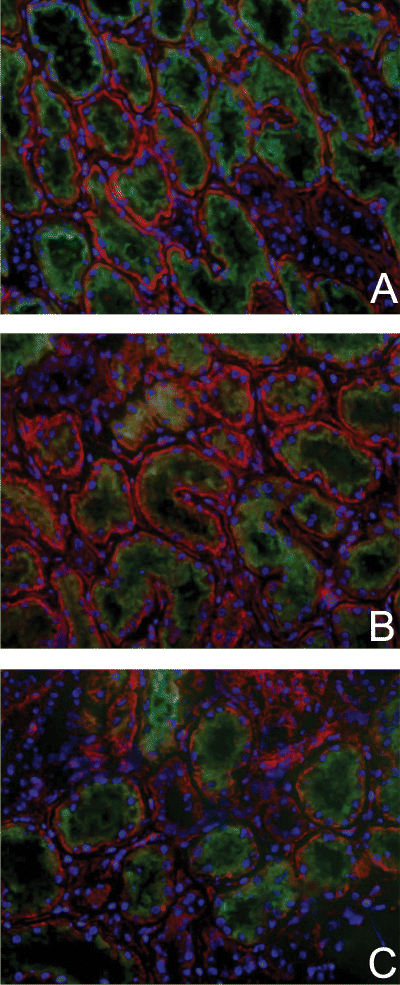
Immunofluorescence staining of Bcl-2 during renal normothermic ischemia. Renal Bcl-2 staining was most abundant (red) in proximal tubules of control tissue (A) and in kidneys rendered ischemic for 35 min (B) and 80 min respectively (C). Less staining was detected in distal tubules in both control and ischemic tissue. Proximal tubules were identified by FITC Lotus Lectin staining (green). Red staining, Bcl-2 protein (Texas Red); green staining, Lotus Lectin (fluorescein isothiocyanate); blue staining, nuclei (4,6-diamidino(2)phenylindole). Magnification: x200.
Collectively, enhanced Bax protein levels combined with decreased Bcl-2 levels suggests that normothermic ischemia enhances susceptibilityfor the activation of the intrinsic pathway. In this context we determined the activation of initiator caspase-9. We observed a significant correlation between activation of caspase-9 (34/37 kDa) and the normothermic ischemia time (r = 0.79, p < 0.001, Spearman correlation analysis) (Figure 5). Taken together, these data show that already during normothermic ischemia, activation of the intrinsic pathway of the apoptotic cascade occurs.
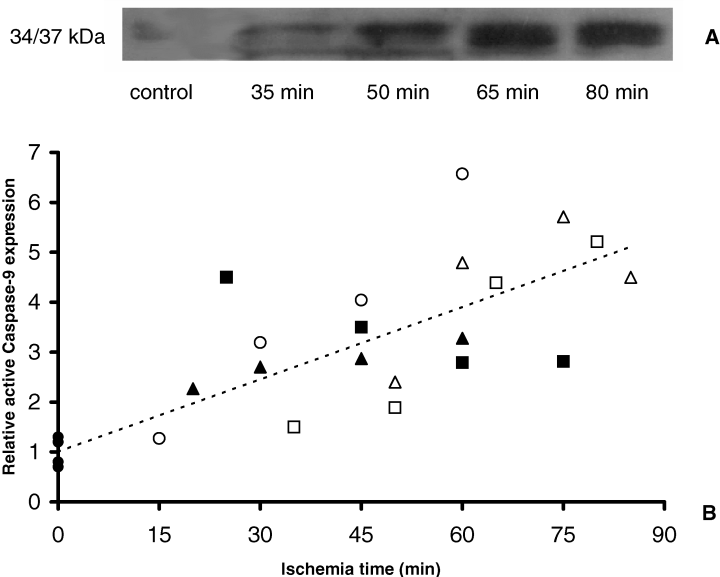
Ischemia induces intrarenal caspase-9 activation. (A) Representative immunoblot analysis of activated caspase-9. (B) Densitometric analysis of caspase-9 immunoblotting with ischemic (n = 5) and control kidneys (n = 4). Data of ischemic kidneys are expressed as fraction of the mean of control data. Individual ischemic kidneys are displayed by different symbols (,,,,) and control kidneys by a filled circle. Activation of caspase-9 (34/37 kDa) is significantly correlated with the ischemia time (r = 0.79, p < 0.001, Spearman correlation analysis). Dotted line gives trendline of all data.
Initiation of the extrinsic pathway during normothermic renal ischemia
Next, we examined whether, besides initiation of the intrinsic pathway, also the extrinsic pathway was activated during ischemia. Activation of this route is regulated by the cytoplasmic protein cFLIP, a competitive inhibitor of caspase-8 (17). We therefore measured cFLIP levels during ischemia. Here we show that cFLIP (55 kDa) was normally expressed in the human kidney and got depleted during normothermic ischemia (Figure 6). Interestingly, the reduction of cFLIP levels significantly correlated with the duration of ischemia (r = 0.78, p < 0.001, Spearman correlation analysis). Subsequently, we determined whether cFLIP depletion results in activation of caspase-8, the initiator caspase of the intrinsic pathway. Similar levels of the pro-form of caspase-8 (43/44 kDa) were found in control and ischemic tissue (Figure 7A). In line, activated caspase-8 fragments were not detectable during normothermic ischemia (data not shown). Thus, although cFLIP was depleted during ischemia, no active caspase-8 was detected.
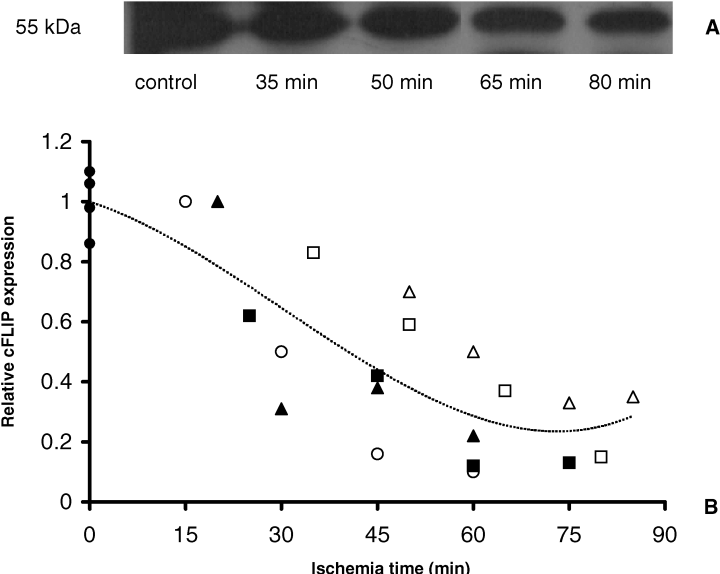
Depletion of cFLIP during normothermic renal ischemia. (A) Representative immunoblot analysis of intrarenal cFLIP. (B) Densitometric analysis of cFLIP immunoblotting with ischemic (n = 5) and control kidneys (n = 4). Data of ischemic kidneys are expressed as fraction of the mean of control data. Individual ischemic kidneys are displayed by different symbols (,,,,) and control kidneys by a filled circle. cFLIP (55 kDa) expression levels reduces in time with induction of the length of ischemia and this correlation is statistically significant (r = 0.78, p < 0.001, Spearman correlation analysis). Dotted line gives trendline of all data.
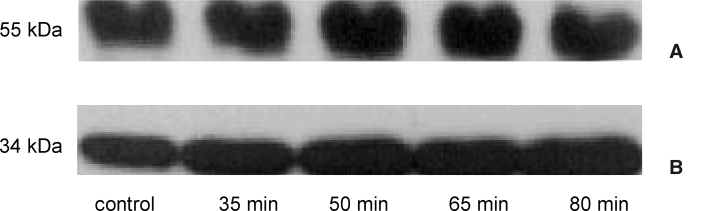
Processing of caspase-8 and caspase-3 during renal ischemia. (A) The 54/55 kDa inactive isoforms of caspase-8 are detected in ischemic and control tissue. The 43/44 kDa fragment compromising the intermediate caspase-8 cleavage fragments are not detected (data not shown). A representative blot is shown. (B) 34 kDa pro-form caspase-3 was present in healthy and ischemic tissue. Activation of caspase-3 could not be detected in any samples (data not shown). A representative blot is shown.
Activation of effector caspases during normothermic renal ischemia
To evaluate whether the observed activated intrinsic pathway leads to execution of the apoptotic process, processing of the central effector caspase, caspase-3 was determined. The caspase-3 proform (34 kDa) was detected in control tissue. The basal expression levels did not change during ischemia (Figure 7B). In line, fragments representing active caspase-3 were not observed. Taken together, our data implicate that during ischemia the circumstances are created for activation of the apoptotic cascade upon reperfusion.
Discussion
In the current study, we investigated the initiation of apoptotic cell death during normothermic renal ischemia. In renal transplantation, normothermic warm ischemia occurs in NHB donation, especially in category 2 donors. This warm ischemia is considered to be the major cause of delayed graft function and or primary non-function. Also cold ischemia as occurs during preservation of donor organs can lead to organ damage. In cadaveric donors, cold ischemia time was associated with the numbers of apoptotic cells, present 1 h after onset of reperfusion/transplantation (5,14). In vitro experiments showed that cold storage of proximal tubular cells for 48 h resulted in activation of apoptosis, only after re-warming. The apoptotic process was not activated during cold storage (18). Here we investigate initiation of the apoptotic process during normothermic ischemia in an ex vivo model. Initiation of the apoptotic cascade occurs either via activation of the extrinsic or the intrinsic pathway. Because regulation of the intrinsic pathway is accomplished in part through the stoichiometric balance of pro-apoptotic and anti-apoptotic Bcl-2 proteins (12,19), we studied alterations in levels of anti-apoptotic (Bcl-2) and pro-apoptotic (Bax) members of this protein family. Using a human model for renal normothermic ischemia we demonstrated that depletion of monomeric Bcl-2, that is able to prevent dimerization of the pro-apoptotic Bax, significantly correlated with the extent of ischemia. Our findings further extend recent data from Schwarz et al. (20). They showed that low mRNA levels for Bcl-2 in proximal tubular epithelial cells in pre-transplant cadaveric donor kidney biopsies are associated with apoptosis and subsequent ARF and delayed allograft function (20). These data support our findings that epithelial cells of the proximal tubules are the predominant site of renal Bcl-2 protein expression and that ischemia leads to reduced Bcl-2 levels. Next, we showed that protein levels of monomeric Bax are strongly enhanced by normothermic ischemia, predominantly in the early ischemic phase. These data are supported by findings from the group of Hébert, who showed in an experimental rat model elevation of renal Bax protein expression, 10 min after the induction of cardiac arrest (21). Moreover, these Western blotting data are completed by immunohistochemistry experiments with an ab that detects active Bax. Immunohistochemistry data revealed that renal ischemia also resulted in activated Bax protein expression. Activated Bax was primarily detected in distal tubules. This increased distal active Bax expression and the decreased proximal Bcl-2 expression suggests a pro-apoptotic state in both distal and proximal tubular epithelium. At this stage there is no explanation for the observed diffuse expression of apoptotic proteins. Many mechanisms could be responsible such as differences in ‘stress’ in the different parts of the tubule during ischemia. It is generally said that the proximal part is more sensitive to ischemia and reperfusion with more necrotic cell death while the distal tubules suffer more from apoptotic cell death (9). However, it is not completely elucidated as yet which part of the nephron suffers the most severe after an ischemic insult (22,23).
Next, for the first time we showed that a significant correlation exists between activation of caspase-9 and the duration of normothermic renal ischemia. The observed activation of caspase-9 in the present study did, however, not result in activation of the downstream caspase-3 during normothermic ischemia. Activation of this central effector caspase-3 is essential for cleavage of different substrates resulting in execution of the apoptotic process. Previous published findings in experimental models have been contradictory as to whether caspase-3 is induced during ischemia or rather during reperfusion (24–27). We do not have an explanation for the observed data that activated caspase-9 fails to activate caspase-3 at this stage. However, it is known that next to caspases, also XIAP, an inhibitor of caspase-3, is recruited to the apoptosome (28). How the inhibitory effect of XIAP is overcome and cellular caspase-3 activation and cell death can take place is at this stage unknown. Possibly reperfusion is necessary as a major insult to overcome the protective effect of XIAP, resulting in cell death.
Besides activation of the intrinsic pathway, we also demonstrate that the extrinsic pathway is already initiated during ischemia. In particular, we show that depletion of the competitive caspase-8 inhibitor, cFLIP, significantly correlated with the length of normothermic renal ischemia. These findings further extend data from Rasper et al. who showed significant cFLIP depletion after ischemia in the reperfused heart (17). Since cFLIP prevents death-receptor-mediated apoptosis by precluding recruitment of caspase-8 to the DISC (17,29), reductions of the cFLIP content facilitate caspase-8 activation. However, in our model cFLIP depletion was not accompanied by caspase-8 activation during ischemia, indicating that low cFLIP levels alone are insufficient to activate the intrinsic pathway. Presumably, activation of the extrinsic pathway needs conditions that are only met during reperfusion. Indeed, in a rat cardiac I/R model it has been demonstrated that caspase-8 activation occurs in the reperfusion phase, but is not processed by ischemia alone (30).
Taken together, we show in a human model for renal normothermic ischemia that the intrinsic as well as the extrinsic pathway of apoptosis are initiated during ischemia. These data indicate that during ischemia the circumstances are created that facilitate execution of apoptosis upon reperfusion. This hypothesis is supported by data obtained in our murine model of renal I/R. In this model, we confirmed the observation that initiation of the apoptotic process emerges during ischemia. Furthermore, the length of the ischemic period corresponds with the extent of apoptosis and the concomitant organ function during reperfusion (unpublished findings). Potentially, assessment of proteins involved in the early apoptotic process in pre-transplant kidney biopsies might be helpful to estimate ischemic damage and predict concomitant graft function. Evidence that apoptosis is crucially involved in clinical transplantation has been provided by different groups, showing that apoptotic cell death is enhanced in post-reperfusion biopsies from cadaveric allografts compared with living-related renal transplants (5,14). However, these studies report apoptotic cell death in brain-death donors. In these donors, besides ischemia, other factors such as periods of diminished organ perfusion, hemodynamic instability and activation of the immune-system affect post-transplant organ function (31). In contrast, our model is restricted to the effect of ischemia itself, thereby reflecting the situation in NHB organ donation, in which warm ischemia forms the major determinant of graft outcome. Therefore, our novel findings that ischemia alone, already initiates the apoptotic process, might explain the increased risk for NHB graft dysfunction.
In conclusion, in a human model for renal normothermic ischemia we demonstrate that the apoptotic cascade is initiated during ischemia. For the first time, the time-dependent regulation of proteins responsible for the onset of the apoptotic cascade in the course of normothermic ischemia is shown. Determination of these processes might be beneficial in assessing the degree of ischemic injury in NHB donor kidneys and other organs. Furthermore, these new insights can be explored for the development of specific anti-apoptotic treatment to prevent organ damage upon transplantation due to ischemic injury.
Acknowledgment
This study was supported by the Dutch Kidney Foundation, Grant C99.1840.




