Mycophenolic Acid Inhibits Platelet-Derived Growth Factor-Induced Reactive Oxygen Species and Mitogen-Activated Protein Kinase Activation in Rat Vascular Smooth Muscle Cells
Abstract
Vascular smooth muscle cell (VSMC) proliferation is the major pathologic feature associated with chronic allograft nephropathy, and mycophenolic acid (MPA) inhibits VSMC proliferation. Since the role of inosine monophosphate dehydrogenase (IMPDH)-dependent de novo guanosine synthesis is limited in VSMCs, we examined the effects of MPA on platelet-derived growth factor (PDGF)-induced cellular ROS and mitogen-actived protein kinases (MAPK) activation in VSMCs. Primary cultured rat VSMCs were stimulated with PDGF-BB in the presence or absence of MPA. Cell proliferation was assessed by [3H]-thymidine incorporation, ROS by flow cytometry and MAPK activation by Western blot analysis. PDGF increased cell proliferation, cellular ROS and extracellular-regulated protein kinase (ERK) 1/2 and p38 MAPK activation by 3.4-, 1.6-, 3.3- and 3.9-fold, respectively. MPA at above 1 μM inhibited PDGF-induced cellular ROS and ERK 1/2 and p38 MAPK activation, as well as proliferation. Structurally different anti-oxidants and inhibitor of ERK or p38 MAPK blocked PDGF-induced proliferation. Anti-oxidants also inhibited ERK 1/2 and p38 MAPK activation. Exogenous guanosine partially recovered the inhibitory effect of MPA on VSMC proliferation. These results suggest that MPA may inhibit PDGF-induced VSMC proliferation partially through inhibiting cellular ROS, and subsequent ERK 1/2 and p38 MAPK activation in addition to inhibiting IMPDH.
Abbreviations:
-
- ERK
-
- Extracellular-regulated protein kinase;
-
- FBS
-
- Fetal bovine serum;
-
- IMPDH
-
- Insoine monophosphate dehydrogenase;
-
- MAPK
-
- Mitogen-activated protein kinases;
-
- MEM
-
- Minimum essential medium;
-
- MPA
-
- Mycophenolic acid;
-
- PBS
-
- Phosphate buffered saline;
-
- PDGF
-
- Platelet-derived growth factor;
-
- p38 MAPK
-
- p38 mitogen-activated protein kinase;
-
- p38 I
-
- p38 MAPK inhibitor [2-(4-Chlorophenyl)-4-(4-fluorophenyl)-5-pyridin-4-yl-1,2-dihydropyrazol-3-one];
-
- ROS
-
- Reactive oxygen species;
-
- VSMC
-
- Vascular smooth muscle cell.
Introduction
Despite improvements in the short-term success of kidney transplantation during recent years, the rate of long-term graft attrition remains high. Abnormal vascular remodeling has been proposed to be the major pathogenesis of chronic allograft dysfunction. The final events of vascular diseases such as chronic allograft vasculopathy (1–4), atherosclerosis (5) and restenosis (6) include the serial events of vascular smooth muscle cell (VSMC) proliferation, migration and accumulation of extracellular matrix. Immunologic or non-immunologic injury of the endothelium and the subsequent vascular response may initiate the release of cytokines and growth factors and lead to the aforementioned serial events of VSMCs.
Mycophenolic acid (MPA), a selective and uncompetitive inosine monophosphate dehydrogenase (IMPDH) inhibitor, inhibits not only lymphocyte proliferation, but also the proliferation of VSMCs (7), mesangial cells (8) and myofibroblasts (9), suggesting that MPA may have the potential to prevent and perhaps treat chronic allograft vasculopathy. The inhibition of de novo guanosine synthesis has been shown to be the major mechanism, whereby MPA suppresses lymphocyte (10,11) and mesangial cell (8) proliferation. However, the manner in which MPA inhibits VSMC proliferation has not yet been clearly understood. Cells and tissues can be arranged in terms of their dependency on the de novo or salvage pathways involved in guanosine synthesis, with lymphocytes at one extreme, brain cells at the other, and other cells occupying intermediate positions (12). It is therefore possible that other mechanisms than IMPDH inhibition may play a significant role in the inhibition of VSMC proliferation by MPA.
Recently, reactive oxygen species (ROS), especially the superoxide anion and hydrogen peroxide, have been proposed to be important signaling molecules in many biological events, including cell proliferation (13–17). ROS serve as second messengers that activate multiple cellular signaling proteins and redox-sensitive transcription factors, which in turn lead to alterations of many genes that have important functional roles in the biologic behavior of VSMCs. In fact, hydrogen peroxide has been suggested to mediate platelet-derived growth factor (PDGF)-induced VSMC proliferation (18).
The mitogen-activated protein kinase (MAPK) family, which includes extracellular-regulated protein kinase (ERK), c-jun N-terminal protein kinase (JNK) and p38 MAPK, is a ubiquitous cellular serine-threonine kinase, which plays a fundamental role in a variety of cellular responses to diverse extracellular stimuli in many different cells (19). In fact, ERK 1/2 mediates PDGF-induced VSMC proliferation and migration (20). The p38 MAPK was recently found to participate in VSMC proliferation in response to thrombin and balloon injury (21,22).
We, therefore, hypothesized that MPA may inhibit PDGF-induced VSMC proliferation by inhibiting cellular ROS and subsequent ERK 1/2 and p38 MAPK activation, in addition to inhibiting de novo guanosine synthesis.
Materials and Methods
Chemicals and instruments
All chemicals and tissue culture plates were obtained from the Sigma Chemical Company (St. Louis, MD, USA) or from Becton Dickinson Labware (Lincoln Park, NJ, USA), respectively, unless otherwise stated.
Cell cultures
Rat VSMCs were isolated and characterized as previously described (23). Briefly, primary cultured rat VSMCs were obtained by the collagenase dissociation of the aortas of Sprague-Dawley rats weighing 200–250 g. Cells were grown in MEM supplemented with 10% FBS (GIBCO, Grand Island, NY, USA), 30 mM NaHCO3, 50 mM HEPES, 5 μg/mL insulin, 5 μg/mL transferrin, 100 U/mL penicillin (GIBCO) and 100 μg/mL streptomycin (GIBCO) at 37°C in a 5% CO2 humidified incubator. At confluence, the cells exhibited a characteristic ‘hill and valley’ morphology. Immunocytochemical study confirmed that the cells stained positively for anti-α1-actin antibody (DAKO Japan Co., Kyoto, Japan). Passages 5–9 were used in the study.
Drug treatments
Near confluent rat VSMCs were incubated with serum-free MEM for 48 h to arrest and synchronize cell growth. Media were then replaced with fresh serum-free MEM containing 10 ng/mL of PDGF-BB, and cells were incubated for up to 48 h. PDGF-BB is the most potent mitogen among PDGF-AA, -AB and -BB (24). The concentration of PDGF-BB used was determined by preliminary study (data not shown). MPA was administered 1 h before the addition of PDGF. Anti-oxidants [5 mM N-acetylcystein (NAC) or 500 μM trolox], 50 μM PD98059 (a known MEK, direct ERK 1/2 upstream kinase, inhibitor; Calbiochem, San Diego, CA, USA) and 10 μM p38 MAPK inhibitor [2-(-4-chlorophenyl)-4-(4-fluorophenyl)-5-pyridin-4-yl-1,2-dihydropyrazol-3-one; Calbiochem], were administered 1 h before PDGF to determine the roles of ROS, ERK 1/2 and p38 MAPK on PDGF-induced proliferation, respectively. The p38 MAPK inhibitor used in the present study has been reported to be 8-fold more potent than SB203580 as an inhibitor of p38 MAPK (25). Guanosine 100 μM with or without MPA was administered 1 h before PDGF to investigate the role of de novo synthesis of guanosine on PDGF-induced VSMC proliferation.
[3H]-Thymidine incorporation
DNA synthesis was assessed by [3H]-thymidine incorporation. VSMCs were allowed to proliferate in response to 10 ng/mL of PDGF-BB. One μCi/mL of [3H]-thymidine (Du Pont Co., Wilmington, DE, USA) was added to each well for the last 12 h of experimental periods. The cells were then washed twice and trypsinized before harvesting with a cell harvester (Titertek Cell Harvester 550, Flow Laboratories, Irvine, Scotland, UK) onto glass-fiber filters (Flow Laboratories, Irvine, Scotland, UK). They were then placed in a 3 mL scintillation cocktail solution, and their radioactivities were measured using a β-counter (TL 5000s, Beckman Instruments Inc., Fullerton, CA, USA).
Flow cytometry
5-(and 6-) chloromethyl-2′,7′-dichlorodihydro-fluorescein diacetate (DCFH-DA; Molecular Probes, Eugene, OR, USA)-sensitive cellular ROS was measured by flow cytometry (Becton Dickinson Labware). The cells were washed twice with cold PBS and incubated with 5 μM DCFH-DA for 20 min. After washing unincorporated dye, fluorescence was determined by flow cytometry (excitation; 485 nm, emission; 530 nm).
Western blot analysis
ERK1/2 and p38 MAPK activation was measured by Western blot analysis. Briefly, cells were washed with cold PBS and lysed in a lysis buffer containing 1.0% Triton X-100, 10% glycerol, 20 mM Tris-HCl (pH 7.0), 137 mM sodium chloride, 5 mM EDTA, 20 μM leupeptin, 1 μg/mL aprotinin, 1 mM PMSF, 1 mM sodium orthovanadate, 10 mM sodium fluoride, 1 mM EGTA (pH 8.0), 1 mM pyrophosphate and 1 mM β-glycerophosphate. Insoluble materials were removed by centrifugation at 12000 rpm for 15 min at 4°C and the concentration of cellular protein was measured using the Bio-rad protein assay kit (Bio-rad, Hercules, CA, USA). Aliquots containing 20 μg of protein, for ERK 1/2 determination, and 50 μg of protein for p38 MAPK determination, were mixed with loading buffer (60 mM Tris-HCl, 25% glycerol, 2% SDS, 14.4 mM 2-ME, 0.1% bromophenol blue), separated in a 10% SDS-PAGE and transferred to a nitrocellulose membrane (Bio-rad). After being blocked for 1 h with 5% non-fat dry milk, the membranes were incubated with anti-ERK 1/2 or anti-p38 MAPK phosphorylated antibody (Cell Signaling, Beverly, MA, USA). Blots were washed and then incubated with horseradish-peroxidase (HRP)-conjugated anti-rabbit antibody for 1 h at room temperature. Sites of antibody binding were visualized by using an enhanced chemiluminescence (ECL; Amersham, Buckinghamshire, UK) Western blotting detection system, and quantified using a densitometer. All ERK and p38 MAPK measurements were normalized versus total ERK 1/2 or total p38 MAPK, which were reprobed using the respective specific antibodies (cell signaling).
Statistical analysis
All results were calculated by relative ratio against control (without any agent), and were expressed as means ± SE. The mean values obtained from each group were compared by ANOVA and subsequently using Fisher's least significance difference method. p-Values of <0.05 were accepted for statistical significance.
Results
MPA inhibited PDGF-induced rat VSMC proliferation
[3H]-thymidine incorporation was measured after 48 h in the presence or absence of PDGF at 10 ng/mL. PDGF increased [3H]-thymidine incorporation by 3.4-fold (from 397.0 ± 51.7 cpm basal value to 1367.3 ± 327.2 cpm) that of the control (Figure 1, p < 0.05, n = 5). MPA at concentrations higher than 100 nM significantly inhibited PDGF-induced [3H]-thymidine up-regulation (Figure 1, p < 0.05, n = 5). The IC50 of MPA was between 100 nM and 1 μM.

MPA inhibited PDGF-induced rat VSMC proliferation at 48 h and exogenous guanosine partially overcame the inhibition of MPA. Cell proliferation was measured by [3H]-thymidine incorporation after exposing quiescent VSMCs to serum-free media containing different concentrations of MPA, in the presence or absence of 100 μM guanosine, which were added before the addition of 10 ng/mL of PDGF-BB. The experimental protocol is detailed in the ‘Materials and Methods’ section. Data are presented as means ± SE of five experiments. *p < 0.05 versus control. †p < 0.05 versus PDGF without MPA.
Exogenous guanosine 100 μM did not affect basal [3H]-thymidine incorporation but partially overcame the inhibition of MPA on PDGF-induced [3H]-thymidine incorporation (Figure 1, p < 0.05, n = 5). [3H]-thymidine incorporation of VSMCs in the presence of PDGF, MPA 10 μM and guanosine 100 μM was 2.4-fold (957.4 ± 115.6 cpm) that of the control. This was significantly higher than PDGF and MPA 10 μM, but still lower than PDGF alone.
MPA inhibited PDGF-induced cellular ROS in rat VSMCs
PDGF at a concentration that induced cell proliferation, rapidly (at 5 min) increased cellular ROS by 1.6-fold that of the control (Figure 2A, p < 0.05, n = 5). MPA at above 10 nM inhibited PDGF-induced cellular ROS in a dose-dependent manner (Figure 2B, p < 0.05, n = 5). Cellular ROS in VSMCs cultured under PDGF and 10 μM of MPA was 1.1-fold that of the control.
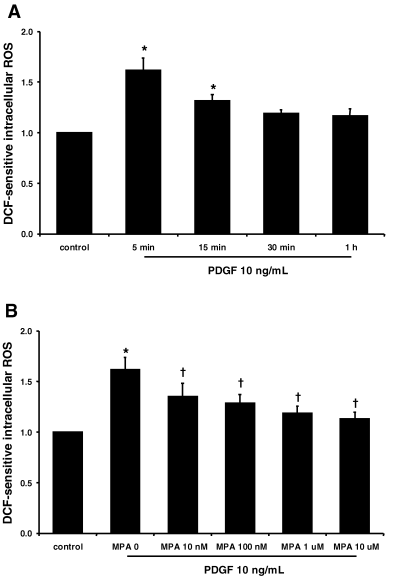
MPA inhibited PDGF-induced cellular ROS generation in rat VSMCs. (A) Time course of PDGF-induced cellular ROS generation. (B) Effects of MPA on PDGF-induced cellular ROS generation at 5 min. The experimental protocol is detailed in the ‘Materials and Methods’ section. Data are presented as means ± SE of five experiments. *p < 0.05 versus control. †p < 0.05 versus MPA 0.
MPA inhibited PDGF-induced ERK 1/2 and p38 MAPK activation in rat VSMCs
PDGF at a concentration that induced cell proliferation also increased both ERK 1/2 and p38 MAPK activation (Figure 3). PDGF-induced ERK 1/2 activation peaked at 15 min by 3.3-fold that of the control (p < 0.05, n = 4). PDGF-induced p38 MPAK activation at 5 min was 3.9-fold higher than control (p < 0.05, n = 4).
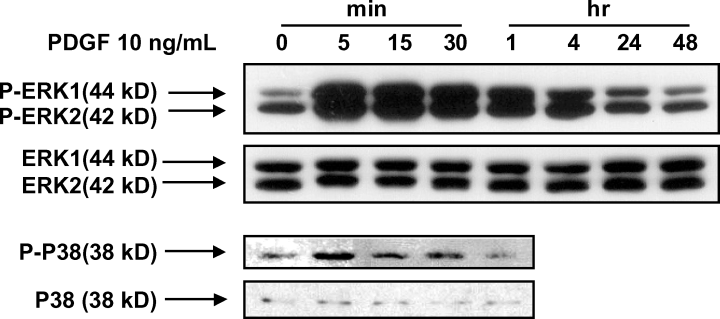
PDGF increased ERK 1/2 and p38 MAPK activation in rat VSMCs. The experimental protocol is detailed in the ‘Materials and Methods’ section. Data are presented as representative Western blot pictures of five experiments.
MPA at above 1 μM significantly reduced PDGF-induced ERK 1/2 (Figure 4A, B, p < 0.05, n = 4) and p38 MAPK activation (Figure 4A, C, p < 0.05, n = 4) in a dose-dependent manner. ERK 1/2 and p38 MAPK activation in the presence of PDGF and MPA 10 μM were 2.4-fold and 2.4-fold that of the control, respectively. However, ERK 1/2 activation remained statistically higher than control.
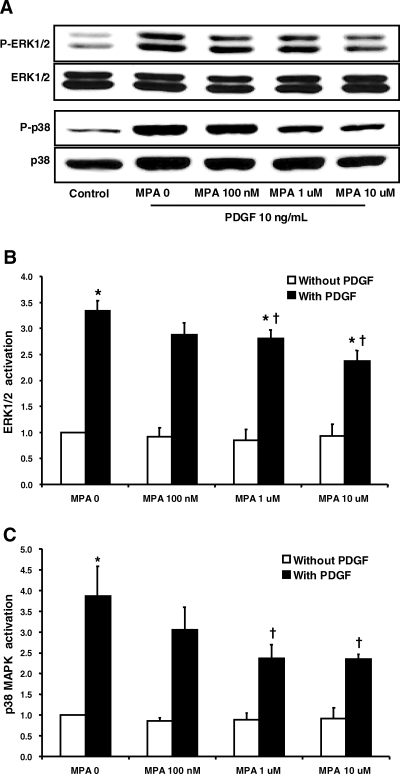
MPA inhibited PDGF-induced ERK 1/2 and p38 MAPK activation in rat VSMCs. (A) Representative Western blot analysis of ERK 1/2 and p38 MAPK activation. Each protein band was quantified by densitometry. (B) Effects of MPA on PDGF-induced ERK1/2 activation. (C) Effects of MPA on PDGF-induced p38 MAPK activation. The experimental protocol is detailed in the ‘Materials and Methods’ section. Data are presented as means ± SE of four experiments, *p < 0.05 versus MPA 0 without PDGF. †p < 0.05 versus PDGF without MPA.
Cellular ROS generation and the activation of ERK 1/2 and p38 MAPK are required for the PDGF-induced proliferation of rat VSMCs
Structurally different anti-oxidants (NAC 5 mM or trolox 500 μM), the ERK inhibitor (PD98059 50 μM) and the p38 MAPK inhibitor (p38 I 10 μM) all effectively inhibited PDGF-induced [3H]-thymidine up-regulation (Figure 5, p < 0.05, n = 5). The anti-oxidants also inhibited PDGF-induced ERK 1/2 (Figure 6A, B, p < 0.05, n = 5) and p38 MAPK activation (Figure 6A, C, p < 0.05, n = 5), suggesting that ERK 1/2 and p38 MAPK activation are downstream molecules of cellular ROS in VSMCs cultured under PDGF.

ROS, ERK and p38 MAPK are required for the PDGF-induced proliferation of rat VSMCs. Proliferation was measured by determining [3H]-thymidine incorporation after exposing quiescent VSMCs to serum free media containing NAC, trolox, PD98059 and p38 MAPK inhibitor (p38 inhibitor), which were added 1 h before the addition of PDGF 10 ng/mL. The experimental protocol is detailed in the ‘Materials and Methods’ section. Data shown are the means ± SE of five experiments. *p < 0.05 versus control without PDGF. †p < 0.05 versus PDGF control.
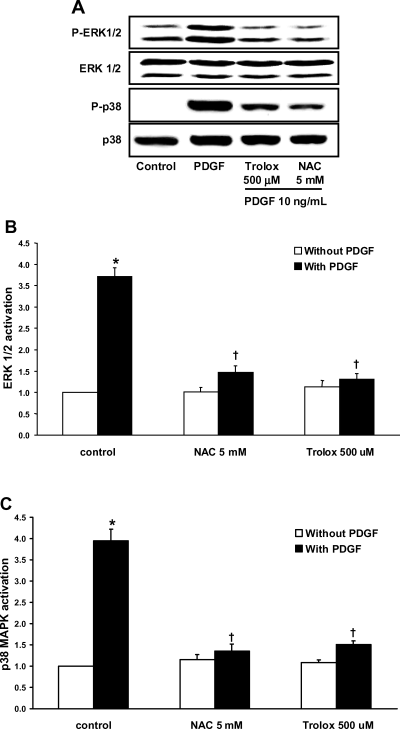
ROS mediates PDGF-induced ERK 1/2 and p38 MAPK activation. (A) Representative Western blot analysis of ERK 1/2 and p38 MAPK activation. Each protein band was quantified by densitometry. (B) Effects of NAC and trolox on PDGF-induced ERK 1/2 activation. (C) Effects of NAC and trolox on PDGF-induced p38 MAPK activation. The experimental protocol is detailed in the ‘Materials and Methods’ section. Data are the means ± SE of five experiments. *p < 0.05 versus control without PDGF. †p < 0.05 versus PDGF control.
Discussion
In this study, we observed that; (1) PDGF increased proliferation, cellular ROS and the activation of ERK 1/2 and p38 MAPK in VSMCs; (2) MPA at concentrations inhibiting PDGF-induced VSMC proliferation blocked PDGF-induced cellular ROS, and partially but significantly inhibited the activation of ERK 1/2 and p38 MAPK; (3) Structurally different anti-oxidants, ERK 1/2 inhibitor and p38 MAPK inhibitor blocked PDGF-induced VSMC proliferation; (4) The anti-oxidants also blocked PDGF-induced ERK 1/2 and p38 MAPK in cultured VSMCs; and finally, (5) Exogenous guanosine partially overcame the anti-proliferative effect of MPA. These observations suggest that MPA may inhibit PDGF-induced VSMC proliferation partially through inhibiting cellular ROS and subsequent ERK 1/2 and p38 MAPK activation, in addition to inhibiting de novo guanosine synthesis.
The present observation that treatment with PDGF at 10 ng/mL for 48 h increased rat VSMC proliferation confirmed the findings of our previous study (7). MPA effectively inhibited PDGF-induced VSMC proliferation in a dose-dependent manner (0.1–10 μM) at 48 h. In the present study, the IC50 of MPA for the PDGF-induced DNA synthesis in VSMCs was 100 nM to1 μM, which agrees with the findings of Mohacsi et al (26). Clinically attainable concentrations of MPA (1–10 μM) were reported to inhibit the human arterial smooth muscle cell and fibroblast proliferation in culture (12).
MPA inhibited PDGF-induced cellular ROS in a dose-dependent manner. The quantification of PDGF-induced ROS by flow cytometry in the present study agrees with a previous report by Sundarasen et al. (18), in which ROS was quantified by confocal microscopy. While the mechanisms involved in the inhibition of cellular ROS by MPA are not clear at present, PDGF increases cellular ROS through NAD(P)H oxidase through Gi1,2 and Ras family proteins (27,28). PDGF activates heterotrimeric G-proteins or small G-proteins, such as Rac, Ras and Rho (29). Tiazofurin, another IMPDH inhibitor, down-regulated the expression of heterotrimeric G-protein or small G-protein (30–32). The effects of MPA on the PDGF-induced heterotrimeric G-proteins or small G-proteins need to be addressed in future studies. In the present study, MPA inhibited PDGF-induced cellular ROS at a 100-fold lower concentration than that required with inhibiting cell proliferation. This suggests that an anti-oxidative effect of MPA may be necessary but not sufficient to inhibit VSMC proliferation. To the best of our knowledge, the inhibitory effect of MPA on cellular ROS generation has not been previously reported.
PDGF significantly increased ERK 1/2 activation, and MPA inhibited PDGF-induced ERK 1/2 activation in a dose-dependent manner. MPA at above 1 μM significantly inhibited ERK 1/2 activation. The observation that PD98059 effectively inhibited VSMC proliferation, suggests that the inhibitory effect of MPA on PDGF-induced VSMC proliferation may be due to the inhibition of ERK 1/2 activation. However, the inhibition of ERK 1/2 activation by MPA remained significantly higher than control (Figure 4A, B) at the face of total blockade of proliferation. This result suggests that there may be threshold for the activation of ERK 1/2 involved in PDGF-induced VSMC proliferation.
PDGF was found to significantly increase p38 MAPK activation, and MPA blocked p38 MAPK activation at concentrations above 1 μM (Figure 4A, C). The finding that p38 MAPK inhibitor, a selective inhibitor of p38 MAPK, inhibited PDGF-induced proliferation, suggests that p38 MAPK may also play an important role in PDGF-induced VSMC proliferation. The role of p38 MAPK in the proliferation of VSMCs has recently been suggested in thrombin-induced VSMC proliferation (21) and balloon injury-induced neointimal hyperplasia (22).
Effects of anti-oxidants on PDGF-induced ERK 1/2 and p38 MAPK activation suggest that ERK 1/2 and p38 MAPK activation are downstream of ROS in VSMCs cultured in the presence of PDGF.
We further tested whether guanosine restores the inhibitory effect of MPA on rat VSMC proliferation, since that anti-proliferative effect of MPA is dependent on the inhibition of IMPDH in lymphocytes (10,11), and that MPA effectively decreased mesangial cell proliferation by guanosine depletion (8). Exogenous guanosine partially overcame the inhibition of MPA on PDGF-induced cell proliferation suggesting that the inhibition of IMPDH may play a role in anti-proliferative effect of MPA on VSMC proliferation but not be the sole mechanism.
In conclusion, our findings suggest that MPA may inhibit PDGF-induced rat VSMC proliferation partly through inhibiting cellular ROS and subsequent ERK 1/2 and p38 MAPK activation in addition to inhibiting de novo guanosine synthesis. The relative importance of these effects of MPA on PDGF-induced signaling molecules and guanosine depletion on the inhibition of VSMC proliferation remains an issue for further study.
Acknowledgments
This work was supported by the Korea Research Foundation Grant (KRF-2002-042-E00045).




