Distinct Transcriptional Changes in Donor Kidneys upon Brain Death Induction in Rats: Insights in the Processes of Brain Death
Abstract
Brain death affects hormone regulation, inflammatory reactivity and hemodynamic stability. In transplant models, donor organs retrieved from brain dead (BD) rats suffer from increased rates of primary non-function and lower graft survival. To unravel the mechanisms behind brain death we have performed DNA microarray studies with kidney-derived RNA from normo- and hypotensive BD rats, corresponding with optimal and marginal BD donors, respectively. In kidneys from normotensive donors 63 genes were identified as either up- (55) or down-regulated (8), while 90 genes were differentially expressed (67 up-regulated) in hypotensive BD donor kidneys. Most genes were categorized in different functional groups: metabolism/transport (including the down-regulated water channel Aqp-2), inflammation/coagulation (containing the largest number (16) of up-regulated genes including selectins, Il-6, α- and β-fibrinogen), cell division/fibrosis (including KIM-1 involved in tubular regeneration) and defense/repair (with the cytoprotective genes HO-1, Hsp70, MnSOD2). Also, genes encoding transcription factors (including immediate early genes as Atf-3, Egr-1) and proteins involved in signal transduction (Pik3r1) were identified. Summarizing, the use of DNA microarrays has clarified parts of the process of brain death: Brain-death-induced effects ultimately lead, via activation of transcription factors and signal transduction cascades, to differential expression of different “effector” genes. Not only deleterious processes such as inflammation and fibrosis occur in brain dead donor kidneys but genes involved in protection and early repair processes are activated as well. These findings can be used to introduce specific cytoprotective interventions in the brain dead donor to better maintain or even increase organ viability.
Introduction
Organs derived from brain dead donors are the main source for kidney transplantation. In addition, kidneys from living (un)related and, to a lesser extent, from non-heart-beating donors are used. Living donor–recipient combinations have been known to have superior results over cadaveric kidney transplantation in terms of function, rejection and prolonged transplant survival, even when an inferior matching grade, as in living unrelated transplantation, was present (1). In the United States, living unrelated transplantation was reported to have a 5-year graft survival of 72% with a half-life of the graft of 13 years, while in cadaveric transplantation 5-year graft survival is 62% and the transplant half-life is 9 years only (2). In the past years it has become clear that this difference in success can, primarily, be attributed to the pathophysiological changes that take place during the process of brain death and is not due to differences in cold ischemia times (3–5).
Studies in animal brain death models as well as in humans have clearly shown that brain death influences the hemodynamic stability and hormone regulation in the donor. Also, increased inflammatory reactivity in donor organs has been shown; we and others have found up-regulation of cell adhesion molecules such as ICAM and selectins associated with a progressively increased influx of T-cells, macrophages and polymorphonuclear cells (PMNs) in liver, kidney, heart and lung (6–9). These profound changes in potential donor organs will ultimately lead to histological damage, decreased function and lower graft survival (10,11). Although we can speculate about the role of, e.g. organ hypoperfusion or specific neural factors from the dying brain, the exact cause(s) and/or mechanisms leading to decreased organ viability have not been unraveled yet.
In this study, we have analyzed the issue of brain-death-related decreased organ viability and attempted to identify biological changes that take place in the donor organ-to-be, by gene expression profiling using DNA microarrays. Gene expression profiling allows the identification of individual differentially expressed genes and also enhances our overall insight into activated or down-regulated pathways and cascades. A better knowledge of the key components of brain death will provide us with clues on the triggering factors during progressive brain injury, ultimately leading to the observed decrease in organ viability. By using oligonucleotide microarrays representing 4854 unique genes, we investigated brain-death-induced gene expression changes in rat kidneys. An animal brain death model, simulating acute intracranial trauma as seen in many human donors, was used (9). Normo- and hypotensive 6-hour brain dead rats, corresponding with optimal and marginal brain dead donors, respectively, were included.
Materials and Methods
Animals
Adult male Wistar rats (HSD.Cpb:WU, 300–350 g) were used. Animals received care in compliance with the guidelines of the local animal ethics committee according to the Experiments on Animals Act (1996) issued by the Netherlands Ministry of Public Health, Welfare and Sports.
Experimental design of brain death
Rats were randomly divided into three groups. All animals were anesthetized and intubated. A frontolateral trepanation was made and a balloon catheter inserted in the extradural space. Inflating the catheter during 1 minute led to increased intracranial pressure and induced rapid brain injury leading to immediate brain death, simulating a condition comparable to acute isolated cerebral trauma in human. Animals in the normotensive group (n = 9) received hemodynamic support to achieve normotension whereas the hypotensive brain dead group did not receive donor management (n = 7) and remained hypotensive after brain death induction. After brain death induction all animals were ventilated for 6 hours with O2/air. Previously, we were not able to detect differences between untreated and 1- or 6-hour ventilated control rats (unpublished and (9)). In the current study control animals (n = 9) were sham operated and ventilated for 6 hours. Just before termination of the experiment, serum was taken for biochemical analyses. Next, kidneys were harvested and flushed with UW preservation solution to remove blood cells that could interfere with the expression analyses, and snap frozen. Detailed description on anesthesia and ventilation, surgical procedures, induction of brain death, donor management and biochemical determinations have been presented before (9).
RNA isolation and array experimental set-up
Frozen kidney tissue was homogenized in liquid N2, using mortar and pestle. Total RNA was isolated using the SV Total RNA isolation kit (Promega, Leiden, The Netherlands) according to the manufacturer's protocol. The integrity of total RNA was analyzed by gel electrophoresis and RNA samples were verified for the absence of genomic DNA contamination by performing RT-PCR reactions. Each experimental condition (control, normotensive BD and hypotensive BD dead rats) was divided into two groups. In addition, to minimize eventual biases due to biological variation of the individual animals, each group contained equal amounts of pooled RNA from 3 to 5 rat kidneys. Furthermore, each sample was analyzed in two-fold either being labeled with Cy3 or Cy5.
Microarrays
Microarrays contained the complete Rat oligonucleotide library (Sigma-Genosys/Compugen) printed in triplicate on GAPSII slides (Corning). The rat oligonucleotide library consists of 4854 65-mer oligos representing genes from a diverse range of functionalities. Positive and negative controls were also represented on the arrays. Microarrays were obtained from the Department of Anthropogenetics at the University of Nijmegen, The Netherlands.
Probe construction, hybridization and data acquisition
Labeling of cDNA molecules was performed essentially according to a protocol described at http://pga.tigr.org/sop/M004_1a.pdf. Briefly, for each RNA sample, first-strand amino-modified cDNA was synthesized by oligo-dT primed reverse transcription from 20 μg total RNA using Superscript II reverse transcriptase (Invitrogen, Breda, The Netherlands) in the buffer provided and in the presence of 0.5 mM of dATP, dCTP, dGTP, 0.3 mM dTTP and 0.2 mM amino-allyl dUTP (Sigma, Zwijndrecht, The Netherlands). After 16 hours of incubation at 42°C the RT reaction was stopped and cDNA was purified as described. cDNA was fluorescently labeled with Cy3 or Cy5 fluorophores (Amersham Biosciences, Roosendaal, The Netherlands), as described (12). Labeled cDNA was purified using Microcon YM-30 columns (Millipore, Amsterdam, The Netherlands).
Each probe was put together by mixing the labeled cDNA reactions from the appropriate samples together with 15 μg poly-dA DNA (Qiagen, Hilden, Germany) and 7.5 μg human Cot-1 DNA (Invitrogen) and was heated at 95°C for 3 minutes, cooled to room temperature, mixed with an equal volume of preheated (42°C) hybridization buffer to a final concentration of 25% formamide, 5× SSC and 0.1% SDS. Hybridizations were performed under lifterslips (Erie Scientific, Portsmouth, UK) within hybridization chambers (Telechem, Sunnyvale, CA, USA) in a waterbath at 42°C for 16 hours, simultaneously. After hybridization the slides were washed, dried and scanned at 10 μm resolution in a GMS 428 laser scanner (Affymetrix, Santa Clara, CA, USA). Image intensity data for each array feature was extracted by ImaGene 4.2 software (BioDiscoveries, Marine Del Rey, CA, USA).
Data analysis
For each array, raw median signal intensity data for each spot and each fluorophore was collated and filtered to exclude irregular and empty spots as determined by the ImaGene software. Initial data reduction analysis using principal component analysis (SPSS version 10) revealed no dependence between single-color readings from both fluorophores and therefore these readings were treated separately. For each contrast, respective single color data from one sample was combined with its respective single color data from another sample. To account for dye bias, resulting data were normalized by intensity-dependent regression (Lowess) of the log-transformed ratios by BRB ArrayTools version 3.0.1 as developed by Dr. Richard Simon and Amy Peng Lam http://linus.nci.nih.gov/BRB-ArrayTools.html).
The resulting log-transformed ratios for each gene were analyzed by the statistical package SAM (13). Only genes were analyzed with no more than 25% missing values. For each array triplicate observations for each gene were averaged. For each contrast four individual observations per gene were collated. Missing data were imputed using the K-nearest neighbor method and complete permutation of the data was performed to determine the false discovery rate. One class response analysis was performed to identify genes significantly regulated between both classes and in total 24 permutations of the samples were performed to determine the false discovery rate. A list of genes significantly up- or down-regulated containing less than one, according to SAM algorithm false-positive gene was used for validation experiments and further analysis.
Significant genes were assigned to different functional clusters based on known or putative biological function of the encoded proteins, as determined by searches on PubMed and by using GeneOntology (Gene Ontology Consortium) classifications.
Semi-quantitative RT-PCR
For a selected number of genes microarray results were verified by RT-PCR. cDNA synthesis was performed from 1 μg total RNA using T11VN oligos and M-MLV Reverse Transcriptase, according to the supplier 's protocol (Invitrogen). Two microliters of cDNA was amplified by PCR in a buffer consisting of 0.2 mM dNTPs, 1.5 mM MgCl2, 1× PCR buffer and 1 U Taq DNA polymerase (Invitrogen). Gene-specific primer pairs (0.5 μM each) were added after 3 min at 94°C (hot start). PCR cycles consisted of 94°C for 40 seconds, 56°C for 40 seconds and 72°C for 40 seconds. The number of cycles was selected to allow amplification within the linear range. Primer sequences, the number of cycles and PCR fragment lengths are given in Table 1. Primer pairs were developed using Primer3 (http://bioinformatics.weizmann.ac.il/cgi-bin/primer/primer3.cgi). Ethidium bromide-stained agarose gels were scanned on Image Master® VDS (Amersham Biosciences, UK) using LISCAP sofware. PCR product abundance was quantified using Imagemaster 1D Elite (Amersham) and normalized for the abundance of the β-actin signal from the same cDNA.
| Gene | Primer sequences | Number of cycles | Fragment size (bp) |
|---|---|---|---|
| β-actin | 5′-AACACCCCAGCCATGTACG-3′5′-ATGTCACGCACGATTTCCC-3′ | 24 | 253 |
| E-selectin | 5′-CAACGTGCACGTTTGACTGT-3′5′-AGGTCAAGGCTTGAACACTG-3′ | 32 | 506 |
| ICAM-1 | 5′-GGGTTGGAGACTAACTGGA-3′5′-AGCACTACTGAGAGCTGTG-3′ | 30 | 271 |
| MCP-1 | 5′-TTCACAGTTGCTGCCTGTAGC-3′5′-GTGCTGAAGTCCTTAGGGTTGA-3′ | 28 | 306 |
| KC-protein | 5′-AGACAGTGGCAGGGATTCAC-3′5′-TACTTGGGGACACCCTTTAGC-3′ | 31 | 181 |
| Egr-1 | 5′-TTCAGTCGTAGTGACCACCT-3′5′-TGTCTGAAAGACCCGTTGAG-3′ | 28 | 440 |
| HO-1 | 5′-ACTTTCAGAAGGGTCAGGTGTCC-3′5′-TTGAGCAGGAAGGCGGTCTTAG-3′ | 28 | 523 |
| Hsp-70 | 5′-CTGACAAGAAGAAGGTGCTGG-3′5′-AGCAGCCATCAAGAGTCTGTC-3′ | 25 | 302 |
| Aqp-2 | 5′-TCAGATCCATAGCCTTCTCCC-3′5′-CACATAGAAGGCAGCTCGAAG-3′ | 23 | 260 |
| KIM-1 | 5′-ACTCCTGCAGACTGGAATGG-3′5′-CAAAGCTCAGAGAGCCCATC-3′ | 34 | 214 |
Statistical analysis
Statistical analysis was performed using the Student t-test, with p<0.05 regarded as significant.
Results
Serum Biochemistry
Changes in biochemical serum parameters do reflect organ (dys)function and damage after brain death induction (8). Compared to control sera, sodium and potassium levels did not change much, except in the marginal donor group where a twofold increase in potassium levels was found (Table 2). Also creatinine and LDH levels were significantly increased in this group. The same parameters were also elevated in sera of optimal normotensive donor rats, however to a lesser extent than in the hypotensive brain death group.
| Parameters | Control group (n = 9) | Optimal donors (n = 9) | Marginal donors (n = 7) |
|---|---|---|---|
| Na+ (mmol/L) | 140 ± 0.7 | 145 ± 1.7* | 139 ± 1.6 |
| K+ (mmol/L) | 4.9 ± 0.2 | 5.9 ± 0.5 | 9.6 ± 1.2*,** |
| Creatinine (μmol/L) | 43 ± 2.2 | 98 ± 25* | 178 ± 37*,** |
| LDH (IU/L) | 410 ± 82 | 1660 ± 421* | 3037 ± 1008* |
- Values are expressed as mean ± SEM.
- *p < 0.05 compared to controls; ** p < 0.05 compared to optimal donors.
Hybridization oligonucleotide arrays
When expression profiles of kidneys from normotensive brain dead rats were compared with control rats, regarding a twofold change in expression as the cut-off point of a sequence being differentially expressed, we were able to identify 63 genes that were either up or down regulated. Similarly, in kidneys derived from marginal donors (hypotensive rats) 90 genes were differentially expressed. In the normotensive group expression of 56 genes ranged between 2 and 5, whereas 7 genes had differential values between 5 and 10. Most genes (55) were up regulated. In the hypotensive group 84 genes were up or down regulated with values ranging between 2 and 5. Six genes showed expression changes of more than fivefold. Again, most genes (67) appeared to be up regulated. Most of the differentially expressed genes from the normotensive group (79%) were found in the hypotensive group as well.
Confirmation of microarray data by RT-PCR
To confirm our microarray results we selected nine genes, assigned to several different gene clusters, and analyzed gene expression changes by semi-quantitative RT-PCR (Figure 1). All tested genes showed significantly changed expressions (E-selectin, MCP-1, ICAM-1, KC-protein, Egr-1, KIM-1, HO-1 and HSP70 all up regulated and Aqp-2 down regulated) compared to control kidneys. Only HSP70 up regulation (on average almost 3-fold) in the optimal BD donor group did not reach significance (p = 0.11), due to the large spread of the data. This was also observed for the HSP70 microarray data, nevertheless, here significance was reached. RT-PCR analyses for ICAM-1 was included since several studies have shown up regulation of ICAM-1 in BD donor organs, whereas our microarray data did not. RT-PCR revealed a significant up regulation of ICAM-1 (p < 0.05) however, 2-fold elevated mRNA levels were not reached, which is in line with the outcome of the microarray experiments.

Confirmation of differential expression, as shown in Table 3 , of a selected number of genes by RT-PCR. *p < 0.05 and **p < 0.005 when compared to sham operated controls.
The relevance of our findings was further confirmed after immunohistochemical analyses in which increased amounts of HO-1 and HSP70 protein were seen in brain dead rat kidneys, already within this 6-hour period of brain death (results not shown).
Genes differentially expressed
The brain-death-responsive genes were clustered according to the putative or known biological function of their encoded proteins, as shown in Table 3. With regard to the optimal BD donor kidneys, the most frequent changes were observed in expression of cell adhesion and cytoskeletal markers, such as E- and P-selectin and α- and β-fibrinogen (cluster 6). Together with the up regulation of a substantial number of cytokines and chemokines (cluster 4), including the significantly up regulated genes Il6, MCP-1, Mob-1 and KC, these changes primarily reflect a massive and intense immune activation of optimal normotensive BD donor kidneys. The same effect of brain death is also seen in marginal donor kidneys, however, the number of differentially expressed genes is lower in these clusters.
| Gene ID | Gene description | Optimal/control | Marginal/control |
|---|---|---|---|
| 1. Metabolism | |||
| J05571 | S-adenosylmethionine synthetase | 1.2 | 0.48 |
| NM_017074 | CTL target antigen (Cth) | 0.74 | 0.45 |
| AB013732 | UDP-glucose dehydrogenase | 1.4 | 2.4 |
| AB006138 | Alpha 1,2-fucosyltransferase (FTB) | 1.5 | 2.2 |
| AF144756 | Adipocyte lipid-binding protein (ALBP) | 0.30 | 0.30 |
| U27518 | UDP-glucuronosyltransferase | 1.1 | 2.5 |
| NM_017127 | Choline kinase (Chk) | 1.2 | 2.1 |
| 2. Protein synthesis | |||
| K01594 | 5S ribosomal RNA | 0.46 | 1.7 |
| 3. Transporters | |||
| NM_019134 | Solute carrier family 12 (bumetanide-sensitive sodium-[potassium]-chloride cotransporter) (Slc12a1) | 0.65 | 0.45 |
| U54699 | Epithelial sodium channel alpha subunit (rEnaca) | 0.57 | 0.45 |
| AF142439 | Phosphohippolin (PHP) | 0.56 | 0.48 |
| AJ223355 | Mitochondrial dicarboxylate carrier | 0.87 | 0.47 |
| D63149 | Proton-coupled peptide transporter PEPT2 | 0.68 | 0.43 |
| D79981 | Kidney specific organic anion transporter OAT-K1 | 0.62 | 0.48 |
| NM_012654 | Solute carrier family 9 (sodium/hydrogen exchanger 3) (Slc9a3) | 0.64 | 0.44 |
| NM_012833 | Canalicular multispecific organic anion transporter (Cmoat) | 1.4 | 4.8 |
| X13295 | Alpha-2u globulin-related protein | 4.3 | 4.8 |
| AB000489 | RPHO-1 | 1.5 | 2.4 |
| AF157026 | Type IIb sodium-phosphate transporter | 2.7 | 2.2 |
| NM_012909 | Aquaporin 2 (Aqp2) | 0.40 | 0.35 |
| 4. Cytokines, chemokines and related receptors | |||
| M86536 | KC protein | 8.5 | 3.3 |
| U17035 | Mob-1 | 5.5 | 4.1 |
| U45965 | Macrophage inflammatory protein-2 | 1.8 | 2.1 |
| AF058786 | JE/MCP-1 | 7.3 | 2.8 |
| NM_012589 | Interleukin 6 (Il6) | 5.0 | 4.5 |
| L18891 | Intercellular calcium-binding protein (MRP8) | 2.2 | 1.8 |
| M98820 | Interleukin 1-beta (Il1-beta) | 2.5 | 1.7 |
| M80367 | Isoprenylated 67 kDa protein | 2.6 | 1.6 |
| NM_012881 | Sialoprotein (osteopontin) (Spp1) | 5.5 | 5.2 |
| 5. Cytokine processing and signal transduction | |||
| AF239157 | DEXRAS1 (Dexras1) | 3.3 | 3.1 |
| NM_013005 | Phosphoinositide 3-kinase p85 (Pik3r1) | 2.7 | 2.1 |
| AF234260 | Heterotrimeric guanine nucleotide-binding protein alpha q subunit | 1.4 | 2.1 |
| AF205438 | G-protein-coupled receptor induced protein GIG2 (Gig2) | 2.6 | 2.4 |
| M74295 | RhoB gene | 2.4 | 3.5 |
| NM_012911 | Arrestin, beta 2 (Arrb2) | 2.1 | 2.1 |
| NM_019292 | Carbonic anhydrase 3 (Ca3) | 0.48 | 0.55 |
| AF220760 | Tissue-type liver thioredoxin reductase 1 | 1.3 | 2.1 |
| J03627 | S-100 related protein clone 42C | 1.7 | 2.1 |
| NM_012817 | Insulin-like growth factor-binding protein 5 (Igfbp5) | 0.75 | 0.42 |
| NM_013126 | Diacylglycerol kinase 3 gamma (Dgkg) | 0.77 | 0.48 |
| 6. Cell adhesion, cytoskeleton and related genes | |||
| M35601 | Alpha-fibrinogen | 6.5 | 8.4 |
| M35602 | Beta-fibrinogen | 2.9 | 4.7 |
| U05675 | Fibrinogen B beta chain | 2.1 | 4.7 |
| NM_012794 | Glycosylation dependent cell adhesion molecule 1 (Glycam1) | 2.9 | 2.1 |
| L25527 | E-selectin | 3.1 | 1.3 |
| NM_013114 | P-Selectin | 2.1 | 1.4 |
| AF035963 | Kidney injury molecule-1 (KIM-1) | 2.8 | 7.3 |
| M12201 | Alpha-2 type I collagen segment 2 | 0.49 | 0.38 |
| Z78279 | Collagen alpha1 type I | 0.48 | 0.45 |
| Gene ID | Gene description | Optimal/control | Marginal/control |
|---|---|---|---|
| NM_019153 | Fibulin 5 (Fbln5) | 0.48 | 0.45 |
| NM_017184 | Troponin I skeletal slow 1 (Tnni1) | 5.9 | 6.3 |
| V01224 | Alpha-actin | 2.5 | 4.3 |
| J02705 | Oncomodulin | 2.0 | 1.8 |
| 7. Transcription factors | |||
| NM_017086 | Early growth response 3 (Egr3) | 2.8 | 2.6 |
| AF134773 | LIM protein (FHL1) | 0.51 | 0.44 |
| X63369 | TIS 11 | 3.6 | 3.3 |
| NM_019242 | Interferon-related developmental regulator 1 (Ifrd1) | 3.8 | 3.6 |
| AF009330 | Enhancer-of-split and hairy-related protein 2 (SHARP-2) | 2.3 | 2.1 |
| AF252627 | Activating transcription factor (Atf-4) | 1.8 | 2.7 |
| NM_012591 | Interferon regulatory factor 1 (Irf1) | 2.7 | 2.4 |
| NM_012912 | Activating transcription factor 3 (Atf-3) | 2.5 | 3.8 |
| AF001417 | Zinc finger protein (Zf-9) | 2.1 | 3.2 |
| M57235 | Interleukin-6-dependent binding protein (IL-6DBP) | 1.8 | 2.3 |
| NM_013086 | CAMP responsive element modulator (Crem) | 2.2 | 2.7 |
| NM_012551 | Early growth response 1 (Egr1) | 2.6 | 2.5 |
| NM_012953 | Fos-like antigen 1 (Fosl1) | 1.5 | 2.4 |
| AB025431 | GILZ | 0.55 | 0.47 |
| 8. Hormone and growth factor regulated sequences | |||
| NM_019216 | Growth differentiation factor 15 (Gdf15) | 2.1 | 3.2 |
| X95094 | Parathyroid hormone regulated sequence | 2.8 | 2.8 |
| U53184 | Estrogen-responsive uterine mRNA | 1.8 | 2.4 |
| NM_013043 | Transforming growth factor beta stimulated clone 22 (Tgfb1i4) | 3.3 | 3.4 |
| S74327 | Clone E512, estrogen induced gene | 0.47 | 0.45 |
| 9. Growth and cell cycle | |||
| U36994 | GADD153 | 1.7 | 2.1 |
| L32591 | GADD45 | 3.0 | 3.0 |
| AB020978 | GADD45gamma | 2.6 | 2.3 |
| AF036548 | RGC-32 (RGC-32) | 0.53 | 0.33 |
| NM_017172 | Butyrate response factor 1 (Brf1) | 1.9 | 2.1 |
| NM_017259 | B-cell translocation gene 2, anti-proliferive (Btg2) | 2.9 | 3.9 |
| L27843 | Tyrosine phosphatase (PRL-1) | 1.3 | 2.2 |
| 10. Defense and repair | |||
| AF075383 | Suppressor of cytokine signaling-3 (SOCS-3) | 3.3 | 2.7 |
| NM_012580 | Heme oxygenase (HO-1) | 4.2 | 10.9 |
| L16764 | Heat shock protein 70 (HSP70) | 4.1 | 2.8 |
| Z75029 | Hsp70.2 | 3.1 | 2.2 |
| NM_012620 | Plasminogen activator inhibitor (Pai1) | 3.6 | 3.3 |
| NM_017051 | Superoxide dismutase 2, mitochondrial (Sod2) | 2.3 | 1.9 |
| 11. Apoptosis | |||
| NM_017180 | T-cell death associated gene (Tdag) | 2.3 | 3.6 |
| NM_013091 | Tumor necrosis factor receptor (Tnfr1) | 1.9 | 2.1 |
| 12. Proteases | |||
| AF149118 | A disintegrin and metalloproteinase with thrombospondin motifs 1 (ADAMTS-1) | 4.6 | 4.3 |
| D87336 | Bleomycin hydrolase | 2.4 | 3.2 |
| AF198087 | Adrenal secretory serine protease precursor | 4.6 | 4.3 |
| L05175 | Serine protease | 2.4 | 1.9 |
| 13. Organogenese and development | |||
| D38056 | B61 | 1.4 | 2.1 |
| 14. Miscellaneous | |||
| NM_012603 | Avian myelocytomatosis viral (v-myc) oncogene homolog (Myc) | 2.1 | 3.0 |
| M26758 | Major acute phase protein (alpha1-MAP) | 2.4 | 1.7 |
| U95001 | Developmentally-regulated cardiac factor (DRCF-5) | 0.65 | 0.49 |
| AF106659 | Deubiquitinating enzyme Ubp69 (ubp69) | 0.70 | 0.37 |
| NM_017363 | Placental lactogen 1 (Pl1) | 1.9 | 2.1 |
| Gene ID | Gene description | Optimal/control | Marginal/control |
|---|---|---|---|
| L37380 | Apical endosomal glycoprotein | 2.1 | 2.2 |
| AF091577 | Isolate HAF-TP1 olfactory receptor | 4.7 | 5.1 |
| NM_019282 | Cysteine knot superfamily 1BMP antagonist 1 (Cktsf1b1) | 0.55 | 0.42 |
| NM_012752 | CD24 antigen (Cd24) | 2.5 | 2.7 |
| NM_012862 | Matrix Gla protein (Mgp) | 1.9 | 2.1 |
| NM_020074 | Proteoglycan peptide core protein (Pgsg) | 1.9 | 2.2 |
With regard to the marginal hypotensive donor kidneys, the most frequent changes were found in the transcription factor cluster (cluster 7). A relevant number of these transcription factors belong to the group of so called immediate early genes (IEGs): Egr-1, Egr-3, TIS 11, Atf-3, Atf-4 and Zf-9. Although signal transduction cascades primarily act via activation or phosphorylation of the proteins involved, we also found a substantial number of genes encoding signal transduction proteins to be differentially expressed (mostly up regulated). Of these, Pik3r1, depending on stimulus and cell type, is involved in transmission of signals such as induction of immediate early gene expression (Egr-1) (14) or inhibition of apoptosis (15).
The largest differences between optimal and marginal brain dead donor kidneys were found in genes encoding proteins involved in metabolism and transport (clusters 1 and 3). In hypotensive BD kidneys 18 genes had changed expressions. Remarkably, most genes in the metabolism and transporter clusters were down regulated, whereas all the other clusters primarily contained up regulated genes. Since one of the main functions of the kidney is to maintain sodium and potassium homeostasis, the differential expression of four dedicated sodium and potassium transporters might reflect (early) dysfunction of BD donor kidneys.
Most of the above mentioned changes in gene expression represent deleterious processes, already present during brain death in donor kidneys. In contrast, processes suggesting defense- and repair mechanisms were observed as well, as seen, e.g. in the intense up regulation of the heat-shock genes HO-1 and HSP70. In addition, elevated mRNA levels for genes like osteopontin, KIM-1 or GADD45 (assigned to other gene clusters) were not only just markers for cell damage, but strongly suggest that repair processes have already started in BD damaged kidney tissue.
Discussion
In the past years it has become clear that brain death affects post-transplant function and graft survival in organ transplantation. We and others have shown in animal models that brain death has definite effects on hemodynamic stability, hormone regulation and inflammatory reactivity (16). Nonetheless, it still remains unclear what the individual contributions of these detrimental factors to organ quality are and if so, how these factors influence each other and whether there are (still) undiscovered additional factors influencing BD donor organ quality and transplantation outcome. Therefore, to elucidate the complex mechanisms of brain death we have performed DNA microarray experiments to identify pertinent gene expression changes in BD donor kidneys.
Brain-death-induced gene expression changes were investigated in normo- and hypotensive 6-hour BD rats, mimicking the optimal and marginal human BD donor, respectively. In kidneys derived from marginal donors (hypotensive rats) 90 genes were identified as being significantly differentially expressed whereas in the normotensive group 63 genes had changed expressions. The majority of the differentially expressed genes from the normotensive group (79%) were observed in the hypotensive group as well. In this analysis we found a number of genes which, in previous studies, had been identified as differentially expressed (e.g. Il-1beta, Il-6, E-selectin). Also, from a selected number of genes expression changes were confirmed with RT-PCR.
The significance of our results however, lies primarily in the identification of a number of previously unidentified genes involved in the process of brain death. In Figure 2 a scheme is shown, presenting the complex effects of brain death on donor kidneys. This scheme is based on the array analyses of normotensive BD rats (Table 3) as this group probably reflects best the direct effects of brain death on donor kidneys. One group of genes that are seriously affected by the deleterious processes of brain death are the genes involved in immune activation and coagulation (Table 3). In recent years many researchers have focused on the effect of brain death on the immunological status of the future graft. Adhesion molecules and cytokines were found to be clearly up regulated in BD donor kidneys from rats (7,8,17,18) and man (19,20). Our results confirm these findings and, in addition, show that immune activation is massive and even more intense and involves many (this group contains the highest number (16) of up regulated genes) adhesion molecules (E-selectin, P-selectin and Glycam 1), chemokines (KC-protein, MIP-2, MCP-1, Mob-1 and Osteopontin) cytokines (Il-6, Il-1beta and MRP8) and coagulation factors (α-fibrinogen, β-fibrinogen and fibrinogen B). It is obvious that immunological activation, which is associated with recruitment of leukocytes, as has been shown by our group and others (7,8,18), will have profound deleterious effects on organ quality and function before and after transplantation. Therapeutical intervention, preferably in the donor, aiming at the prevention of up-regulation of these genes, is therefore a worthwhile approach to decrease immunogenicity and increase graft viability (7,21).
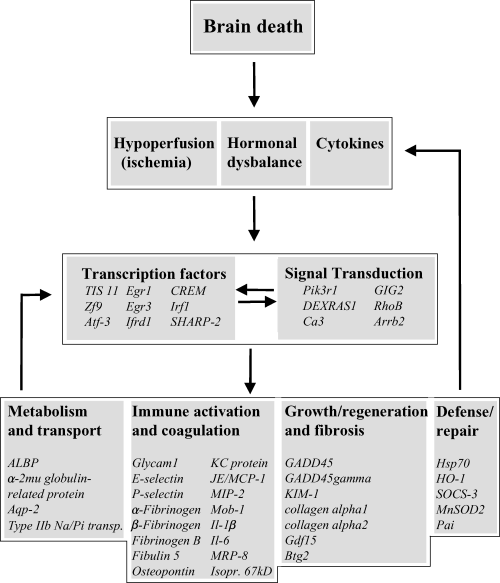
Schematic representation of the pathophysiological and molecular changes that take place in brain-dead donor kidneys. Brain death has definite effects on hemodynamic stability, hormone regulation and messenger molecules such as cytokines. These effects ultimately lead, via activation of signal transduction cascades and the induction of transcription factors, to differential expression of “effector” genes involved in metabolism, immune activation, growth and regeneration, and repair. Some of these, such as Il-1β, Il-6 and SOCS-3, have shown to influence again the expression and activity of cytokines, transcription factors and signal transduction proteins (28,40,41).
In spite of the deleterious processes that occur during brain death only few genes involved in metabolism and transport and indicative of kidney function are affected in optimal BD donor kidneys. In contrast, in marginal donors 11 genes involved in metabolism and transport have changed expression, the majority of them down-regulated. This is in line with the results of the serum biochemical parameters (Table 2) indicating more kidney dysfunction and damage in marginal donors than in optimal donors. In addition, the dysregulation of sodium and potassium transporters may explain the elevated potassium levels found in serum of marginal BD donors (Table 2). Another intriguing observation is the down-regulation of the transporter aquaporin-2 (Aqp-2) in BD donor kidneys. Aqp-2 is a water channel mainly located in renal collecting duct cells and is involved in the reabsorption of water. It plays a critical role in urine concentration (22,23). Interestingly, one of the consequences of brain death is a markedly increased urine production, better known as diabetes insipidus. In this respect, the decreased Aqp-2 expression could therefore, at least in part, explain the increased urine production in BD donors.
So far, we have described a number of genes involved in ongoing harmful processes. On the other hand we have detected a group of genes involved in defense and repair, which are clearly up-regulated. Both, HO-1 and HSP70 have cytoprotective properties and are induced after diverse forms of stress such as ischemia, heat and due to toxins (24–26). MnSOD2 protects cells against oxide radicals (27) whereas SOCS-3 (suppressor of cytokine signaling-3) renders cells less susceptible against the deleterious effects of cytokines such as Il1-β (28). Numerous studies have shown that artificial up-regulation of HSP70, HO-1 and MnSOD2, prior to the event, induces cellular protection against various forms of stress such as ischemia/reperfusion injury (29–31). The brain-death-induced stress response seems to be insufficient to protect kidney cells against the harmful processes of cerebral injury and therefore should be merely regarded as a reflection of the stress and possibly early protection.
In addition, other differentially expressed genes indicative for initiating regeneration processes, were found. Growth differentiation factor 15 (Gdf15) and kidney injury molecule-1 (KIM-1) are known to be severely up-regulated after injury and have been suggested to be involved in regeneration (32–34). KIM-1 was recently cloned as a gene encoding a type-I transmembrane glycoprotein, highly expressed on de-differentiating renal proximal tubular cells undergoing regeneration after toxic or ischemic injury. The exact role of KIM-1 in the regeneration process is still under investigation but the finding of up-regulated KIM-1 in BD donor kidneys suggests at least damage of tubular epithelial cells. Interestingly, our RT-PCR analyses showed that KIM-1 was significantly higher expressed in marginal than optimal BD donor kidneys, suggesting a direct correlation between kidney injury (Table 2) and the level of KIM-1 gene expression.
Until now, genes encoding “effector” proteins have been discussed. Induction or down-regulation of these genes is mediated via signal transduction cascades and/or transcription factors (Figure 2). Indeed, quite a number of these types of genes were differentially expressed. Nevertheless, it seems rather complicated to directly link up-regulation of one of those genes to differential expression of “effector” genes, primarily due to the fact these complex interactions mostly are cell type and stimulus dependent. However, a transcription factor putatively regulating “effector” genes in BD donor kidneys is the transcription factor Zf-9. Zf-9 was found to be up-regulated during early hepatic fibrosis and responsible for the regulation of expression of collagens (35), such as collagen α1 (Figure 2). Another example is the possible involvement of Atf-3 in increasing HO-1 expression (36). It is remarkable, however, that most of the up-regulated transcription factors (Egr-1, Egr-3, TIS 11, Atf-3, Atf-4 and Zf-9) belong to the group of immediate early genes. IEGs respond, via signal transduction routes, to extracellular stimuli such as mitogens, hormones and stress after which their expression is rapidly and transiently induced (37–39). This again indicates that brain death induces numerous processes, probably cross-talking with each other, ultimately leading to differential expression of entire sets of genes as indicated in Figure 2.
In summary, we feel that the use of DNA microarrays has led to the identification of a substantial number of differentially expressed genes in BD donor kidneys and has given us a new and broader insight in (parts of) the process of brain death. The picture as presented in Figure 2, is that the brain-death-induced effects on hemodynamic stability, hormone regulation and messenger molecules such as cytokines ultimately lead, via activation of signal transduction cascades and the induction of transcription factors, to differential expression of different “effector” genes. Remarkably, not only deleterious processes as inflammation and fibrosis are induced in BD donor kidneys but also genes involved in protection and repair processes are activated as well. It should be kept in mind that the changes we have observed now are most likely just a tip of the iceberg, as we might have missed local changes in specific cell types since total RNA was used from whole-kidney-derived homogenized tissue. Nevertheless, these findings open up ways for focussed research related to the mechanism of brain death. In addition, our results will allow us to explore targeted cytoprotective interventions in the brain dead donor to stabilize or even increase organ viability prior to preservation and transplantation.
Acknowledgment
This study was supported in part by the ESOT-Wyeth Research Scholarship 2003.




