Thiopurine S-Methyltransferase Genotype Predicts Azathioprine-Induced Myelotoxicity in Kidney Transplant Recipients
Abstract
Azathioprine (AZA) is an immunosuppressive prodrug that undergoes metabolism by thiopurine S-methyltransferase (TPMT). Eighty to ninety-five percent of low or deficient TPMT enzyme activity is genetically determined by the presence of three nonfunctional mutant alleles: TPMT*2, TPMT*3A and TPMT*3C. Using TPMT as a pharmacogenetic paradigm, we explored the association between these genetic mutations and development of adverse drug effects in an ethnically diverse renal transplant population receiving azathioprine. Biochemical and clinical data were retrospectively evaluated during the first four weeks after kidney transplantation. TPMT nonfunctional mutant alleles were identified by polymerase chain reaction-based methods. Of 89 patients initially consented, 36 met inclusion criteria for this retrospective study. Five patients possessing a single TPMT nonfunctional mutant allele were identified: TPMT*3A: n = 2 Caucasians; TPMT*3B: n = 1 Caucasian; TPMT*3C: n = 2 African-Americans. TPMT nonfunctional mutant alleles were associated with significant reductions in hematological indices and a significant increase in cyclosporine plasma concentrations in the first month post-transplant. TPMT genotype was an independent predictor for hemoglobin, hematocrit and red blood cell changes while mean azathioprine dose (mg/kg/day), azathioprine dose (mg/kg/day) at day 30 and cyclosporinemia at day 30 were not. Prospective application of pharmacogenetic principles may assist in optimization of immunosuppressive drug therapy and minimize drug toxicities.
Introduction
Azathioprine (AZA) is a synthetic purine analog used for prevention of rejection in organ transplantation and treatment of autoimmune disorders (1–5). Azathioprine is a prodrug of 6-mercaptopurine (6MP), an agent used in chemotherapeutic regimens to treat acute lymphoblastic leukemia (ALL) (6,7). In transplantation, AZA continues to be a widely used agent in Europe although its use in the United States for graft rejection prophylaxis has declined. According to United Network for Organ Sharing (UNOS) data from 2000, immunosuppressive drug use of AZA in heart (19%), lung (54%) and heart-lung (43%) transplantation surpassed use in kidney, pancreas, pancreas after kidney, kidney-pancreas, liver and intestine transplantation (<10% each) in the first year post-transplant (8). Immunosuppressive benefits do not come without risk. Myelosuppression is a significant toxicity of AZA and has resulted in reduction or discontinuation of azathioprine or 6MP doses and septic death (9–12).
AZA metabolism has had biochemical and genomic elucidation. AZA is a prodrug that undergoes nonenzymatic conversion to 6MP which is inactivated by xanthine oxidase (XO) to thiouric acid or by thiopurine S-methyltransferase (TPMT) to methyl-mercaptopurine (Figure 1). By a third biotransformation pathway, hypoxanthine phosphoribosyl transferase (HPRT) intracellularly converts 6MP to thioinosine monophosphate (TIMP), which results in active methyl-mercaptopurine nucleotides and thioguanine nucleotides (TGN) to exert immunosuppressive effects through inhibition of DNA, RNA and protein synthesis. TPMT S-methylates 6MP to inactive 6MP, which shunts 6MP away from activation to toxic TGN (6,7,9,13,14).
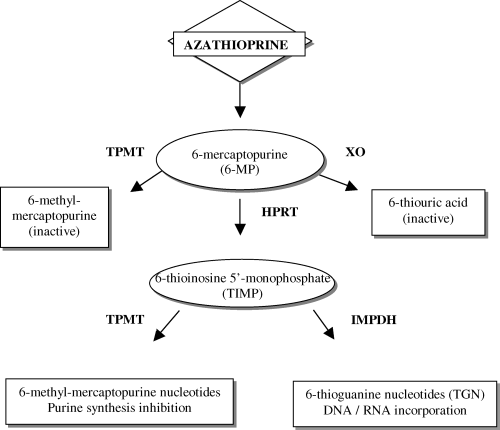
Thiopurine metabolic pathways. Enzymatic biotransformations are catalyzed by thiopurine methyltransferase (TPMT), xanthine oxidase (XO), and hypoxanthine phophoribosyl transferase (HPRT). After transformation from 6MP, TIMP is converted by inosine monophosphate dehydrogenase (IMPDH) to active TGN or methylated by TPMT to methyl-mercaptopurine metabolites.
Myelotoxicity has been associated with administration of azathioprine and 6MP in individuals with nonfunctional genetic mutations of TPMT. TPMT enzyme activity in humans demonstrates genetic variability (14). One in 300 individuals (0.3%) has low to absent TPMT enzymatic activity (homozygous TPMTlow), approximately 10% have intermediate activity (TPMThigh/TMPTlow), and 90% have normal to high activity (homozygous TPMThighor wild type) (14). The basis for reduced TPMT activity has been identified as genetic point mutations. Three genetic mutations account for 80–95% of intermediate or low enzyme activity: TPMT*2 (G238C), TPMT*3A (G460A and A719G) and TPMT*3C (A719G) (9). TPMT*3C is more prevalent in African-Americans and TPMT*3A and TPMT*2 are more common in Caucasians (15). Reduced clearance of active TGN metabolites may result in accmulation and an increased risk of myelotoxicity (12); however, individuals with high TPMT activity may be at risk for therapeutic failure due to inadequate levels of TGN metabolites resulting in relapses of childhood leukemia and inflammatory bowel disease and poor graft outcomes in solid organ transplantation (1,2,16–20). Thus, TPMT genotype determines TPMT enzymatic activity phenotype, which in turn regulates TGN concentrations that are ultimately translated to subtherapeutic, therapeutic or toxic effects.
Large inter-individual differences occur in response to drug therapy; similar drug dosages given to different individuals may result in drug efficacy, toxicity or failure. Genetic contribution to variability in drug disposition and metabolism has been investigated to explore the impact of genetic variability for metabolic enzymes, transporters and drug targets (21,22). Pharmacogenomic research aims to discover the genetic basis for inter-individual differences in efficacy and toxicity of therapeutic drugs and to determine optimal drug therapy in a prospective manner (21,22). TPMT presents an opportunity to evaluate important relationships in renal transplant recipients using a well-described, functional pharmacogenomic model (9). The aim of our retrospective study was to explore associations between TPMT nonfunctional mutations and development of hematological toxicities in a diverse kidney transplant population receiving azathioprine.
Materials and Methods
Patients and biochemical/clinical data collection
Eighty-nine primary kidney transplants performed at this institution were retrospectively evaluated from 1991 to 2000. Peripheral venous blood samples were obtained for the genetic study during routine clinic visits. Clinical data were obtained from hospital inpatient and outpatient clinic computer systems. Primary kidney transplant recipients received azathioprine as part of standard triple immunosuppressive drug therapy (cyclosporine/tacrolimus and corticosteroids). Patients were excluded if AZA was replaced by mycophenolate mofetil or if treated with allopurinol. Fifty-three of 89 patients were excluded from evaluation: simultaneous organ transplantation (1 kidney/liver, 1 kidney/pancreas), loss to follow-up (23 patients), investigational drug protocols using nonstandard triple therapy immunosuppression (14 patients), failed kidney transplant before day 30 (3 patients), secondary renal transplant (7 patients), mycophenolate mofetil (3 patients) and no kidney transplant (1 patient). Of the three patients who were switched from AZA to mycophenolate mofetil (MMF) therapy, one individual had a positive B-cell crossmatch with the donor organ and received daclizumab induction and MMF; one individual had slow kidney function but no documented rejection; and one individual had leukopenia and anemia unrelated to AZA genetic mutations under study. Thirty-six patients (22 Caucasians, 14 African-Americans; 17 women, 19 males) met study criteria. Twenty-one patients had received a deceased donor renal transplant and 15 had a living donor renal transplant. Laboratory and clinical information were collected from the initial 30 days post-transplant. Whole blood parent cyclosporine and tacrolimus trough concentrations were evaluated during the first 30 days after transplantation with fluorescent monoclonal antibody technology. Leukopenia was defined as white blood cell (WBC) count < 3000 cells/mm3; thrombocytopenia as platelets (PLT) < 100,000/mm3. Although the normative red blood cell (RBC) range for adult males was 4.5–5.5 million/mm3 and 4.0–4.9 million/mm3 for adult females, RBC < 4 was million/mm3 defined as below normal limits for men and women in this study. Hemoglobin (HGB) <11 g/dL (hematocrit < 33%) in pre-menopausal females and hemoglobin <12 g/dL (hematocrit < 37%) in adult males and post-menopausal females was defined as the anemic threshold to initiate evaluation based on the 2000 National Kidney Foundation/Dialysis Outcomes Quality Initiative (NKF-K/DOQI) clinical practice guidelines for treatment of anemia of chronic kidney disease; for the purpose of this study, hemoglobin <11 g/dL and hematocrit < 33% was defined as the threshold to initiate an anemic workup in men and women based on NKF-K/DOQI guidelines (23). Institutional normative values for adults for other hematological indices included: mean corpuscular volume, 80–100 (MCV, cumicron); mean corpuscular hemoglobin, 26–34 (MCH, pg); mean corpuscular hemoglobin concentration, 31–37 (MCHC, gldL); iron, 65–150 (mcg/dL); ferritin, 13–300 (ng/mL); transferrin, 175–400 (mg/dL); total iron binding capacity, 250–420 (TIBC, mcg/dL); percent transferrin saturation 20–55, (TSAT,%); and folate, 3.6–20 (ng/mL). Physicians caring for transplant patients were blinded to TPMT status. Patients provided informed, written consent to participate in the study. The institutional review board approved all research procedures.
Genotyping
Identification of the TPMT*2 variant allele was performed using a previously described PCR method to detect the G238C mutation (10). Final volume (26 μL) included: 30–60 μg (2 μL) genomic DNA, 0.5 μL of specific primers, 23 μL of Platinum PCR SuperMix™ (Invitrogen, Carlsbad, CA). PCR condition was as follows: an initial denaturation of 94°C for 2 min, denaturing at 94°C for 30 sec, annealing at 58.1°C for 30 sec, extending at 72°C for 30 sec for 30 cycles with a final extension at 72°C for 4 min. PCR products were stained with ethidium bromide and analyzed using 1.5% agarose gel.
The presence of TPMT*3 variant alleles was determined using previously described PCR-RFLP methods to detect G460A and A719G mutations (10). RFLP reactions were performed with Mwo I (New England Biolabs, Beverly, MA) and Acc I (New England Biolabs, Beverly, MA) to identify G460A and A719G polymorphisms, respectively. Digestion products were visualized with 8% polyacrylamide gel.
TPMT*3 PCR reactions used final volume (40 μL): 60–120 ng (4 μL) genomic DNA, 0.8 μL of specific primers and 34.4 μL of Platinum PCR SuperMix™ (Invitrogen, Carlsbad, CA). PCR condition was as follows: an initial denaturation of 94°C for 2 min, denaturing at 94°C for 30 sec, annealing at 58.1°C for 30 sec, extending at 72°C for 30 sec for 35 cycles with a final extension at 72°C for 4 min. PCR products were stained with ethidium bromide and analyzed using 1.5% agarose gel.
Statistics
Means were compared using analysis of variance (ANOVA) tests. Genetic frequencies and genotype interactions with infections and rejections were compared using chi-squared or Fisher's tests. Multivariate regression was performed to evaluate the relationship between hematological effects and genotype. The dependent variables were defined as hemoglobin, hematocrit and RBC. Independent variables were mean azathioprine dose, cyclosporinemia and TPMT genotype. Statistical analyses were performed using SAS® and StatView® computer software (SAS Institute, Inc., Cary, NC). p-values < 0.05 were considered statistically significant.
Results
Demographic characteristics of the 36 kidney transplant recipients are described in Table 1. Five individuals with heterozygous nonfunctional TPMT mutant alleles were identified: two individuals with TPMT*3A (Caucasian), one with TPMT*3B (Caucasian) and two with TPMT*3C (both African-Americans). No individuals were identified with TPMT*2 mutation or homozygous nonfunctional TPMT mutations. Thirty-one individuals did not have detected TPMT mutations. The two groups had similar demographic characteristics with allele frequencies in Hardy-Weinberg equilibrium. Both groups received similar doses of AZA (mg/kg/day) at initiation of therapy. Figure 2 shows that patients with one nonfunctional TPMT mutant allele demonstrated a slightly lower AZA dose versus patients without detected TPMT mutations (1.8 ± 0.8 vs. 2.1 ± 0.4 mg/kg/day, p = 0.1510) at day 30 but the difference was not statistically significant. Patients with one nonfunctional TPMT mutant allele had a significantly higher cyclosporine whole blood trough level than patients without detected TPMT mutations at day 30 (467 ± 232 vs. 290 ± 111 ng/mL, p = 0.0099). Cyclosporine levels were similar between the two groups at initiation of immunosuppression therapy (497 ± 385 vs. 413 ± 72 ng/mL, p = NS). All patients were on hemodialysis prior to transplant; therefore, baseline creatinine clearances were not available. Thirty days after transplantation, calculated creatinine clearance was slightly higher in patients without detected TPMT mutations compared to patients with a TPMT nonfunctional mutation (56 ± 17 vs. 44 ± 17 mL/min, p = NS); however, the difference was not significantly different. Hemodialysis was not performed secondary to poor renal function. One patient received tacrolimus-based immunosuppression; thus, the cyclosporine trough levels and creatinine clearance were evaluated in 35 patients.
| Baseline WT | V | Day 30 WT | V | |
|---|---|---|---|---|
| Caucasian/African-American | 19/12 | 3/2 | – | – |
| Male/Female | 16/15 | 3/2 | – | – |
| Age (years) | 44 ± 14 | 45 ± 8 | – | – |
| Weight (kg) | 70 ± 15 | 82 ± 12 | – | – |
| AZA dose (mg/kg/day) | 2.5 ± 0.6 | 2.4 ± 0.4 | 2.1 ± 0.4 | 1.8 ± 0.8 |
| Cyclosporine trough* concentration (ng/mL) | 497 ± 385 | 413 ± 72 | 290 ± 111 | 467 ± 232† |
| Creatinine clearance* (calc, mL/min) | – | – | 56 ± 17 | 44 ± 17 |
- WT: patients without detected TPMT mutations, V: patients with one nonfunctional mutant allele.
- *One patient received tacrolimus-based immunosuppression first month post-transplant, N = 35. At baseline, all patients received hemodialysis.
- †Significant at day 30 (p < 0.05).
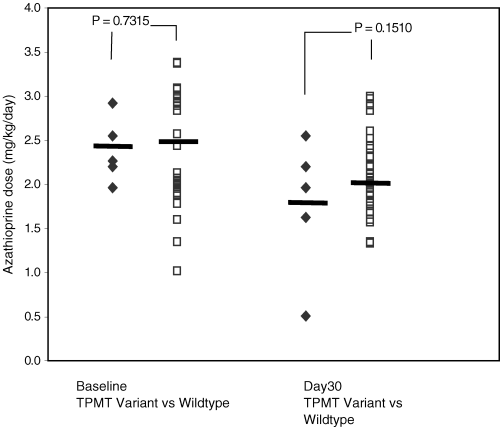
Azathioprine dose requirement (mg/kg/day) at baseline and 30 days post-transplantation in adult kidney transplant patients separated by thiopurine S-methyltransferase genotypes. The horizontal line represents the mean value. Open squares represent patients without detected TPMT mutations, and closed diamonds represent patients with one nonfunctional mutant allele.
Table 2 shows that hematopoetic profiles including WBC, PLT, RBC, HGB and HCT of patients with one nonfunctional TPMT mutant allele and patients without detected TPMT mutations were not significantly different at baseline and at day 30 of AZA therapy. At baseline, patients with one nonfunctional TPMT mutation did not demonstrate mean HGB <11 g/dL or HCT < 33% (12.5 ± 1.9 g/dL and 36.7 ± 5.7%, respectively) whereas patients without detected TPMT mutations did (10.8 ± 1.7 g/dL and 32.0 ± 4.9%, respectively) (23). After thirty days of azathioprine, both groups met NKF-K/DOQI guidelines for initiation of anemic workup (23). Patients with one nonfunctional TPMT mutant allele demonstrated a significant change in red blood cell (p = 0.0205), hematocrit (p = 0.0059) and hemoglobin (p = 0.0084) values over a 30-day period versus patients without detected TPMT mutations. The change in white blood cells and platelets over time was not statistically different between the two groups. No patient developed severe leukopenia. One patient with one nonfunctional TPMT*3C mutant allele developed thrombocytopenia with a platelet count of 70,000. Multivariate regression analysis showed that TPMT genotype was a significant predictor of hemoglobin (p = 0.008), hematocrit (p = 0.004) and red blood cell (p = 0.020) changes over the initial 30 days post-kidney transplant, independent of other potentially confounding factors: mean azathioprine dose (mg/kg/day), mean azathioprine dose (mg/kg/day) at day 30 and cyclosporinemia at day 30. Cyclosporine 12-hour whole blood trough concentration was not found to be an independent predictor for change of hemoglobin (p = 0.097), hematocrit (p = 0.050) and red blood cells (p = 0.142) 30 days after renal transplant.
| Baseline WT | V | Day 30 WT | V | |
|---|---|---|---|---|
| WBC (thou/mm3) | 10.0 ± 4.6 | 9.6 ± 3.3 | 8.3 ± 2.8 | 7.3 ± 2.8 |
| PLT (thou/mm3)* | 274 ± 135 | 214 ± 41 | 272 ± 143 | 194 ± 80 |
| RBC (million/mm3) | 3.65 ± 0.8 | 4.03 ± 0.6 | 3.47 ± 0.5 | 3.01 ± 0.7† |
| HGB (g/dL) | 10.8 ± 1.7 | 12.5 ± 1.9 | 10.8 ± 1.5 | 9.6 ± 2.4† |
| HCT (%) | 32.0 ± 4.9 | 36.7 ± 5.7 | 31.9 ± 4.3 | 28.0 ± 7.2† |
| MCV (cumicron) | 89.1 ± 9.1 | 91.2 ± 7.2 | 92.4 ± 8.7 | 92.9 ± 7.9 |
| MCH (pg) | 30.0 ± 3.5 | 31.2 ± 2.9 | 31.2 ± 3.4 | 31.6 ± 2.8 |
| MCHC (g/dL) | 33.6 ± 1.0 | 34.1 ± 0.8 | 33.8 ± 0.8 | 34.1 ± 0.5 |
- WT: patients without detected TPMT mutations, V: patients with one nonfunctional mutant allele.
- *One patient did not have platelet measures performed first month post-transplant, N = 35.
- †Significant change from baseline to day 30 (p < 0.05).
Normative values of MCV, MCH and MCHC were not statistically different between TPMT genotypes and did not significantly change over 30 days reflecting normocytic and normochromic cells. In retrospective review of the cohort of 36 patients, only 11 patients had full laboratory evaluation of hematological indices during the initial 30 days post-transplant: mean iron 121 mcg/dL (range 33–242), mean ferritin 483.7 ng/mL (range 46.3–1204.6), mean transferrin 172.2 mg/dL (range 97–262), mean TIBC 218.3 mcg/dL (range 123–304) and mean TSAT 51.8% (range 21–97). Only four patients had folate levels performed with mean value 8.4 ng/mL (range 4.5–14.3). Two patients without detected TPMT mutations and one patient with one nonfunctional TPMT mutant allele received packed red blood cells during admission for renal transplant (2 units, 8 units and 2 units, respectively). No patient received angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) during the initial 30 days post-transplant. By the end of the first month after renal transplantation, iron supplementation was initiated in 10 of 36 patients and exogenous erythropoietin injections were prescribed to three of 36 patients.
TPMT genotype was not associated with two clinically relevant outcomes in this kidney transplant population receiving azathioprine. In patients without detected TPMT mutations, 3/31 (10%) had an infection requiring the initiation of antibiotic therapy within the first 30 days post-transplantation (p = NS, data not shown). Patients with one nonfunctional TPMT mutation required an antibiotic to treat infection in 2/5 (40%) individuals that was not statistically different than development of infection in patients without detected TPMT mutations (p = NS, data not shown). No patient had active cytomegalovirus infection. Five of 31 (16%) patients without detected TPMT mutations had a documented acute rejection by biopsy and were subsequently treated with steroid pulse therapy (three biopsies prior to Banff 1997 criteria and one Banff borderline rejection) and a single patient (Banff I rejection) required subsequent treatment with monoclonal CD3 antibody preparation. One of five (20%) patients with one nonfunctional TPMT mutation had documented acute rejection by biopsy (Banff IIa rejection) requiring treatment with steroids and monoclonal CD3 antibody preparation. No TPMT genotype and rejection interactions were identified (p = NS, data not shown). No patient received polyclonal antibody preparations (thymoglobulins) for induction or treatment of rejection. Routine cytomegalovirus and pneumocystis carinii pneumonia drug prophylaxes were prescribed as standard of care.
Discussion
Pharmacogenomics is a rapidly expanding field that aims to discover the genetic basis for differences in individual drug efficacy and toxicity. Identifying genetic contribution to drug disposition including metabolizing enzymes, drug receptors and drug transporters is the goal of pharmacogenetics in order to predict drug efficacy and to avoid drug-induced toxicity (21,22). TPMT provides a clinical pharmacogenetic paradigm for investigation of hematological side effects (24,25). The metabolic pathway for detoxification of thioguanine nucleotides has been described and genetic variants have been identified which confer a susceptibility to thioguanine toxicity (9,13,14).
Our retrospective study explored associations between development of azathioprine-induced adverse drug effects and presence of TPMT nonfunctional mutant alleles. In kidney transplant recipients with a nonfunctional TPMT mutant allele, there was a reduction of hemoglobin <11 g/dL and hematocrit <33%, values that would suggest patient evaluation for anemia (23). Patients without detected TPMT mutations did not demonstrate significant changes over the first month post-kidney transplant; however, this group did not demonstrate hemoglobin and hematocrit recovery during the initial thirty days post-kidney transplant and should also have been evaluated for anemia based on the 2000 NKF-KDOQI guidelines (23). While the hematopoietic indices observed in the cohort of 36 patients were not associated with hematotoxicity warranting discontinuation of azathioprine, neither group showed early laboratory evidence of hematological improvement one month after successful renal transplantation.
Anemia is a common phenomenon after kidney transplantation but remains poorly understood (26–28). Commonly identified causes of anemia include allograft dysfunction, purine synthesis inhibitors (AZA and MMF), angiotensin converting enzyme inhibitors and angiotensin-II receptor antagonists. Azathioprine can cause bone marrow suppression and has been associated with leukopenia, thrombocytopenia, macrocytosis without megaloblastic anemia and rarely pure red cell aplasia (29). Individuals with genetic mutations of the TPMT enzyme inadvertently initiated on azathioprine or 6MP therapy have manifested clinical intolerance resulting in reduction or discontinuation of azathioprine dosages (4,10–12,30).
Anemia after kidney transplantation has clinical importance and may affect long-term outcomes in kidney transplant recipients. Clinical signs and symptoms of anemia include exercise intolerance, cardiac dysfunction and cognitive impairment (23,26,31). In chronic renal failure (CRF), blood hemoglobin >11 g/dL and hematocrit > 33% have been associated with lower risk of death, lower hospitalization rates and improved clinical parameters including physical performance, cognitive function, and brain oxygenation (23). Late post-transplant anemia (PTA) has recently been identified as an under-recognized problem in kidney transplant recipients 5 years post-transplant. Early and late PTA remains poorly understood in kidney transplant recipients (26–29,32). In CRF, long-term negative effects of anemia are reduced quality of life and increased cardiovascular risk due to left ventricular hypertrophy and increased cardiac output (23,28,31). After successful kidney transplant, hemoglobin increases to normal values within 8–12 weeks; however, this study demonstrated that the group without detected TPMT mutations and the group with detected nonfunctional TPMT mutations did not demonstrate improvement of hemoglobin values during the initial month after transplant (33).
While the hematological toxicities of the antimetabolite agents have been acknowledged, the effect of cyclosporine on erythropoiesis has been debated. Cyclosporine has been associated with stimulatory effects on bone marrow and early erythroid progenitors and has shown an inhibitory effect on erythroid progenitors and epoietin secretion (34–38). Previous in vitro investigations involving cyclosporine have demonstrated concentration-dependent inhibition of erythropoietin release and proliferation of hematopoietic stem and progenitor cells (35,38). Azathioprine has been postulated to interfere with erythroid proliferation and erythropoietin receptor expression or interaction resulting in alterations to erythropoietin sensitivity (37). This study identified azathioprine-treated renal transplant patients possessing a nonfunctional TPMT mutant allele with significantly elevated cyclosporine whole blood trough concentrations. In this setting, AZA and cyclosporine may have effected synergistic inhibition on erythroid indices resulting in significant changes. An inability to clear toxic AZA metabolites due a nonfunctional TPMT mutant allele may have contributed to accmulation of toxic metabolites resulting in additional bone marrow suppression, poor proliferation of erythroid cells and further alterations to erythropoietin sensitivity.
Several factors potentially associated with PTA were retrospectively evaluated: allograft dysfunction, ACE inhibitors and ARBs, iron biochemical markers, and health professional prescription pattern of oral iron supplementation or erythropoietin injections. Transplant recipients in this study had limited renal allograft dysfunction based on mean calculated creatinine clearances and no observed hemodialysis episodes after renal transplant. No patient received ACE inhibitors or ARBs during the initial 30 days after kidney transplantation. Both TPMT genotype groups had normochromic and normocytic red cells based on MCV, MCH and MCHC, and the values did not change significantly over the first month. Of the 36 patients, only 11 had complete iron biochemical evaluations performed after kidney transplant that did not suggest iron deficiency anemia based on normative ferritin and transferrin saturation values. Of the entire cohort, 10 patients were initiated on oral iron supplementation, and three were initiated on erythropoietin. Only one-third of the patients had iron biochemical studies performed despite demonstrating mean hemoglobin and hematocrit values below thresholds for anemic workup according to the 2000 NKF-DOQI guidelines, and one-fourth of the patients were initiated on replacement therapies (23). Post-transplant anemia has been identified as a common occurrence early after renal transplantation, and has increasingly been identified as a late post-transplant phenomenon (26–29,32). Both short-term and long-term PTA have poorly understood implications on morbidity and mortality of renal transplant patients, and it is undetermined whether aggressive management of anemia after renal transplant may improve long-term outcomes.
Cyclosporinemia and creatinine clearance were evaluated in both TPMT genotype groups. Unexpectedly, we observed that patients with one nonfunctional TPMT mutant allele had significantly higher cyclosporine whole blood trough concentrations than patients without detected TPMT mutations. Calcineurin inhibitors such as cyclosporine demonstrate concentration-dependent nephrotoxicity secondary to potent vasoconstriction of afferent arterioles of renal glomeruli (39,40). Creatinine clearance was examined after 30 days of azathioprine therapy and was similar between the two groups. Additionally, cyclosporinemia was not found to be an independent predictor of erythroid cell changes in both TPMT groups over the first month after renal transplant.
This study supports a previous report of nonfunctional TPMT mutant allele frequencies in African-Americans and Caucasians (15). Specific variant alleles differed between the two groups as previously described (15). Interestingly, TPMT*3B is a rare variant, and population frequencies of this variant have not been well described (15).
This retrospective pharmacogenetics study had limitations. This study encompassed a small, highly-selected study population. Stringent study inclusion criteria were employed and only those individuals who were able to tolerate AZA for 30 days duration were evaluated for associations between TPMT genotype and biochemical and clinical data. Consequently, this study population did not demonstrate a high frequency of adverse drug reactions including neutropenia and thrombocytopenia, in contrast to patients in other pharmacogenetic studies who were unable to tolerate AZA or 6MP and required reduced dosages or discontinuation of therapy (4,10–12,30). As a retrospective study neither intended to examine nor powered to determine differences in PTA, this study has identified that individuals with a detected TPMT mutation had a higher likelihood to have changes in erythroid indices that was not associated with AZA dose or cyclosporinemia. We assumed appropriate care and monitoring of iron, folate and vitamin B12 by a physician directing hemodialysis. Measurements of erythropoietin levels were not routinely performed at our institution, and erythropoietin resistance and deficiency were not evaluable. The number of patients who met inclusion criteria was small, and in order to detect a meaningful difference in major outcomes such as infection and rejection, a larger study group would be required. Measurements of TPMT enzyme activity and TGN concentrations were not routinely performed at our institution during kidney transplantation occurring during the 1990s. During this time, evidence accmulated to advocate genotyping for TPMT mutations and phenotypic evaluation of TPMT enzyme activity or TGN concentrations in individuals receiving AZA. Although AZA has a reduced role in transplantation today, the application of pharmacogenomic principles to identify individuals with genetic mutations and measurement of TGN concentrations remains a relevant issue for heart and lung transplant populations (8). Although phenotypic measurements of TPMT enzyme activity may be used to monitor development of AZA toxicity, evaluation of individuals with one nonfunctional TPMT mutant allele may offer advantages over phenotypic methods (3,16). Nonfunctional TPMT mutant alleles have been shown to correlate with low TPMT enzymatic activity allowing prospective and reliable identification; TPMT genotyping is not influenced by recent erythrocyte transfusions, hemodialysis, uremia or enzyme induction by administration of AZA (2,9,41). Finally, hematotoxicity may occur in the absence of TPMT*2, TPMT*3A or TPMT*3C variants due to presence of other rare TPMT variants or other factors including viral infections, drugs or environment (42). Prospective, well-controlled studies are needed to evaluate relationships between TPMT genetic mutations, AZA and cyclosporine and their potential contributions to post-kidney transplant anemia.
Significantly, this study has clinically relevant implications suggesting that azathioprine-induced toxicity is not limited to the leukocytes and neutrophils in individuals, especially in individuals with identified nonfunctional TPMT mutant alleles. Preemptive strategies to identify individuals possessing TPMT nonfunctional mutant alleles have been advocated in transplantation and non-transplantation populations prior to initiation of azathioprine or its active metabolite 6MP (3–5,12,24,43). Reduced azathioprine or 6MP doses have been advised for individuals possessing nonfunctional TPMT mutant alleles to prevent severe myelosuppression; however, specific guidelines have not been prospectively validated in any population, including transplantation (3–5,12,24,43). Prospective TPMT genotyping and phenotyping have been advocated by drug manufacturers to assist in identifying individuals with low or absent TPMT activity who are at risk for severe, life-threatening myelosuppression (44). Recently, the US Food and Drug Administration heard arguments to address TPMT genetic variability in product package information to address patients with poor or intermediate TPMT enzymatic activity and to encourage TPMT status testing. (45). It was further suggested that labeling could be improved by additional information indicating that patients with poor or intermediate TPMT enzymatic activity may tolerate only 1/10th to 1/2 of the average 6MP dose (45). To optimize patient care in the clinical setting to avoid azathioprine myelotoxicity and to evaluate thiopurine therapeutic efficacy, pharmacogenomic evaluation of TPMT genotype and phenotypic monitoring of TPMT enzyme activity or TGN concentration measurement should be used to augment regular hematological monitoring.
Conclusion
The use of pharmacogenomics to predict individual responses to different drugs may allow for immunosuppressive regimen tailoring. Further pharmacogenomic studies involving metabolism of immunomodulating agents used in transplantation may optimize immunosuppressive drug regimens, improve clinical outcomes and minimize drug toxicity. In addition, further prospective studies in post-transplant anemia may result in improved understanding of underlying anemic mechanisms and the relationships between short-term and long-term renal transplantation outcomes.
Acknowledgments
We thank Mariko Ono, Edith St. Pierre, Tuan Luu, Son Nguyen, Matthew Huentelman and Bill Farmerie for their excellent technical assistance and insight. This work was supported in part by an American College of Clinical Pharmacy (ACCP) fellowship grant.




