Cellular Immune Responses to Cytomegalovirus in Renal Transplant Recipients
Abstract
Control of CMV replication depends primarily on anti-CMV T lymphocyte activity. However, the functional T-cell responses to CMV in immunosuppressed solid organ transplant recipients are not well understood. In this study we employed cytokine flowcytometry (CFC) using pooled CMV peptides and viral lysates to detect CMV-specific T-cell responses in 17 healthy controls, 33 stable renal transplant recipients (Tx recipients) and 6 transplant recipients with active CMV infection (CMV(+)). We found that pooled peptides and lysates provide optimal detection of IFNγ production in anti-CMV CD8+ and CD4+ T cells, respectively. In both healthy controls and Tx recipients, CMV-specific T-cell levels strongly correlated with serostatus. Seropositive Tx recipients have significantly higher levels of CMV-specific CD8+ T-cell responses compared to healthy controls, which may signify an effort to control enhanced viral replication in immunosuppressed Tx recipients. In some individuals, absence of anti-CMV T-cell response may correlate with lack of viral clearance by ganciclovir therapy, even when CMV isolates are not ganciclovir resistant. Thus, monitoring cellular immunity with CFC along with viral load by PCR merits further exploration for identification of patients at the risk of developing CMV disease, tailoring prophylactic and therapeutic decisions and preventing complications.
Introduction
CMV infection is still the most common opportunistic infection following solid organ transplantation. CMV infection can initiate endothelial cell activation and vascular injury that may facilitate acute rejection, chronic rejection, atherosclerosis, transplant glomerulopathy or thrombotic microangiopathy (1). Donor (+) Recipient (−) serostatus and Thymoglobin® induction have been reported as the most important risk factors for CMV disease in the immediate post-transplant period (2). CMV infections are primarily controlled by antigen-specific cytotoxic (CD8+) and helper (CD4+) T lymphocytes. Studies on the precise role of CMV-specific T cells in immunocompromised solid organ recipients are limited, but should improve understanding of CMV-specific immune responses and allow better prevention and management of this infection.
The recent development of three major techniques—MHC tetramers, ELISPOT and cytokine flowcytometry (CFC)—has significantly increased our understanding of the role of T cells in regulating CMV and other viral infections. Tetramers detect HLA-restricted CD8+ or CD4+ T-cell response to a single peptide derived from viral proteins. HLA restriction, limited availability of defined peptide epitopes at present, especially for Class II tetramers, and lack of functional correlation prevents widespread clinical use of this technique. CFC enables non-HLA-restricted high-resolution, multi-parameter flow cytometric analysis of the nature and frequency of virus-specific cells, which cannot be obtained by ELISPOT. Whole blood when incubated with viral lysate leads to proteolytic processing and presentation of specific viral peptides to CD4+ and CD8+ T cells by the peripheral blood antigen presenting cells (APC), which stimulates cytokine secretion. Cytokine production by antigen-specific T cells, commonly IFNγ and TNFα, can be visualized by the use of Brefeldin A that inhibits granule secretion and leads to intracellular accumulation of cytokines (3).
Studies using MHC tetramers have reported that recovery of CMV-specific CD8+ T-cells reduces the risk of developing CMV disease in the immediate post-transplant period in allogeneic stem cell transplantation (SCT) (4), although SCT recipients with very few circulating lymphocytes cannot be directly compared to solid-organ recipients who have essentially normal lymphoid organs. Other investigators have reported similar frequencies of CMV-specific CD8+ T cells in immunosuppressed solid organ recipients using MHC tetramers as compared to healthy controls (5,6). Although several studies have examined the frequency of functional CD8+ and CD4+ responses to CMV infection in solid-organ recipients, as determined by CFC, the results are not conclusive (7–9). CMV viral lysate or individual HLA-specific viral peptide was used for detection of CD4+ and CD8+ T-cell responses in these studies. Recently, more sensitive techniques to determine CMV-specific T-cell responses using pooled overlapping peptides from CMV pp65 protein have been described (10). CFC using pooled overlapping peptides is independent of HLA type and does not require prior knowledge of T-cell epitopes. Data comparing the efficacy of pooled peptides and viral lysates to determine anti-CMV CD4+ and CD8+ T-cell responses in solid-organ transplant recipients are not available.
In this study we employed CFC using both pooled peptides from CMV pp65 protein and whole CMV viral lysates as antigens to detect CMV-specific T-cell responses. Using this technique, we investigated the functional T-cell responses to CMV infection in healthy controls, asymptomatic renal transplant recipients and renal transplant recipients with active CMV infections.
Methods
Subjects
Thirty-three asymptomatic renal transplant recipients (Tx recipients), six renal transplant recipients with CMV infection (CMV+ Tx recipients) and 17 normal healthy subjects (Healthy Controls) were included in the study after approval from the Institutional Review Board. The transplant patients received renal allografts between October 2001 and October 2003 at Cedars-Sinai Medical Center. Immunosuppressive regimen consisted of calcineurin inhibitor, mycophenolate mofetil and prednisone. Blood samples were collected 12 h after the last dose of immunosuppressants and processed for CFC assay within 4 h. Anti-viral prophylaxis consisted of oral-Ganciclovir (o-GCV) 1 g t.i.d. for 3 months for high risk (D+R−), o-Acyclovir (o-ACV) 800 mg qid for 3 months for moderate risk (D+R+ and D−R+) and 800 mg qid for low risk patients (D−R−). Anti-viral treatment for CMV disease consisted of reduction of calcineurin inhibitors, withholding MMF if required and o-val-GCV or intravenous ganciclovir (iv-GCV) for 2–4 weeks. Patients who had persistently high viral loads despite treatment with o-val-GCV/iv-GCV, were treated with 0.15 g/kg CMV-Ig. The frequency of CMV-PCR surveillance is bi-weekly for the first 3 months and subsequently once every 3 months for the first year.
Antigenic stimulation and cytokine flowcytometry
CFC assays using antigen-specific stimulation of whole blood was carried out as previously described (10,11). Sucrose density purified CMV viral lysate (CMV lysate) (Advanced Biotechnologies, Columbia, MD) or an overlapping peptide mixture of 138 peptides spanning the sequence of CMV protein pp65 (CMV peptides) (BD Biosciences, San Jose, CA) were used for antigenic stimulation (10). Each peptide consisted of 15 amino acid residues and overlaps the consecutive peptide by 11 amino acid residues.
Anti-CD28/49d (1 μg/mL, BD Biosciences) was added to heparinized blood for co-stimulation. 0.2 mL of blood was aliquoted into four microcentrifuge tubes and CMV peptides (2 μg/peptide/mL) or CMV lysates (1 μg/mL) were added. Positive and unstimulated negative control tubes employed Staphylococcal Enterotoxin B (SEB, 1 μg/mL, Sigma-Aldrich, St. Louis, MO) or no additive, respectively. Finally, Brefeldin A was added to all tubes at 10 μg/mL before starting incubation. Preliminary experiments showed that addition of Brefeldin A at the beginning enables maximum sensitivity for detection of CD8+ responses although the efficiency of detection of CD4+ responses was slightly reduced. After incubation for 6 h at 37°C, 20 μL of 20 mM EDTA was added to each tube to detach adherent cells. Red blood cells were lysed in FACS Lysis solution (BD Biosciences) for 10 min at room temperature. After permeabilization with 0.5 mL FACS Perm 2 solution (BD Biosciences), cells were stained with an antibody cocktail containing antibodies to CD3, CD8 (Caltag, Burlingame, CA) and IFNγ (BD Biosciences), and submitted for flow cytometry.
At least 100 000 CD3+ cells were acquired and analyzed with Cell Quest software (BD Biosciences). CD3+ cells were further gated as CD8+ or CD8− populations and separately analyzed for IFNγ production (12). CD3+ CD8− T cells were considered as practically equivalent to CD4+ population since similar results were obtained in CD3+CD8− and CD3+CD4+ cells in pilot experiments. Further, this gating strategy precludes double positive CD4+ CD8+ T cells from being included twice on separate CD4+ and CD8+ gates. Response regions were defined using positive and negative controls for each sample as described (13). Cytokine positive cells were determined using visual cluster analysis (14). Positive events in the antigen stimulation tubes were subtracted from unstimulated negative controls to obtain specific response for each sample. Results are expressed as the ratio of IFNγ+ CD4+ or IFNγ+ CD8+ cells to the total CD4+ or CD8+ population, respectively.
Quantitative CMV-PCR
A quantitative PCR for detection of CMV DNA previously developed in our laboratory was used for all PCR tests (15). Briefly, total DNA was isolated from EDTA blood using a DNA extraction kit (Qiagen, Valencia, CA) and 500 ng of total DNA was used per CMV-PCR. PCR was performed using a thermal cycler (Perkin Elmer Cetus, Norwalk, CT). The CMV-specific primers used have been described previously (15) and were specific for the immediate early gene of CMV. After separation of the PCR product by PAGE followed by ethidium bromide staining, the intensity of PCR products in test DNA samples was compared with those in the control plasmid, and the copy number in test DNA samples were calculated. CMV-PCR assay was performed twice for each sample, and both results were similar. The sensitivity of our CMV-PCR assay is five copies of CMV DNA/500 ng total DNA.
ELISA for CMV-IgG
CMV serostatus was determined by a commercial CMV IgG ELISA Kit (BioCheck, Inc., Burlingame, CA) according to the manufacturer's instructions.
UL97 mutation analysis
Mutation analysis was performed at the University of Washington Laboratory Medicine/Clinical Molecular Virology Laboratory by PCR sequencing.
Statistical analysis
The two-sided Mann-Whitney U test was used for analysis of differences between groups; for correlations, the Spearman non-parametric correlation test was used. p-values less than 0.05 were considered statistically significant.
Results
Detection of T-cell responses to CMV antigens by CFC
Stimulation of whole blood with CMV lysate or CMV peptides for 6 h in the presence of Brefeldin A leads to intracellular accumulation of IFNγ. Figure 1 shows a typical result of anti-CMV CD4+ and CD8+ response in a whole blood CFC assay. Among CD8+ T cells stimulated with CMV antigens, peak IFNγ production was observed in CD8dim cells.
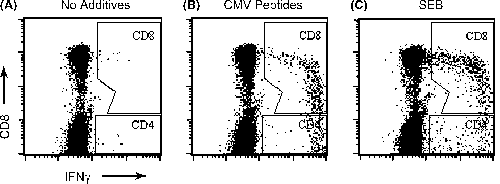
Detection of CMV-specific CD8+ and CD4+ T cells by cytokine flowcytometry. Shown are typical responses from a seropositive transplant recipient in the absence of antigen (A), with CMV antigen (B) and Staphylococcal Enterotoxin B (C). Following data acquisition, samples were gated on CD3+ events, and the percentages of IFNγ+ CD8+ and IFNγ+ CD4+ events were determined, respectively, as shown, after subtracting percentage positive events in unstimulated controls. Axes show log fluorescence intensity.
For all further experiments described below, unless specified otherwise, CD8+ and CD4+ responses measured using CMV peptides and CMV lysate respectively, are shown since these antigens provide optimal conditions for detection of antigen-specific T cells in the CFC assay.
CMV-specific responses in healthy controls and asymptomatic transplant recipients
CMV-specific T-cell responses were measured in 17 healthy controls (10 seropositive/7 seronegative), 33 asymptomatic Tx recipients (24/9) and 6 CMV+ Tx recipients (3/3). Three of nine seronegative asymptomatic Tx recipients had seropositive donors. In 33 asymptomatic transplant recipients, the post-transplant duration at the time of the assay were 168 ± 198 days (Range 6–732 days) for seropositive and 131 ± 131 days (range 17–368 days) for seronegative Tx recipients. Of these 33 patients, only two seropositive recipients had documented evidence of CMV infection prior to the date of CFC assay. In healthy controls and Tx recipients, a strong correlation was noted between serostatus and IFNγ+ CD8+ or IFNγ+ CD4+ levels. IFNγ+ CD8+ cells in all seronegative healthy controls and Tx recipients were below 0.1% (Figure 2A). Most seropositive healthy controls and Tx recipients had higher CD8+ responses to CMV than those who were seronegative. Compared to healthy controls, Tx recipients showed significantly higher levels of IFNγ positive CD8+ cells (1.95 ± 1.77% vs. 0.51 ± 0.71%, p = 0.004). All seronegative healthy controls and Tx recipients showed CD4+ responses below 0.05% (Figure 2B). CMV-specific CD4+ levels in most seropositive healthy controls and Tx recipients were greater than 0.05% and similar in both groups (1.27 ± 1.32% and 1.04 ± 1.51%, respectively) (Figure 2B).
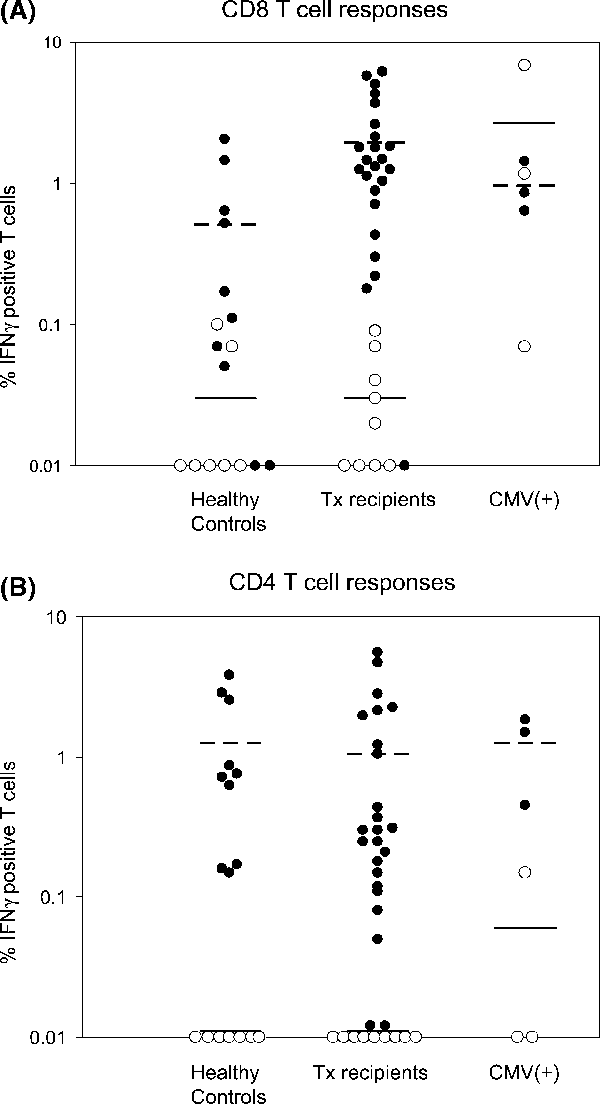
CMV-specific T-cell responses in healthy controls, asymptomatic transplant recipients (Tx recipients) and Tx recipients with CMV infection (CMV(+)). IFNγ responses to pooled CMV pp65 peptides and CMV lysate are shown for CD3+ CD8+ T cells (A) and CD3+ CD4+ T cells (B), respectively. Open circles (○) and closed circles (•) denote seronegative and seropositive subjects in each group. Mean values for seropositive subjects (dotted lines) and seronegative subjects (solid line) are also shown.
We next attempted to correlate CD8+ and CD4+ responses in seropositive healthy controls and Tx recipients. Higher levels of CMV-specific CD4+ T cells compared to CD8+ T cells were noted in healthy controls whereas CD8+ T cells were dominant in Tx recipients. CD8+ responses similar to or higher than CD4+ responses were observed in 19/24 seropositive Tx recipients (1.95 ± 1.77% vs. 1.04 ± 1.51%) (Figure 3B), while only three healthy controls showed higher CD8+ responses than CD4+ responses (0.51 ± 0.71% vs. 1.27 ± 1.32 (Figure 3A). There was no significant correlation between CMV-specific CD8+ and CD4+ T-cell frequency in both groups.

Relationship between CMV-specific CD8+ and CD4+ T cells in 10 seropositive healthy control (A) and 24 seropositive transplant recipients (B).
CMV-specific responses in Tx patients with CMV infection
CMV levels in peripheral blood were monitored by CMV-PCR in 5/6 patients who developed CMV infection except one (patient no. 4), who had biopsy-proven gastrointestinal CMV disease but did not develop viremia. Treatment with valganciclovir led to prompt control of infection in all subjects except patient no. 6 (Figure 4).
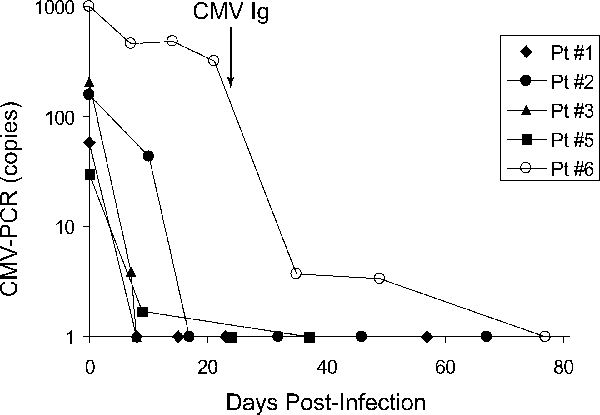
Longitudinal analysis of viral load and correlation with CMV-specific cellular immunity in five transplant recipients who developed CMV infection. Patient no. corresponds to that in Table 1.
Samples obtained from these patients at time points indicated in Table 1 were examined for anti-CMV T-cell responses. CMV serostatus in samples obtained pre-infection were confirmed by CMV-IgG ELISA. Three seropositive patients had substantial CD4+ and CD8+ responses (Table 1, closed circle in Figure 2, patient nos. 1, 2 and 3 in Figure 4) and showed prompt clearance of CMV DNA after anti-viral therapy. Patient nos. 4 and 5 who were seronegative showed predominant CD8+ responses with minimal CD4+ responses (open circle in Figure 2, patient no. 5 in Figure 4) and recovered in 1–2 weeks after ganciclovir therapy. The remaining seronegative patient (patient no. 6) did not have detectable T-cell responses at 20 days after PCR positivity, and CMV DNA was detectable for more than 3 weeks despite reduction of immunosuppression and oral valganciclovir therapy at 900 mg b.i.d. (Figure 4). UL97 resistance testing did not show any mutations associated with ganciclovir resistance. This patient was then treated with CMV Ig at 0.15 g/kg that resolved the infection.
| Patient No. | Age | Detection of anti-CMV T cells | Recipient | IFNγ+ T cells | ||
|---|---|---|---|---|---|---|
| Weeks post-Tx | Days post-infection | Serostatus pre-infection | CD8+ | CD4+ | ||
| 1 | 46 | 21 | 0 | + | 0.85 | 0.45 |
| 2 | 55 | 23 | 9 | + | 0.64 | 1.49 |
| 3 | 40 | 28 | 34 | + | 1.43 | 1.83 |
| 4 | 63 | 20 | 41 | − | 1.17 | 0.15 |
| 5 | 28 | 28 | 24 | − | 6.81 | 0.01 |
| 6 | 58 | 74 | 20 | − | 0.07 | 0.01 |
Comparison of peptides and lysates for stimulation of CMV-specific T cells
Viral antigens used for antigenic stimulation directly affect the efficiency and sensitivity of in vitro tests for detection of viral specific T cells. The efficacy of CMV peptides and lysates for stimulation of CMV-specific T cells in CFC is not well defined. In this study, most blood samples were stimulated with both CMV peptides and lysates and the results were compared. CMV-specific CD4+ or CD8+ responses to both antigenic preparations in 10 seropositive healthy controls and 24 seropositive Tx recipients are shown in Figure 5. In healthy controls, CD8+ responses to CMV peptides were similar to or higher than responses to CMV lysate in eight of nine samples (0.51 ± 0.71 vs. 0.08 ± 0.08%) whereas the reverse was true for CD4+ cells in all samples (0.23 ± 0.19% vs. 1.27 ± 1.32%). CD8+ responses in seropositive Tx recipients were also higher when stimulated by CMV peptides compared to CMV lysates (1.95 ± 1.77% vs. 0.30 ± 0.67%). However, unlike healthy controls, CD4+ responses to lysates in Tx recipients were not significantly different from peptide responses (1.04 ± 1.51% vs. 0.93 ± 1.26%).
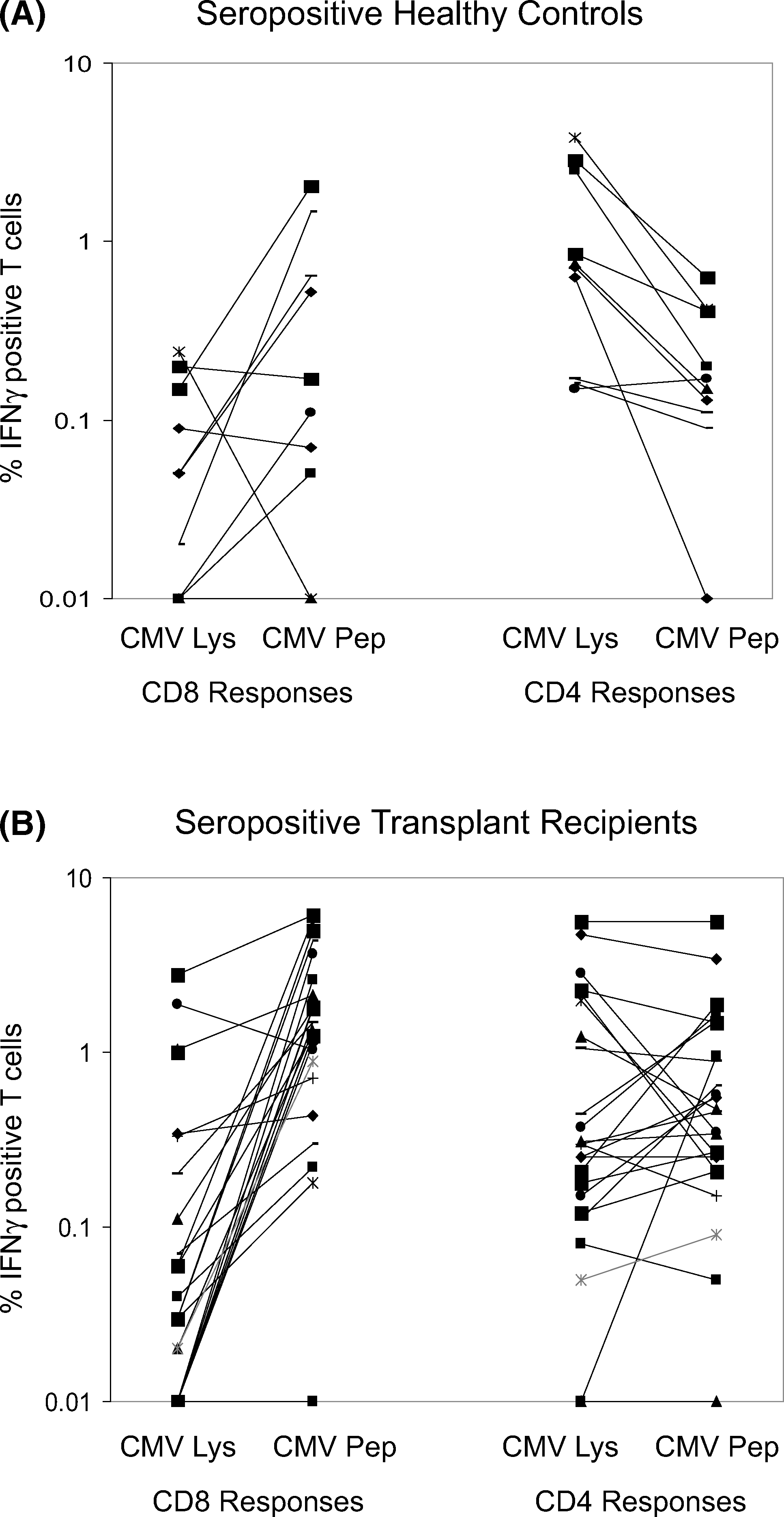
Comparison of peptides and lysates for stimulation of CMV-specific CD8+ and CD4+ T cells in seropositive healthy controls (A) and seropositive transplant recipients (B). IFNγ responses to CMV lysate versus CMV pp65 peptides are shown for CD8+ and CD4+ T cells for each group.
Discussion
In this study, we investigated the CD8+ and CD4+ T-cell responses to CMV in three groups of subjects—healthy controls, asymptomatic renal transplant recipients and renal transplant recipients who developed CMV infection using a CMV-specific CFC assay. Although our understanding of how T cells control CMV and other viral infections has increased considerably due to development of new techniques, such as MHC tetramers, ELISPOT and CFC, the results often do not correlate well. This is partly due to technical differences between methods. CFC has several advantages—it is HLA independent, provides a functional readout, allows simultaneous analysis of multiple cell populations and does not require prior knowledge of HLA specific T-cell epitopes (10). The efficiency and sensitivity of CFC, however, is critically dependent on the type of viral antigen used for the assay. Data comparing the efficacy of various viral antigens such as single or pooled peptides or viral lysates to determine anti-CMV CD4+ and CD8+ T-cell responses have not been established, especially in solid-organ transplant recipients. In this study, pooled CMV pp65 peptides and viral lysates prepared from sucrose-density purified AD169 virions were used to compare CD8+ and CD4+ T-cell responses since pp65 is the major component of both antigenic preparations and another major antigenic protein in CMV, IE-1, is excluded.
All samples were stimulated with pooled CMV peptides, CMV lysates and SEB in our study. In CD8+ T cells, maximal IFNγ production in response to antigenic stimulation was seen in CD8dim T cells, which has been previously shown to contain larger proportion of CD27−, CD45RO+ and CD62L− memory T cells (16). SEB, which directly binds TCR, showed maximal IFNγ production in CD8hi T cells, possibly due to the fact that there is no preferential stimulation for memory T cells. In healthy controls, higher sensitivity to detect CMV-specific CD8+ and CD4+ T cells was obtained using pooled peptides and CMV lysates, respectively. These results are consistent with a previous report (12). Increased sensitivity for detection of CMV-specific CD8+ T cells using pooled peptides observed in this study is probably due to direct loading of peptides on to HLA Class I molecules expressed on cell surface, which leads to more efficient presentation to CD8+ T cells compared to cross-priming, which is known to be inefficient and leads to lower CD8+ T-cell responses (17). Although the reason(s) for more preferential stimulation of CD8+ and CD4+ cells by different viral antigens in CFC are not fully understood, it is clear that pooled peptides and CMV lysate provide optimal stimulation of anti-CMV CD8+ and CD4+ T cells, respectively. Therefore, we used these conditions for further data analysis and interpretation.
Our results clearly show that seronegative individuals had virtually no T-cell responses predisposing them to more severe disease, while most seropositive individuals among healthy controls and Tx recipients showed specific T-cell responses to CMV antigens. The association of CMV serology with CMV-specific CD8+ and CD4+ responses in normal individuals has also been reported by Sester et al. (18). Interestingly, seropositive Tx recipients showed significantly higher levels of CD8+ T cells compared to normal controls in our study, while CMV-specific CD4+ levels were similar to normal individuals. After primary infection, herpes viruses such as CMV enter a state of lifelong latency and chronic latent infection, which induces ongoing T-cell efforts to control persistent viral replication (1). Breakdown of this equilibrium leads to active CMV infection resulting in CMV disease. It is obvious that immunosuppressive drugs directly inhibit T-cell function in transplant recipients. Our results suggest that suppressed T-cell activity in this patient population leads to enhanced CMV replication, resulting in CMV-specific CD8+ T-cell expansion despite immunosuppression.
In contrast to elevated CD8+ levels in Tx recipients, CMV-specific CD4+ levels in these subjects were similar to normal individuals. The significance of this finding is not completely clear. It is possible that CD4+ T-cell expansion may be lower than cytotoxic T-cell proliferation in immunosuppressed individuals due to reduced proliferative capacity or greater susceptibility to apoptosis (19). Alternatively, this may be due to immunosuppressive drug therapy causing sub-optimal antigen processing and presentation by APC (20). This hypothesis is also supported by the fact that in immunosuppressed individuals, CD4+ responses to lysates, which require antigen processing by APC, were similar to peptides, which is different from normal individuals whose CD4+ cell responses were higher to CMV lysates than pooled peptides. In fact, CMV-specific CD4+ responses were elevated in seropositive Tx recipients versus seropositive normal individuals when the results obtained by pooled peptides were used for the analysis (Figure 5). Thus, it is likely that absence of elevation of CD4+ cell responses in Tx recipients compared to normal individuals, based on the responses to CMV lysates, underestimates the actual CD4+ T-cell responses in Tx recipients. However, a definitive answer needs further study.
Sester et al. (9) have reported dominance of CMV-specific CD4+ T cells in immunosuppressed individuals, which is inconsistent with our observations. These investigators used CMV-infected fibroblast lysates to compare CD4+ and CD8+ responses, which could explain the disparity. As shown in Figure 5, anti-CMV CD8+ responses were also lower than CD4+ responses in our subjects when CMV lysates were used as antigens. In addition to inefficient stimulation of CD8+ T cells, fibroblast lysates, which contain all alpha, beta and gamma proteins in addition to CMV structural proteins found in CMV viral lysates, may have led to an over-estimation of CD4+ responses in their study. CD4+ responses determined using CMV viral lysates allow more rational comparisons with CD8+ responses measured with pooled CMV peptides since pp65 is the major component of both antigenic preparations and IE-1 is excluded. Since pooled peptides used in our assay measure CD8+ responses to CMV pp65 protein only, it is possible that cumulative responses to all CMV structural proteins would have even higher magnitude. Higher CD8+ T-cell levels may, therefore, signify clandestine viral replication and increased efforts by the cellular immune system to control viral expansion in transplant recipients as mentioned above.
In three seropositive patients who developed CMV infection, we detected both CMV-specific CD8+ and CD4+ cells. All three patients cleared CMV within 1–2 weeks following o-GCV therapy. In three seronegative patients who developed primary infection, two showed significant CD8+ responses and quickly recovered from the infection after anti-viral treatment. In contrast, the third seronegative patient (patient no. 6 in Figure 4) whose CD8+ responses were minimal, showed persistence of CMV DNA for more than 3 weeks despite ganciclovir therapy. This individual was subsequently treated with CMV-Ig, which rapidly cleared the infection. Patients who fail to respond to GCV may have UL97 or seldom, UL54 mutations causing ‘GCV-resistant’ CMV (21) but the frequency of these mutations are very low (22). Since this individual did not have any UL97 mutations, it is very likely that the lack of response to the GCV therapy observed in this patient was due to the lack of cellular immune response, not drug resistance. This result also suggests that humoral immunity by passive administration of anti-CMV antibodies compensated for deficient cellular immunity. Gratama et al. have shown that CMV-specific CD8+ T cells frequently reach supranormal levels in SCT recipients who have resolved their CMV activations whereas CMV disease developed in all patients who failed to recover CMV-specific CD8+ T cells, in spite of GCV treatment (4). These results and ours strongly suggest that the absence of CD8+ responses leads to prolonged CMV infection and disease that are unresponsive to GCV therapy and usually requires adjunctive therapy. It should be noted that anti-CMV CD4+ response in the three seronegative patients including two patients with anti-CMV CD8+ response were minimal or absent in the immediate post-infection period. In a study investigating the kinetics of primary CMV infection, Gamadia et al. have shown that in symptomatic individuals the CMV-specific effector memory CD4 T-cell response was delayed and only detectable after antiviral therapy suggesting that formation of effector-memory CD4 T cells is necessary for recovery of infection (23).
Overall, our results imply that immunosuppression, though sufficient to prevent alloreactivity, does not affect anti-CMV T-cell immunity, which appears to be enhanced in response to clandestine viral replication. Our study demonstrated that CFC provides a reliable, objective measure of anti-CMV immunity, which can detect sub-clinical viral replication prior to onset of viremia and can predict those likely to develop GCV unresponsiveness. Data from this study also indicate that monitoring anti-CMV T-cell responses with CFC coupled with CMV-PCR may be useful to better predict, prevent and manage CMV infections. Further, it may be possible to tailor CMV prophylaxis or pharmacotherapy for individual patients based on CMV-specific humoral and cellular immunity.
Acknowledgments
We thank Lily King for her expert advice and Lara Hilo for technical assistance.




