Chronic melatonin treatment and its precursor L-tryptophan improve the monoaminergic neurotransmission and related behavior in the aged rat brain
Abstract
Abstract: Melatonin has an important role in the aging process as a potential drug to relieve oxidative damage, a likely cause of age-associated brain dysfunction. As age advances, the nocturnal production of melatonin decreases potentially causing physiological alterations. The present experiments were performed to study in vivo the effects of exogenously administered melatonin chronically on monoaminergic central neurotransmitters serotonin (5-HT), dopamine (DA) and norepinephrine (NE) and behavioral tests in old rats. The accumulation of 5-hydroxy-tryptophan (5-HTP) and L-3,4-dihydroxyphenylalanine (DOPA) after decarboxylase inhibition was used as a measure of the rate of tryptophan and tyrosine hydroxylation in rat brain. Also neurotransmitters 5-HT, DA and NE and some metabolites were quantified by HPLC. In control rats, an age-related decline was observed in neurochemical parameters. However, chronic administration of melatonin (1 mg/kg/day, diluted in drinking water, 4 wk) significantly reversed the age-induced deficits in all the monoaminergic neurotransmitters studied. Also, neurochemical parameters were analyzed after administration of melatonin biosynthesis precursor L-tryptophan (240 mg/kg/day, i.p., at night for 4 wk) revealing similar improvement effects to those induced by melatonin. Behavioral data corresponded well with the neurochemical findings since spatial memory test in radial-maze and motor coordination in rota-rod were significantly improved after chronic melatonin treatment. In conclusion, these in vivo findings suggest that melatonin and L-tryptophan treatments exert a long-term effect on the 5-HT, DA and NE neurotransmission by enhancing monoamine synthesis in aged rats, which might improve the age-dependent deficits in cognition and motor coordination.
Introduction
Besides the known functions of melatonin (N-acetyl-5-methoxytryptamine) as a transducer of the light signal, melatonin may have an important role in the aging process due to its ability to reduce oxidative damage due to aging [1]. It is well known that as age advances, the nocturnal production of melatonin decreases in human beings and animals [2–6 among others]. Melatonin and its metabolites have strong antioxidant properties which act as endogenous buffering agents against oxidative stress [7–9]. This suggests there may be physiological alterations when melatonin’s secretion is reduced on old age. This may in part relate to the free radical theory of aging [10]. Besides being a direct scavenger of radicals, melatonin has indirect antioxidative actions as well [11, 12]. Oxidative damage is considered a likely cause of age-associated brain dysfunction because the brain is believed to be particularly vulnerable to oxidative stress due to a relatively high rate of oxygen free radical generation without suitable levels of antioxidant defenses compared with other somatic tissues [1, 13, 14]. Exogenous melatonin may prevent the increased production of age-related lipid peroxidation products [15] and might have a potential role for retardation of age-related oxidative events.
Neuronal death during brain aging results, at least in part, from the disruption of synaptic connectivity caused by oxidative stress. Chronic melatonin administration to rats improved the dendritic stability [16] and increased hippocampal pyramidal cell number [17, 18], indicating a protective effect of melatonin against hippocampal neurodegeneration. Moreover, it has been shown that melatonin as an antioxidant displays pronounced neuroprotective effects in some age-associated neurodegenerative diseases [1, 19–21]. Also, melatonin metabolites shows neuroprotective effects in many experimental models systems [8, 22]. Monoaminergic neuron systems appear to be vulnerable to reactive oxygen species (ROS), which have also been implicated in some neurodegenerative diseases and intoxication by drugs [23, 24]. Melatonin protects against ROS-mediated neuronal degeneration models. Particularly, these properties have been demonstrated on serotonergic and dopaminergic neurons [25–27].
Monoaminergic neurotransmitters are involved in many functions throughout the brain, which change during aging. Two of the neurotransmitters closely related with cognitive function are 5-hydroxytryptamine (5-HT; serotonin) and dopamine (DA). Both 5-HT and DA are synthesised and released in nerve terminals of brain regions such as the hippocampus and the corpus striatum, which are involved in the organization of diverse cognitive processes, including learning and memory [28].
Therefore, the present study assessed the in vivo effects of chronic melatonin treatment on brain synthesis and turnover of monoamines 5-HT, DA and norepinephrine (NE) in aged rats. The accumulation of 5-hydroxytryptophan (5-HTP) and L-3,4-dihydroxyphenylalanine (DOPA) was used as a measure of the rate of tryptophan or tyrosine hydroxylation, respectively, in the brain in vivo, and also as a measure of monoaminergic neurotransmission activity. Also, intraneuronally stored 5-HT, DA and NE and some metabolites were quantified. The chronic effects of L-tryptophan (a 5-HT and melatonin precursor) on monoaminergic systems were also analyzed. In addition, behavioral tests were performed in aged rats using the eight-arm radial maze and rota-rod to evaluate the effects of chronic melatonin on working memory and motor ability.
Material and methods
Animals
Young (3 months, 220–240 g, n = 6) and old (20 months, 400–450 g, n = 18) male Sprague-Dawley rats (Charles-River, Barcelona, Spain) were used. The animals were individually housed under controlled environmental conditions (20º± 2°C; 70% humidity, and 12-hr light/dark cycle, lights on at 08.00 hr daily), with free access to standard food (Panlab A04, Barcelona, Spain) and tap water. The animals were handled daily for several days before the behavioral and neurochemical experiments to reduce stress during testing. The rats were treated in accordance with the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes (Directive 86/609/EEC) and in agreement with the Bioethical Committee of the University of Balearic Islands.
Drug treatments
Rats were chronically treated with melatonin (1 mg/kg/day, diluted in the drinking water) during 4 wk. Daily preparation of drinking water containing melatonin was adjusted for weight and volume of liquid consumed by the rat. To increase melatonin solubility in water, it was previously solubilized in ethanol (20 mg in 10 mL) to a final concentration of 1% alcohol in the bottle. Control animals also received 1% alcohol in the drinking water. For the chronic treatment with L-tryptophan, a group of rats were injected daily for 4 wk (240 mg/kg in saline, i.p. at 20:00 hr). Chronic treatment with saline to old rats (1 mL/kg, i.p., n = 5) did not modify the synthesis of monoamines compared to the above mentioned control old group rats (e.g. F(1,9) = 0.109 P = 0.749 and F(1,8) = 0.079 P = 0.7860 for 5-HTP and DOPA, respectively, in hippocampus, see Figure legends for statistical comparison by ANOVA). For that only one control group was considered to statistical evaluation of all results. The doses of melatonin and L-tryptophan and the administration time selected were chosen from previous studies [29–31].
After the chronic treatments, rats received NSD 1015 (Sigma-Aldrich, Madrid, Spain) (100 mg/kg, i.p. at 9:30 hr) and then were killed by decapitation after another 30 min, to measure the in vivo accumulation of 5-HTP and DOPA during 30 min (see below). The brains were quickly removed and dissected on an ice-cold plate to separate hippocampus (60–65 mg) and striatum (caudate-putamen) (15–20 mg). The selected brain regions were immediately frozen in liquid nitrogen, and stored at −80°C until the assays.
Tryptophan hydroxylase and tyrosine hydroxylase activities
The in vivo activity of TPH (tryptophan-5-monoxygenase, EC 1.14.16.4) and TH (tyrosine-3-monoxygenase, EC 1.14.16.2) rate-limiting enzymes in the pathway for the synthesis of 5-HT and catecholamines, respectively, were determined simultaneously by measuring the accumulation of 5-HTP and DOPA within 30 min after inhibition of the aromatic L-amino acid decarboxylase (EC 4.1.1.28) by a maximally effective dose of NSD 1015 (3-hydroxybenzylhydrazine HCl, 100 mg/kg, i.p.) [32]. Administration of the L-amino acid decarboxylase inhibitor, shortly before sacrifice, enabled us not only to determine tryptophan hydroxylase (TPH) or tyrosine hydroxylase (TH) activity in different brain areas, but also to quantify the pool of 5-HT, DA or NE unaffected by recent synthesis, primarily intraneuronally stored transmitter. Moreover, the metabolite levels allow to analyze the recent use of these neurotransmitters. The 5-HTP and DOPA accumulation method is the most commonly used assay system to monitor the in vivo rate of tryptophan and tyrosine hydroxylation in the brain. Tryptophan hydroxylase is now known to exist in two isoforms: TPH-1 is mainly expressed in the pineal gland and in gut enterochromaffin cells [33], and TPH-2 is preferentially expressed in the brain where it plays a fundamental role in 5-HT synthesis [34]. Therefore, the present data on the synthesis of 5-HTP refers to the activity of the TPH-2 isoform. The effects induced by melatonin and L-tryptophan on brain were measured in areas enriched in 5-HT and NE (hippocampus) or 5-HT and DA (corpus striatum) nerve terminals. As tyrosine hydroxylation is a common step in the synthesis of catecholamines, the accumulation of DOPA in the striatum preferentially indicated synthesis of DA, and that in the hippocampus was mainly related to the synthesis of NE. The 5-HTP and DOPA formed from endogenous tryptophan and tyrosine, respectively, and the other compounds were then determined by high-performance liquid chromatography (HPLC) with electrochemical detection (ED).
Brain samples and chromatographic (HPLC-ED) analyses
After 4 wk of different treatments, the rats were killed by decapitation (around 10:00 hr) and hippocampus and striatum were immediately frozen in liquid nitrogen, and stored at −80°C until the assays. Brain regions were placed individually into cold tubes containing 1 mL of 0.4 m HClO4, 0.01% K2EDTA and 0.1% Na2S2O5, and then homogenized with an Ultra-Turrax homogenizer (Type Tp 18/10, Janke and Kunkel, Germany). The homogenate was centrifuged at 40,000 g for 15 min at 4°C. The resulting supernatant was filtered through 0.45 μm syringe filters (Spartan-3, Sigma-Aldrich) and then various aliquots were injected into the HPLC system. The contents of precursor amino acids (5-HTP and DOPA), monoamines (5-HT, DA and NE) and metabolites (mainly 5-hydroxy-indole acetic acid, 5-HIAA; homovanillic acid, HVA and 3,4-dihydroxyphenylacetic acid, DOPAC) were determined as described previously [35] (for representative chromatographic analyses from the rat corpus striatum see [36]; also other brain regions in [37]. Briefly, aliquots of the purified supernatants (5–10 μL depending on the brain region) were subjected to HPLC on a reversed-phase column (Spherisorb S3 ODS1 C18; 3-μm particle size range; 4.6 mm × 10 cm) coupled to a Tracer ODS2 C18 (2–5 μm particle size range) pre-column (Teknokroma, Barcelona, Spain). The mobile phase consisted of 0.1 m KH2PO4; 2.1 mm octane sulfonic acid; 0.1 mm K2EDTA; 2 mm NaCl and 12% (vol/vol) methanol (pH 2.7–2.8, adjusted with 85% H3PO4), which were pumped at a flow rate of 0.8 mL/min with a Waters M-510 solvent delivery system (Waters, Barcelona, Spain). The compounds were detected electrochemically by means of a cell with a glassy working carbon electrode with an applied oxidation potential of +0.75 V against an in situ Ag/AgCl reference electrode (ISAAC; Waters Concorde Electrochemical Detector). The current produced was monitored by use of an interphase (Waters busSAT/IN Module) connected to a digital PC. The concentrations of 5-HTP, DOPA, monoamines neurotransmitters and metabolites in a given sample were calculated by interpolating the corresponding peak height into a parallel standard curve using the software Millennium32 (Waters).
Radial maze test
The effect of chronic treatment with melatonin on working memory in aged rats was tested by means of the eight-arm radial maze. The maze (Panlab, S.L., Barcelona, Spain) consisted of an octagonal central platform (32 cm diameter) with eight equally spaced radial arms (50 cm long, 12 cm wide). To test radial maze memory task, rats were allowed to make an arm choice to obtain food pellets until all eight arms had been visited or 20 min had elapsed. Animals (control and melatonin-treated rats n = 13) were previously submitted to 48 hr fasting to achieve a convenient motivational level. Thus, trials were judged complete when rats had chosen all eight baited arms or spent 20 min in the trial (time to achieve performance). The sum of nonvisited arms and re-entry into arms was scored as a working memory error. All procedure was performed during the light period.
Rota-rod test of motor coordination
To examine the effects of chronic treatment with melatonin in aged rats on motor ability and balance was used the rota-rod test. Rats (control and melatonin-treated rats n = 13) were trained to remain on a rota-rod apparatus (Panlab, S.L.) during several training sessions for adaptation. Training was considered sufficient when the performance of the animals was stabilized. Then, rats were placed on an accelerating rota-rod (increasing in speed from 4 to 40 cm/s during a 30 s period). The length of time that the rats remained on the rota-rod was recorded and taken as measure of competency. Each rat performed five separate trials and the results were averaged. All procedure was performed during the light period.
Drugs and reagents
The following drugs and reagents were used: 3-hydroxybenzylhydrazine HCl (NSD 1015; Sigma chemical company, St. Louis, MO, USA); melatonin (N-acetyl-5-methoxytryptamine; Sigma chemical); L-tryptophan-methyl ester (Sigma chemical). Other reagents were from Sigma or Amersham.
Statistics
Results are expressed as means ± S.E.M. of the number of determinations. One-way ANOVA followed by least significant difference (LSD) test was used for the statistical evaluations. The significance level chosen was P ≤ 0.05.
Results
The synthesis and metabolism of 5-HT was measured in two brain region enriched in serotonergic nerve terminals: hippocampus and striatum. 5-hydroxytryptophan accumulated during 30 min after the inhibition of decarboxylase enzyme with NSD, reflects the rate of 5-HT synthesis. Fig. 1 shows the accummulation of 5-HTP, the pool of 5-HT and the metabolite 5-HIAA levels in the hippocampus and the striatum of young and old control rats, and those in old rats after chronic treatment with exogenous melatonin and L-tryptophan. In control animals, an age-dependent variation in tryptophan hydroxylation was clearly observed, with greater accumulation of 5-HTP in young respect to the old rats (1.94 and 1.78-fold higher in hippocampus and striatum, respectively). The 5-HT content followed a similar pattern reaching the maximum in young rats (1.53 and 1.54-fold higher in the same regions, respectively). Also the metabolite 5-HIAA showed the maximum levels at younger rats respect to the old ones (2.14 and 1.86-fold higher in the hippocampus and striatum, respectively) (Fig. 1).
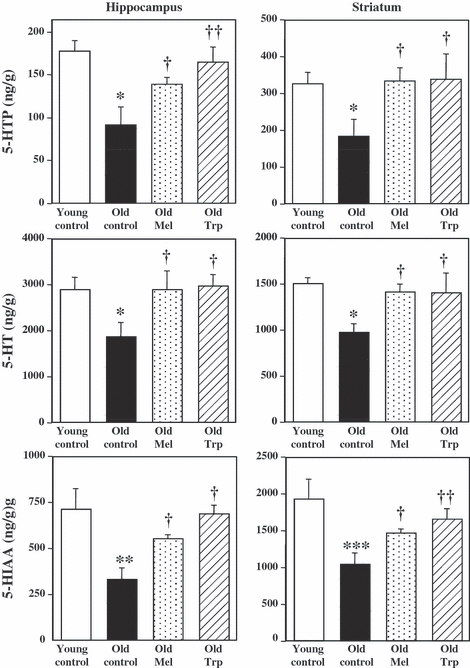
Chronic effects of melatonin and L-tryptophan on the accumulation of 5-hydroxytryptophan (5-HTP), after decarboxylase inhibition (NSD 1015; 100 mg/kg, i.p.) during 30 min, serotonin (5-HT) tissue content and the levels of 5-hydroxy-indole acetic acid (5-HIAA) metabolite, in ng/g of wet tissue, in hippocampus and striatum. Groups of rats were chronically treated with vehicle (Young controls, n = 6 and Old controls, n = 6), melatonin (1 mg/kg/day in drinking water, for 4 wk, n = 6) and L-tryptophan (240 mg/kg, i.p., for 4 wk, n = 6). No significant differences were detected between two old control groups by ANOVA [F(1,9) = 0.109 P = 0.749; F(1,10) = 2.184 P = 0.17; F(1,10) = 2.1162 P = 0.172 for 5-HTP, 5-HT and 5-HIAA, respectively, in hippocampus and F(1,10) = 0.584 P = 0.4623; F(1,8) = 0.908 P = 0.3684; F(1,10) = 0.403 P = 0.539 for 5-HTP, 5-HT and 5-HIAA, respectively, in striatum]. Columns (ng/g) are the mean ± S.E.M. derived from the analysis of n animals per group. One way ANOVA followed by least significant difference (LSD) test was used for statistical evaluation: *P < 0.05, **P < 0.01, ***P < 0.001 when compared with the young control group; †P < 0.05, ††P <0.01, †††P < 0.001 compared to the old control group.
In old rats, chronic melatonin treatment (1 mg/kg/day, 4 wk) increased the synthesis of 5-HTP in hippocampus (53%) and striatum (83%), compared to old control rats. Similarly, the chronic treatment with L-tryptophan, a precursor of both 5-HT and melatonin, also increased serotonin synthesis in the hippocampus (81%), and striatum (84%) of rats when compared with old controls. The mean value of 5-HT content followed a similar pattern, it was increased after melatonin treatment (54% and 45% in hippocampus and striatum of old rats, respectively) and chronic L-tryptophan (57% and 44% in hippocampus and striatum, respectively) versus old control rats. Also the levels of 5-HIAA metabolite were increased after the treatment of old rats with melatonin (65% and 41% in hippocampus and striatum, respectively) and L-tryptophan (105% and 59% in hippocampus and striatum, respectively) indicating the acceleration of 5-HT synthesis and metabolism in the old animals treated with melatonin and L-tryptophan (Fig. 1).
The synthesis of DA was measured in striatum, a brain region enriched in dopaminergic nerve terminals. L-3,4-dihydroxyphenylalanine accumulated during 30 min after the inhibition of decarboxylase enzyme with NSD in striatum, reflects the rate of DA synthesis. Fig. 2 shows the DOPA accumulated, the content of DA, as well as the levels of metabolite HVA in the striatum of young and old control rats, and those in old rats chronically treated with exogenous melatonin or its precursor L-tryptophan. In control animals, a variation in tyrosine hydroxylation with aging was clearly observed, with greater accumulation of DOPA in young respect to old rats (1.51-fold higher). Also, the DA content in the striatum reached higher values in young than in old rats (2.22-fold higher). Similarly, the metabolite HVA content in the striatum showed higher levels in young than in old rats (1.5-fold higher) (Fig. 2) and those of DOPAC followed a similar pattern without statistical significance (data not shown).
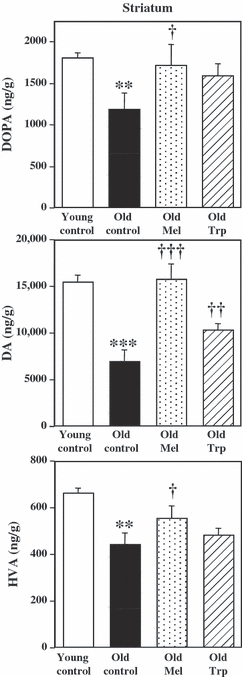
Chronic effects of melatonin and L-tryptophan on the accumulation of L-3,4-dihydroxyphenylalanine (DOPA), after decarboxylase inhibition (NSD 1015; 100 mg/kg, i.p.) during 30 min, dopamine (DA) tissue content, and the homovanillic acid (HVA) metabolite levels, in ng/g of wet tissue, in striatum. Groups of rats were chronically treated with vehicle (Young contros, n = 6 and Old controls, n = 6), melatonin (1 mg/kg/day in drinking water, for 4 wk, n = 6) and L-tryptophan (240 mg/kg, i.p., for 4 wk, n = 6). No significant differences were detected between two old control groups by ANOVA [F(1,10) = 1.58 P = 0.2373; F(1,10) = 0.075 P = 0.789; F(1,10) = 1.316 P = 0.278 for DOPA, DA and HVA, respectively, in striatum]. Columns (ng/g) are the mean ± S.E.M. derived from analysis of n animals per group. One way ANOVA followed by least significant difference (LSD) test was used for statistical evaluation: *P < 0.05, **P < 0.01, ***P < 0.001 compared to the young control group; †P < 0.05, ††P < 0.01, †††P < 0.001 when compared with the old control group.
In old rats, the accumulation of DOPA in the striatum increased (44%) after the chronic melatonin treatment (1 mg/kg/day, 4 wk) but only a slight increase in DOPA accumulation was observed after the chronic treatment with L-tryptophan (27%, P > 0.05). The DA content significantly increased after melatonin (126%) and also after L-tryptophan (57%) treatments. The levels of HVA metabolite were increased after the treatment of old rats with melatonin (26%) but only a more slight increase was observed after L-tryptophan treatment (10%, P > 0.05) (Fig. 2). Changes in DOPAC levels did not show statistical significance [ANOVA(3,16) = 0.5, P = 0.6866] (data not shown).
The synthesis of NE was measured in hippocampus, a brain region enriched in noradrenergic nerve terminals. L-3,4-dihydroxyphenylalanine accumulated during 30 min after the inhibition of decarboxylase enzyme with NSD in hippocampus, reflects the rate of NE synthesis. Fig. 3 shows the DOPA accumulated and the NE levels in the hippocampus of young and old control rats, and those after chronic treatment with exogenous melatonin and its precursor L-tryptophan to old rats. In control animals, tyrosine hydroxylation changed with age, showing greater accumulation of DOPA (4-fold higher) in young respect to the old rats. The NE content in the hippocampus followed a similar pattern reaching the maximum in young (1.57-fold higher) than in old rats (Fig. 3). No metabolite of NE could be measured.
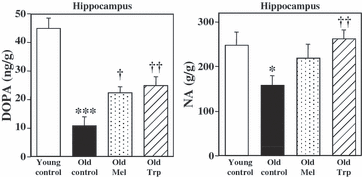
Chronic effects of melatonin and L-tryptophan on the accumulation of L-3,4-dihydroxyphenylalanine (DOPA), after decarboxylase inhibition (NSD 1015; 100 mg/kg, i.p.) during 30 min, and norepinephrine (NE) tissue content, in ng/g of wet tissue, in hippocampus. Groups of rats were chronically treated with vehicle (Young controls, n = 6 and Old controls, n = 6), melatonin (1 mg/kg/day in drinking water, for 4 wk, n = 6) and L-tryptophan (240 mg/kg, i.p., for 4 wk, n = 6). No significant differences were detected between two old control groups by ANOVA [F(1,8) = 0.079 P = 0.786 and F(1,9) = 0.249 P = 0.639 for DOPA and NE, respectively, in hippocampus]. Columns (ng/g) are the mean ± S.E.M. derived from analysis of n animals per group. One way ANOVA followed by least significant difference (LSD) test was used for statistical evaluation: *P < 0.05, **P < 0.01, ***P < 0.001 compared to the young control group; †P < 0.05, ††P < 0.01, †††P < 0.001 compared to the old control group.
The accumulation of DOPA in the hippocampus of old rats, increased after the chronic treatment with melatonin (1 mg/kg/day, 4 wk) (101%) and L-tryptophan (124%). The NE content increased after melatonin treatment (38%, P > 0.05) and also after L-tryptophan treatment (66%) (Fig. 3). No metabolite of NE could be detected.
The effects of chronic treatment with melatonin on working memory task in aged rats were tested by means of the eight-arm radial maze. Rats treated with melatonin showed a reduced performance time to complete the trial (40%, n = 7) and also a reduced number of errors (nonvisited plus repeated visited arms) (36%, n = 7) respect to the control aged rats (Table 1). The results show cognitive improving effects of chronic melatonin treatment in aged rats. On the other hand, the effects of chronic treatment with melatonin in aged rats were assessed on motor coordination and balance by using the rota-rod test. First, impairment of motor coordination was consistently observed in old control rats compared to younger animals. However, a significant improvement in fall off time was observed in rats treated with melatonin which increased the time remained on the rod (73%, n = 7) when compared to control aged rats (Table 2). The results suggest that the repeated treatment with melatonin might aid to improve the descent in motor coordination that normally occurs as a consequence of aging.
| Group and treatment | Performance time (s) | Errors (number) |
|---|---|---|
| Young-saline (n = 6) | 597 ± 57 | 9.4 ± 1.3 |
| Old-saline (n = 6) | 1155 ± 16** | 15 ± 0.37* |
| Old-chronic melatonin (n = 7) | 698 ± 24† | 9.57 ± 0.68†† |
- *P < 0.01, **P < 0.001 compared to young control group; †P < 0.05, ††P < 0.001 compared to old control group by ANOVA.
| Group and treatment | Time on rota-rod (s) |
|---|---|
| Young-control (n = 6) | 26.55 ± 1.06 |
| Old-control (n = 6) | 15.03 ± 1.50* |
| Old-chronic melatonin (n = 7) | 25.97 ± 1.6† |
- *P < 0.01 compared to young control group; †P < 0.05 compared to old control group by ANOVA.
Discussion
The present work found changes in the neurochemical characteristics of monoaminergic systems with age, and studied the effects of melatonin and L-tryptophan chronic treatments on the brain synthesis and metabolism of the neurotransmitters 5-HT, DA and NE. The most conclusive results were the improving effects of chronic melatonin treatment on 5-HT, DA and NE synthesis and metabolism in the aged rat brain in correlation with the restorative effects observed on working memory tasks and motor coordination. Taken together these results seem to be consistent with the reported neuroplastic and neuroprotective actions of melatonin [1, 16, 17, 19–21 among others].
Tryptophan hydroxylation decreased significantly in the hippocampus and striatum of aged rats, indicating an impairment in 5-HT synthesis with age. Also intraneuronal 5-HT content and 5-HIAA metabolite levels showed a concomitant age reduction. In good agreement, the synthesis and metabolism of 5-HT showed a similar decline in the hippocampus and striatum of aged ring doves respect to the young controls [31]. However, when aged rats were repeatedly treated with melatonin, an important increase in tryptophan hydroxylation, 5-HT and 5-HIAA metabolite levels were observed. While an inhibitory effect of acute melatonin on 5-HT synthesis [31] and release [38] was observed during the active phase of animals, in the hippocampus and other brain areas, chronic melatonin treatment significantly increased 5-HT levels in different brain regions (including caudate-putamen) which was consistent with an increased number of serotonergic nerve terminals in the same areas [39]. Moreover, chronic melatonin treatment elevated hippocampal pyramidal cell number in the accelerated senescence prone mouse-8 [17], and increased the protein MAP-2 which improves the stability of the dendritic cytoskeleton and attenuates its decay in the adult rat aging hippocampus [16].
In addition, restorative effects of melatonin on the age-related decline of DA and NE neurochemical activity were observed. It has been reported that long term melatonin treatment significantly stimulated TH expression in different brain areas [40] and increased TH enzymatic activity in the striatum of hamsters [41] and 6-OHDA-lesioned rats [42]. Moreover, a slight improvement in TPH and TH activity and monoamines metabolism in aged rats after nocturnal L-tryptophan administration, consistent with an enhanced melatonin synthesis [43], suggest an indirect melatonin-mediated effect of L-tryptophan treatment on monoaminergic neurochemical data. The essential amino acid L-tryptophan is the precursor in the biosynthesis of the indolamines 5-HT and melatonin. Tryptophan passage across the blood–brain barrier and TPH activity are important for appropriate 5-HT neurotransmission as they affect central 5-HT synthesis [44]. It is well known that brain 5-HT content shows a close physiological dependence on tryptophan levels [43, 45]. As the hydroxylation of tryptophan is the rate-limiting step in the synthesis of 5-HT, TPH determines the effective concentration of 5-HT in vivo, and at night 5-HT is the precursor in the biosynthesis of melatonin. As previously observed, an important increase in diurnal 5-HT and nocturnal melatonin levels occurs after L-tryptophan administration in rats [43]. In good agreement, other works found that circulating melatonin levels showed a dose- and time-dependent increase after the administration of tryptophan and 5-HT releasing drugs to rats [46] and men [47], while the inhibitor of 5-HT synthesis PCPA inhibited rat melatonin release [48]. On the other hand, L-tryptophan administration induced similar changes than melatonin injections on c-fos immunoreactivity at suprachiasmatic nucleus [49], a brain region rich in melatonin receptors.
Aging is associated with learning and memory impairments and a decline in motor coordination. The results obtained after examine working memory tasks and motor ability were consistent with the obtained neurochemical data. An improvement in the aged rats treated with melatonin on motor ability in rota-rod is in good correlation to the DA increase after melatonin treatment in the striatum (a region involved in the control of locomotor function). Taking into account that during the normal process of aging, alterations in dopaminergic function occur, the present results suggest that repeated treatment with melatonin might aid to improve the descent in DA neurotransmission and motor coordination that normally occurs as a consequence of aging. On the other hand, the improvement on task performance in radial maze observed in the aged rats treated with melatonin is in good correlation with the increased neurochemical data observed after melatonin chronic treatment. Learning and memory processes are partially regulated by the neurotransmitter and neuromodulatory activity of 5-HT and DA [28], being the hippocampus and corpus striatum two brain regions involved in the regulation of the adult memory processing [28, 50, 51]. The current findings suggest that the 5-HT and DA enhancing properties of melatonin might contribute to the cognitive improving effects of chronic melatonin treatment in aged rats. These observations correlates with the reported melatonin promotion of neuroplasticity in serotonergic [39] and dopaminergic [25, 26] neurons.
In good agreement with the current work, deficits in noradrenergic signaling seen in aged rats were directly associated to the decline in learning capabilities, and supplementation of animals with high antioxidants diets prevented and/or reverse age-related declines in cerebellar noradrenergic function [52]. Cognitive deficits such as learning impairment and delayed amnesia are the debilitating consequences of aging [53]. Nevertheless, chronic administration of antioxidants are reported to alleviate age-associated cognitive deficits in animals [54, 55]. Melatonin has been confirmed to protect against ROS-mediated neuronal degeneration models [25, 26]. It was shown that chronic melatonin treatment reverses cognitive deficits in aged mice [17], which was associated with its antioxidant properties [1, 56]. Also melatonin as an antioxidant displayed pronounced neuroprotective effects in some age-associated neurodegenerative diseases [1] as Alzheimer’s disease [19–21] or other dementias [57].
In summary, the results from the present study demonstrate that the repeated treatment with melatonin might aid to improve the descent in 5-HT, DA and NE neurotransmission that normally occurs as a consequence of aging, and could be used as a therapy for diseases involving reductions in these neurotransmitters. Particularly, it might be related to the restorative effects of melatonin on the impaired cognitive functions and motor coordination that occurs asociated to aging.
Acknowledgements
This study was supported by the research grants (SAF2007–66878-CO2–02 and BFI2002–04583-C02–029) of the Ministerio de Educación y Ciencia (MEC Madrid, Spain). Author’s contribution. This work was designed and written under the direction of S. Esteban. All remaining authors have equally contributed to its final form.




