Membrane-bound melatonin receptor MT1 down-regulates estrogen responsive genes in breast cancer cells
Abstract
Abstract: Melatonin possesses anti-estrogenic effects on estrogen receptor expressing (ER+) breast cancer cells in culture by reducing cell cycle progression and cell proliferation. There is increasing agreement that on a cellular level the effects of melatonin are primarily induced by the membrane-bound receptor MT1. The participation of a second, nuclear receptor of the group of ligand-dependent transcription factors, called RZRα, is under debate. In this study we used a number of breast cancer cell lines differing in their expression of the estrogen receptor and the two known melatonin receptors. In MCF-7 breast cancer cells transfected with a vector carrying the MT1 gene (MCF-7Mel1a) binding of CREB-protein to the cAMP-responsive element of the breast cancer suppressing gene BRCA-1 was more strongly reduced by treatment with melatonin than in the parental cells. Expression of estrogen responsive genes was determined in serum-starved cells, cells stimulated for 16 hr with estradiol and cells subsequently treated with melatonin. Expression of BRCA-1, p53, p21WAF and c-myc were up-regulated by estradiol. Treatment of the stimulated cells with melatonin counteracted the increase induced by estradiol almost completely. The more MT1 a cell line expressed, the stronger was the reduction of the expression of the estradiol-induced genes. There was no correlation between the expression of the nuclear receptor RZRα and the effects of melatonin on these genes.
Introduction
Breast cancer is the most frequent malignant disease in women. There is good evidence that the pineal gland influences the development and growth of breast cancer by its major hormone melatonin [1–3]. In pinealectomized rats the incidence of mammary carcinoma is increased and hormone replacement with physiological doses of melatonin reversed this phenomenon [4]. Furthermore, the onset of chemically induced breast cancer in rats treated with the carcinogen 7,12-dimethylbenz[a]anthracene was delayed by the administration of melatonin and the number of loci was reduced [5]. Two possible mechanisms may contribute to the in vivo antineoplastic effect of melatonin on breast cancer. Enhanced synthesis of melatonin in the pineal gland at night modulates the secretion of 17β-estradiol by the ovaries [4]. In addition, melatonin has an immunostimulatory effect by inducing the secretion of IL-2 and IL-6 from lymphocytes [6].
In vitro, proliferation of breast cancer cells is inhibited by the treatment with physiological concentrations of melatonin [2, 3, 7]. Conflicting results were reported by some authors who failed to reproduce this observation, although these investigators nominally used the same cell line for their experiments [8, 9]. Most of these experiments were performed with the widely used breast cancer cell line MCF-7, one of the few cell lines that express high numbers of the estrogen receptor, ERα. Ram et al. [10] observed great differences in the responsiveness to melatonin in MCF-7 cells from various laboratories.
The effects of melatonin on the cellular level are triggered by two distinct classes of receptors for melatonin. Activation of the melatonin receptor MT1 inhibits adenylyl cyclase activity via the pertussis toxin-sensitive G-proteins Gi2, Gi3, and Gq/11 [11, 12]. The suppressed cAMP production in turn leads to a decreased PKA activation finally resulting in a diminished phosphorylation of the CREB-protein. CREB phosphorylated at serine 133 recruits the CREB binding protein CBP/p300 to the promoter of cAMP-responsive genes. Treatment of cells with melatonin prevents forskolin induced cAMP synthesis thereby reducing transcription from cAMP-responsive elements (CRE) [12].
A completely different class of melatonin receptors are the ligand-dependent nuclear transcription factors RZR/ROR, homologues to the retinoic acid receptors. RZR/RORα binds as a monomer to the consensus motif AGGTCA in gene promoters [13]. Although the significance of ROR/RZR as nuclear receptors of melatonin is under discussion, a number of reports suggest their involvement in melatonin’s action [14–16].
The membrane-bound melatonin receptor, MT1, and the nuclear receptor, RZR/RORα, clearly differ in their affinities for melatonin. At concentrations below 10−9 m the receptor MT1 is preferentially activated by melatonin, whereas for the activation of RZR/ROR concentrations above 5 × 10−9 m are necessary [7].
Both receptors, MT1 as well as the nuclear receptor RZR/RORα, were independently detected in MCF-7 cells by several laboratories [7, 10]. It is not yet absolutely clear which receptor is responsible for the antiproliferative and anti-estrogenic activity of melatonin. At least, evidence has been provided that the activity of melatonin depends on the presence of estrogen receptors because growth of ER-negative breast cancer cells is not inhibited by melatonin [2, 3]. To date, no convincing clue has been presented as to how the signaling pathways of melatonin cross-talk with transactivation of the estrogen receptors. One promising approach to shed light on the interaction of melatonin and estradiol would be to investigate the influence of melatonin on the expression of genes regulated by estradiol.
The breast cancer susceptibility gene BRCA-1 is a good candidate to study cross-talk between ER and melatonin signal transduction. BRCA-1 expression is up-regulated by estradiol [17, 18], and a CRE in located in the promoter of BRCA-1, -127 bases downstream the transcription start site. Melatonin might be able to down-regulate BRCA-1 expression by reducing CREB-binding to the BRCA-1 promoter via the receptor MT1.
There are numbers of reports describing an antiproliferative effect of melatonin on breast cancer cells but the mechanism by which melatonin suppresses proliferation is not completely elucidated [2, 3]. The c-myc oncogene involved in the regulation of proliferation contains an estrogen responsive element in the promoter and is readily up-regulated by estradiol [19]. It is reasonable that the antiproliferative effect of melatonin is accompanied with an increased expression of genes regulating the cell cycle. Mediavilla et al. [20] observed an increased p53- and p21WAF-expression in MCF-7 cells after treatment with 10−9 m melatonin in serum complemented medium.
In this study we analyzed the effect of melatonin in several breast cancer cell lines on the induction of the breast cancer susceptibility gene BRCA-1 by estradiol and the expression of further estrogen regulated genes.
Material and methods
Cell lines and cell culture
Estradiol and melatonin were purchased from Sigma Chemicals (Steinheim, Germany). The human breast cancer cell line MCF-7 (p181) obtained from ATCC, (Manassas, VA, USA) was provided by Dr W. Körner (Bavarian Environmental Protection Agency, Augsburg, Germany). MCF-7mel1a were MCF-7 cells (ATCC) transfected with a vector carrying the gene MT1 for the membrane-bound melatonin receptor. They were a generous gift of Prof. Steven Hill, Tulane University, New Orleans, LA, USA). The MCF-7mel1a cells were periodically selected under the growth pressure of 1000 μg/mL Zeocin (Invitrogen, Karlsruhe, Germany) to prevent the overgrowth of the nontransfected parental cells. The breast cancer cell lines MDA-MB-435 and MDA-MB-453 established at the MD Anderson Cancer Center (Houston, TX, USA) and the HCC70 breast cancer cells were purchased from ATCC.
The cell line MCU-1 was established in our laboratory from a pleural effusion of a 39-yr-old women with invasive breast cancer. In brief, 50 mL of the pleural effusion was pelleted at 400 g for 15 min, resuspended in 10 mL Dulbecco’s modified EM supplemented with 5% fetal calf serum (Biochrom, Berlin) and plated for 1 hr into a 75 mL Falcon culture flask (BD Biosciences, Heidelberg, Germany) to deplete contaminating fibroblasts by selective adhesion. The supernatant was transferred to a fresh culture flask and grown to 90% confluence. Culture medium was replaced every 5 days. At confluence, cells were detached from the flask by treatment with Trypsin/EDTA (Biochrom) for 15 min at 37°C, resuspended in fresh culture medium and split 1:1. After three passages clones of tumor cells visible between the abundant fibroblasts were selectively removed by trypsin/EDTA using a rubber-policeman and transferred to a fresh culture flask. After ten further passages the cells appeared with homogeneous morphology and were free of contaminating fibroblasts. For the described experiments, the MCU-1 cells were used between passage 15 and 25.
Cells of all breast cancer cell lines used were maintained in Dulbecco’s modified EM supplemented with 5% fetal calf serum (Biochrom), 1 μg/mL transferrin, 2 mm glutamine, 50 U/mL penicillin/streptomycin, 2.5 μg/mL amphotericin B and 1:100 nonessential amino acids (Biochrom) at 37°C, 5% CO2 in an humidified atmosphere.
Stimulation of the breast cancer cells
3 × 105 cells/well were plated in serum containing culture medium into six well plates. The serum containing culture medium was replaced by serum-free culture medium 4 hr later, when the plated cells completely adhered to the plastic dish. The cells were grown in the serum-free medium for further 48 hr. Eighteen hour before harvest, estradiol was added to two wells at a final concentration of 10−11 m. To one of these wells 10−9 m melatonin was added 2 hr before the cells were harvested for RNA-purification.
Purification of RNA
RNA of breast cancer cells grown for 48 hr in serum-depleted medium and cells stimulated for 18 hr by 10−11 m estradiol only and of cells subsequently treated with 10−9 m melatonin for 2 hr was purified using the RNeasy-kit (Qiagen, Hilden, Germany).
RT-PCR
Two-hundred nanogram of RNA were transcribed by 400 u Superscript reverse transcriptase (Invitrogen) in the presence of 0.5 μm oligo-dT primer for 60 min at 37°C. Five microlitre of the resulting cDNA were amplified with 1 u Taq polymerase (Roche, Mannheim, Germany) in the presence of 200 μm dNTPs and 200 nm of the appropriate primers.
Primers: ERα: sense: AATTCAGATAATCGACGCCAG; antisense: GTGTTTCAACATTCTCCCTCCTC; RZRα: sense: ACTTCCCCAACTGTGTCCAT; antisense: GGCACGGCACATTCTGATAA; MT1: sense: TCCTCATCTTCACCATCGTG; antisense: AAGGTGCACGAGTAGATCCT; p53: sense: CGGACGATATTGAACAATGG; antisense: GTCATGTGCTGTGACTGCTT; p21waf: sense: TGAGCGATGGAACTTCGACT; antisense: GCTTCCTCTTGGAGAAGATC; c-myc: sense: GAACTTACAACACCCGAGCA; antisense: TCTTCCAGATGTCCTCGCTG; BRCA: sense: GGAGTTGATCAAGGAACCTG; antisense: ACGGTTTCTGTAGCCCTAC.
Polymerase chain reaction-products were separated in a 2% agarose gel (Type IV, special high EEO, Sigma Chemicals) in 0.5× TBE buffer at 100 V for 30 min. Gels were stained in ethidium bromide (2 μg/mL) for 30 min and photographed on a transiluminator using a CDS camera (TD20; Kodak, Rochester, NY, USA).
Electrophoretic mobility shift assay
The effects of melatonin on the downstream signaling of the membrane-bound melatonin receptor MT1 were analyzed using an electrophoretic mobility shift assay (EMSA). The EMSA used determines changes of the binding of the CREB-protein to a CRE present in the promoter of the BRCA-1 gene. The LightShift™ Chemiluminescent EMSA kit (Pierce, Rockford, IL, USA) was performed according to the instructions of the manufacturer.
Nuclear proteins were purified according to Maniatis from breast cancer cells of various cell lines either kept for 48 hr in serum-free medium or serum-starved and subsequently stimulated for 16 hr by 10−11 m estradiol or stimulated with estradiol and additionally treated with 10−9 m melatonin the last 2 hr before the cells were harvested.
The treated cells were detached after incubation with ice-cold PBS/1 mm EDTA for 5 min using a rubber-policeman. Cells were centrifuged and the cell pellet was resuspended in 250 μL hypotonic buffer (10 mm HEPES pH 7.9, 1.5 mm MgCl2, 10 mm KCl, 0.5 mm DTT, 1:100 dilution of protease inhibitor cocktail (Sigma Chemicals) and centrifuged at 800 g. Cells were resuspended again in 150 μL hypotonic buffer and kept on ice for swelling for 15 min. Subsequently, cells were disrupted by 10 strokes in a Dounce homogenizer. The cell nuclei were pelleted by centrifugation at 3300 g for 15 min. The pellet was resuspended in 25 μL low-salt buffer (20 mm HEPES pH 7.9, 25% glycerol, 1.5 mm MgCl2, 20 mm KCl, 0.2 mm EDTA, 0.5 mm DTT, 1:100 dilution of protease inhibitor cocktail (Sigma Chemicals) and 25 μL high-salt buffer (low-salt buffer containing 1.2 m KCl) were slowly added under gentle agitation. Nuclear proteins were extracted from the nuclei for 30 min on ice. The nuclei were separated at 25,000 g for 30 min and the supernatant dialyzed against dialysis buffer (20 mm HEPES pH 7.9, 400 mm KCl, 0.2 mm DTT, 0.1 mm EDTA).
Protein content was determined by the method of Bradford. Twenty microgram of nuclear proteins in 20 μL were mixed with 5 μL of a master mix containing 2 μL tenfold binding buffer (Pierce), 5 mm MgCl2, 50 ng/μL Poly-IC, 0.05% NP40 and 2 μL Biotin-labeled oligonucleotide probe.
Preparation of the oligonucleotide probe for EMSA
Oligonucleotides end-labeled by Biotin were BRCA-sense: CTTTCCTTTTACGTCATCCGGGGGC; antisense: GCCCCCGGATGACGTAAAAGGAAAG 20 fmol of each oligonucleotide were mixed in 50 μL H2O, denatured at 70°C for 3 min, annealed at 50°C for 5 min and stored on ice until use.
Reaction mixture was kept at room temperature for 20 min. After adding 5 μL loading buffer (Pierce) the mixture was electrophoretically separated in a 6% nondenaturating polyacrylamid gel at 100 V for 45 min.
Oligonucleotides and bound proteins were blotted onto a Nylon-membrane (Hybond-N; GE-Amersham, Freiburg, Germany) and cross-linked at 302 nm on a transiluminator (Stratalinker, Stratagene, Heidelberg, Germany). Nonspecific binding sites were blocked by incubating the membrane in blocking buffer provided by the LightShift™ Chemiluminescent EMSA kit for 15 min before the blots were incubated with a 1:1000 dilution of HRP-Streptavidin (Pierce). Membranes were washed four times with 20 mL wash buffer (Pierce) at room temperature for 5 min and subsequently soaked in an equilibration buffer (Pierce). After washing, membranes were incubated with ECL chemoluminescence reagent (ECL; GE-Amersham, Freiburg, Germany) for 5 min and exposed to X-ray film for various time intervals (BiomaxMR; Kodak). The exposed films were scanned using a flatbed scanner (Hewlett-Packard, R45) and analyzed by the Digital science 1D-software (Kodak).
Densitometric evaluation
Photographs of the PCR-products were analyzed using the Digital science 1D-software (Kodak). The densitometric values of the RT-PCR products were normalized to the values of the house-keeping gene GAPDH. The normalized values of the treated samples were divided by the values of the serum-free control to give the relative expression.
Statistical analysis
The arbitrary units of the densitometric evaluation of the bands obtained from the RT-PCR products of each sample were related to the values of the corresponding band of the house-keeping gene GAPDH. Hence, variations resulting from differences in the purity of the RNA and of the photometric determination of the RNA concentration were eliminated. The resulting normalized values of the treated samples were divided by the normalized value of the control cells (serum-deprived) giving the relative expression compared with the control. The relative expression of all independent experiments entered the calculation of the mean and standard deviation. Analysis for significance of the differences was carried out by Student’s t-test.
Results
Cells of six different breast cancer cell lines (MCU-1, MCF-7p181, MCF-7mel1a, HCC70, MDA-MB-453, and MDA-MB-435) were analyzed for their expression of the estrogen receptor, ERα, the nuclear melatonin receptor, RZRα, and the membrane-bound melatonin receptor, MT1.
Reverse transcriptase-PCR of the mRNA of the breast cancer cell lines was performed using primers specific for the three analyzed genes (Fig. 1). As the abundance of the genes varied strongly in the cell lines under investigation the number of cycles had to be adapted for each cell line. ERα is most strongly expressed in the MCF-7 cell lines, MCF-7p181, MCF-7mel1a, and the newly established cell line MCU-1. For these cell lines 27 PCR-cycles were sufficient to give a visible signal on ethidium bromide stained agarose gels. Expression of ERα decreased in the order MCF-7p181, MCU-1, and MCF-7mel1a. cDNA of the cell lines HCC70 and MDA-MB-435 needed to be amplified by 35 cycles to obtain a visible band. In MDA-MB-453 cells no ERα mRNA was detectable.
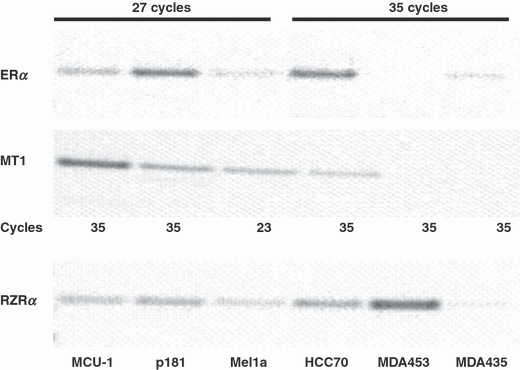
Expression of estrogen receptor (ERα) and melatonin receptors, RZRα and MT1. RNA of six different breast cancer cell lines were analyzed for expression of ERα, RZRα, and MT1 by RT-PCR. For ERα, cDNA of the cell lines MCU-1, MCF-7p181, and MCF-7mel1a was amplified by 27 cycles, for the cell lines HCC70, MDA-MB-453, and MDA-MB-435 35 cycles were applied. For RZRα, cDNA of all cell lines was amplified by 31 cycles. MT1 in MCF-7mel1a was amplified by 23 cycles and all other cell lines by 35 cycles.
For the analysis of the expression of the nuclear melatonin receptor, RZRα, the cDNA of each cell line was amplified by 31 cycles. RZRα is most strongly expressed in the cell line MDA-453 followed by HCC70 and MCF-7p181. MCU-1 and MCF-7mel1a expressed much less RZRα than the aforementioned cell lines. With the cDNA of the cell line MDA-MB-435 only a very faint band of RZRα is visible.
The membrane-bound melatonin receptor, MT1, as expected, is most strongly expressed in the MCF-7mel1a cells that were transfected with a vector carrying the MT1 gene. Twenty-three cycles were sufficient to give a clearly visible band in the agarose gel. All other cDNAs were amplified by 35 cycles for the analysis of the MT1 expression.
Of the nontransfected cell lines, MCU-1 expressed MT1 most strongly followed by MCF-7p181 and HCC70. In the cell lines MDA-MB-453 and MDA-MB-435 no PCR-product of MT1 was detectable even after 35 cycles of duplication.
Melatonin is known to elicit its effect by binding to either the membrane-bound receptor MT1 and/or to the nuclear receptor RZRα. Signal transduction of the receptor MT1 involves the activation of the heterotrimeric G-protein Giα inhibiting the adenyl cyclase with the consequence that the protein kinase A is less activated. This finally leads to a lower phosphorylation of the CREB-protein and a reduced transcription of genes containing a CRE in their promoter. One gene of great importance for breast cancer containing a CRE is the tumor suppressor gene BRCA-1.
Electrophoretic mobility shift assays were performed with nuclear proteins isolated from cells of the breast cancer cell line MCF-7 and from MCF-7mel1a cells transfected with a vector carrying the MT1 gene. The cells were serum starved for 48 hr (C) or subsequently stimulated for 16 hr with 10−11 m estradiol (E2) or additionally treated for 2 hr with 10−9 m melatonin (Mel).
In the EMSA using a 25 bp oligonucleotide homologous to the promoter of the BRCA-1 gene containing a CRE DNA-protein two complexes formed having a lower mobility than the unbound oligonucleotides (Fig. 2).
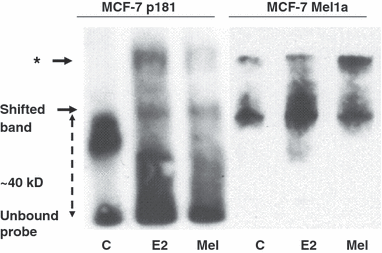
Electrophoretic mobility shift assay of melatonin treated breast cancer cells. Oligonucleotides containing the cAMP-responsive element of the BRCA-1 promoter were incubated with nuclear proteins of the cell lines MCF-7 p181 and MCF-7Mel1a stimulated with 10−11 m estradiol (E2) and subsequently treated with 10−9 m melatonin (Mel). After electrophoresis in a 6% polyacrylamid gel labeled oligonucleotides were detected using peroxidase-coupled streptavidin. One band shifted by 40 kD represents the oligonucleotides bound to activated CREB-protein a second band with lower mobility (*) has bound proteins of yet unknown origin.
Gel mobility shift analysis with the CRE of the BRCA-1 promoter revealed a retarded band specific for the CREB-protein shifted by approximately 40 kD, the molecular weight of CREB-protein. An additional signal of unknown origin detected on the gel is indicated by an asterisk. In serum-starved MCF-7 cells (lanes C), in particular, in the nontransfected wild-typ cells (p181) the signal at 40 kD is weak. Stimulation of both cell lines by estradiol leads to an increased intensity of the shifted band at 40 kD (lanes E2). This increase of the shifted band is stronger in the MCF-7 cells transfected with MT1. Treatment of the E2-stimulated cells with 10−9 m melatonin for 2 hr reduces the intensity of the shifted band almost to control levels.
According to the analysis of the expression of receptors important for the activity of melatonin in the various breast cancer cell lines, four cell lines that differed in their expression of ERα, RZRα, and MT1 were selected for further analysis of the role of the melatonin receptors in the regulation of the estrogen-responsive genes, BRCA-1, p53, p21WAF, and c-myc.
Of these four cell lines, MCF-7p181 expressed the highest level of ERα and moderate amounts of RZRα and MT1. MCF7mel1a expressed the membrane-bound melatonin receptor, MT1, most abundantly and average amounts of ERα and RZRα. MCU-1 cells expressed less ERα than MCF-7p181 but higher amounts of MT1. Finally, MDA-MB-453 expressed almost no ERα and MT1 but most of RZRα (see Fig. 1).
The cells of these four cell lines were serum starved for 48 hr and subsequently stimulated for 16 hr with 10−11 m estradiol and then treated for 2 hr with 10−9 m melatonin. RNA was purified either from serum-starved cells, from cells stimulated with estradiol only or after sequential stimulation with estradiol and melatonin.
RNA of the differently treated cells was analyzed for the expression of four different estrogen-responsive genes: the breast cancer specific suppressor gene BRCA-1, the tumor suppressor gene p53, the cell cycle regulator p21WAF and the protooncogene and proliferation marker c-myc.
RT-PCR for BRCA-1 and p53 was performed by 24 cycles, p21WAF was amplified by 28 cycles, for c-myc only 22 cycles were necessary to give clearly visible signals (3-6).
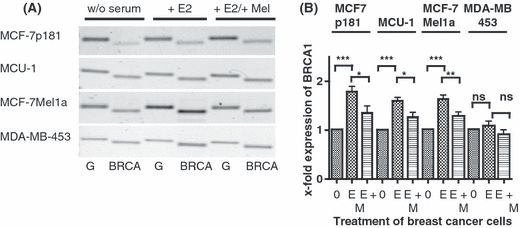
Effect of melatonin on expression of BRCA-1 in various breast cancer cell lines. (A) Serum starved cells (0) were stimulated by 10−11 m estradiol (E) and subsequently treated with 10−9 m melatonin (M). RT-PCR was performed with primers specific for BRCA-1(B). In parallel, PCR of GAPDH (G) shows equal loading of mRNA.Cell lines: MCF-7p181, MCU-1, MCF-7Mel1a, and MDA-MB-453. (B) Densitometric evaluation of changes in gene expression. Expression values of BRCA-1 were normalized to expression of GAPDH. Each point is the mean ± S.E.M. of six to eight independent determinations. (0) cells serum starved for 24 hr; (E) serum-starved cells stimulated with 10−11 m estradiol; (M) cells treated with 10−9 m melatonin after stimulation with estradiol (*P < 0.05; **P < 0.01; ***P < 0.001).
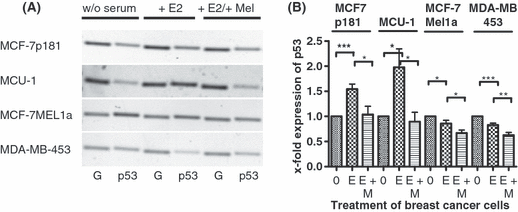
Effect of melatonin on expression of p53 in various breast cancer cell lines. (A) Serum-starved cells (0) were stimulated by 10−11 m estradiol (E) and subsequently treated with 10−9 m melatonin (M). RT-PCR was performed with primers specific for p53. In parallel, PCR of GAPDH (G) shows equal loading of mRNA. Cell lines: MCF-7p181, MCU-1, MCF-7Mel1a, and MDA-MB-453. (B) Densitometric evaluation of changes in gene expression. Expression values of p53 were normalized to expression of GAPDH. Each point is the mean ± S.E.M. of six to eight independent determinations (0) cells serum starved for 24 hr; (E) serum-starved cells stimulated with 10−11 m estradiol; (M) cells treated with 10−9 m melatonin after stimulation with estradiol (*P < 0.05; **P < 0.01; ***P < 0.001).
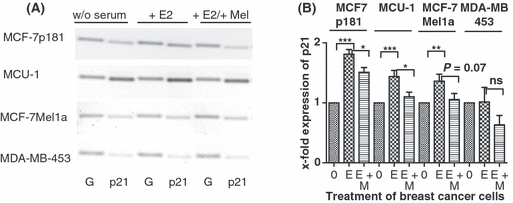
Effect of melatonin on expression of p21WAF in various breast cancer cell lines. (A) Serum-starved cells (0) were stimulated by 10−11 m estradiol (E) and subsequently treated with 10−9 m melatonin (M). RT-PCR was performed with primers specific for p21. In parallel, PCR of GAPDH (G) shows equal loading of mRNA. Cell lines: MCF-7p181, MCU-1, MCF-7Mel1a, and MDA-MB-453. (B) Densitometric evaluation of changes in gene expression. Expression values of p21WAF were normalized to expression of GAPDH. Each point is the mean ± S.E.M. of six to eight independent determinations. (0) cells serum starved for 24 hr; (E) serum-starved cells stimulated with 10−11 m estradiol; (M) cells treated with 10−9 m melatonin after stimulation with estradiol (*P < 0.05; **P < 0.01; ***P < 0.001).
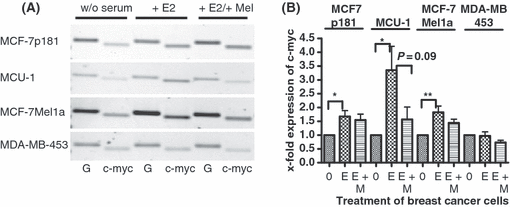
Effect of melatonin on expression of c-myc in various breast cancer cell lines. (A) Serum-starved cells (0) were stimulated by 10−11 m estradiol (E) and subsequently treated with 10−9 m melatonin (M). RT-PCR was performed with primers specific for c-myc. In parallel, PCR of GAPDH (G) shows equal loading of mRNA. Cell lines: MCF-7p181, MCU-1, MCF-7Mel1a, and MDA-MB-453. (B) Densitometric evaluation of changes in gene expression. Expression values of c-myc were normalized to expression of GAPDH. Each point is the mean ± S.E.M. of six to eight independent determinations. (0) cells serum starved for 24 hr; (E) serum-starved cells stimulated with 10−11 m estradiol; (M) cells treated with 10−9 m melatonin after stimulation with estradiol (*P < 0.05; **P < 0.01; ***P < 0.001).
The bands of the PCR-products of gels of 6–8 repetitions of stimulation were evaluated densitometrically and the densitometric data were statistically analyzed and plotted (3-6).
BRCA-1 expression was most strongly increased by estradiol in the MCF-7p181 cells followed by the MCU-1- and MCF-7mel1a cells. In MDA-MB-453 cells the increase of BRCA-1 mRNA by estradiol was nonsignificant and only about 10% above control (Fig. 3B).
Treatment of the estradiol stimulated breast cancer cells by melatonin significantly reduced the BRCA-1 expression half-way back toward control levels except in the MDA-MB-453 cells where BRCA-1 expression was only nonsignificantly suppressed below the value measured in control cells.
The expression of p53 was most strongly increased by estradiol in the breast cancer cell line MCU-1 even though this cell line expressed less ERα than the MCF-7p181 cells (see Fig. 1). There was twice as much p53 mRNA in the estradiol stimulated MCU-1 cells than in the serum-starved cells. In the MCF-7p181 cells the increase by estradiol was only 55%. Although the MCF-7 cells were the parental cells of the MT1-transfected MCF-7mel1a cells, stimulation of MCF-7mel1a cells with estradiol did significantly decrease p53 expression by 14%. This change in expression is in contrast to the changes seen in the MCF-7p181- and MCU-1 cells, probably because of the extremely high expression of the membrane-bound melatonin receptor MT1 (see Fig. 1). In the MDA-MB-453 cells, bare of ERα expression, there was also a significant decrease of p53 expression by 17% after estradiol treatment (Fig. 4B). Of all cell lines tested MDA-MB-453 cells express the highest amount of RZRα but almost no MT1 (see Fig. 1).
If the breast cancer cells were treated with 10−9 m melatonin subsequently to the stimulation by estradiol expression of p53 was dramatically reduced after 2 hr. This effect of melatonin is more obvious in the MCU-1 cell line than in the MCF-7p181 cells that express less MT1 than the MCU-1 cells. In the cell lines MCF-7mel1a and MDA-MB-453, where the expression of p53 already decreased after estradiol stimulation the expression was further significantly lowered by the treatment of the cells with 10−9 m melatonin for 2 hr (Fig. 4B).
The expression of the cell cycle regulator p21WAF significantly increased in MCU-1 cells, in MCF-7p181 cells and the MCF-7mel1a cells after estradiol stimulation. The effect of estradiol was strongest in the MCF-7p181 cell line, increasing p21 expression in these cells by the factor 1.8. In the MDA-MB-453 cells, lacking ERα, p21WAF expression remained almost unchanged after estradiol stimulation (Fig. 5B).
Subsequent treatment with 10−9 m melatonin for 2 hr lowered p21 expression in all four tested cell lines. In the cell lines MCU-1 and MCF-7mel1a the expression of p21 was most strongly decreased. Melatonin lowered p21 expression almost to control level. In the MDA-MB-453 cells the decrease of p21 expression by melatonin was clearly below the level found in the nonstimulated cells but opposite to the other cell lines this change in expression was not significant (Fig. 5B).
By the treatment with estradiol the expression of c-myc was most dramatically increased in the MCU-1 cells by the factor f = 3.4, followed by almost equal values in the two MCF-7 cell lines (1.7- to 1.8-fold). Because of the nondetectable level of ERα in the MDA-MB-453 cells c-myc remained unchanged following estradiol stimulation.
In the MCU-1 cells the increased c-myc expression triggered by the estradiol stimulation was reverted close to control level after melatonin treatment. This decrease of c-myc by melatonin was less pronounced in the MCF-7p181 and the MCF-7mel1a cells. In all three cell lines the effect of melatonin treatment on the increased expression induced by estradiol was significant. In the MDA-MB-453 cells melatonin was even able to suppress c-myc expression below control level (Fig. 6B).
Discussion
In many investigations numerous authors conclusively reported an anti-estrogenic activity of melatonin leading to growth inhibition exclusively in breast cancer cells expressing the estrogen receptor ERα [2, 3]. Melatonin makes (ER+) breast cancer cells less sensitive to estradiol as is exemplified by a shift of the dose-response curve of estradiol to higher concentrations and an increase of the EC50 of estradiol in the presence of 10−9 m melatonin [7]. Despite the enormous efforts, to date, the mechanism of this anti-estrogenic activity of melatonin is not yet clear. In particular, the steps of the signaling pathways of both receptors, ERα and MT1, where the cross-talk of estrogen and melatonin signaling takes place are not identified. Some authors reported a down-regulation of the ERα expression by melatonin but this is obviously not the only mechanism by which melatonin influences the growth of estradiol-dependent breast cancer cells [21, 22].
There is increasing evidence that MT1 is the most important receptor for the activity of melatonin in breast cancer cells [23]. To prove this fact, we performed experiments with a number of breast cancer cell lines that differ in their expression of ERα, the membrane-bound receptor, MT1, and the putative nuclear receptor for melatonin, RZRα. Of these cell lines, the MCF-7Mel1a cells, transfected with a MT1 vector expressed highest amounts of MT1 and lowest amounts of RZRα, MCF-7p181 expressed minute amounts of MT1 and highest amounts of RZRα of all ERα-positive breast cancer cells tested (Fig. 1).
The investigation of the effects of the treatment of these different cell lines with melatonin also enable the evaluation of the participation of the nuclear receptor RZRα on the effects of melatonin on breast cancer cells. Dai et al. [24] determined the expression of RZR/RORα mRNA in some further breast cancer cell lines. RZR/RORα transcripts were most abundantly detected in the cell lines T47D and MDA-MB-231. In addition, we analyzed RZR/RORα expression in the cell lines MCF-7, MCF-7Mel1a, MCU-1, and MDA-MB-453. With MCF-7 being the only cell line examined in both studies the abundance of RZR/RORα expression in breast cancer cell lines could be lined up by increasing expression of RZRα from MCF-7Mel1a, over MCU-1, MCF7, ZR75-1, MDA-MB-453, T47D, to MDA-MB-231 expressing the lowest amount.
Herein, we focused on the effect of melatonin on the breast cancer susceptibility gene BRCA-1. Expression analysis in breast tumors has revealed that sporadic breast tumors contain lower levels of BRCA-1 mRNA in the absence of mutations in the BRCA-1 gene suggesting that disruption of BRCA-1 expression may contribute to the etiology of breast cancer [25].
Induction of BRCA-1 mRNA is a rather late event after E2-stimulation. Jeffy et al. [18] observed a twofold increase of transcriptional activity of the BRCA-1 promoter after treatment of MCF-7 cells with E2 for 24 hr. The proximal BRCA-1 promoter lacks a consensus ERE that binds ERα and upregulation of BRCA-1 requires de novo protein synthesis [26]. On the other hand, there is an AP-1 binding site 27 bases upstream of the transcription start site of BRCA-1 and activation of BRCA-1 transcription is mediated by the tethering of the ERα by the Fos/Jun-complex to this AP-1 site [17].
To investigate the participation of the membrane-bound melatonin receptor MT1 on the anti-estrogenic effects of melatonin in breast cancer cells electrophoretic mobility shift experiments were performed to analyze the binding of nuclear proteins from two breast cancer cell lines expressing different amounts of MT1. The results in Fig. 2 clearly show that the BRCA-1-specific oligonucleotide probe is strongly shifted by 40 kD, the approximate molecular weight of the CREB-protein, that is phosphorylated by protein kinase A in dependence of MT1 stimulation. This effect is most obviously seen in the cell line MCF-7 Mel1a. The subsequent treatment of the E2 stimulated cells with 10−9 m melatonin clearly reduces the amount of shifted BRCA-1 oligonucleotide.
Arizti et al. [27] demonstrated that BRCA-1 expression is also down-regulated in response to p53 induction and vice versa. In our experiments even p53 expression is down-regulated by melatonin. The down-regulation of p53 observed after melatonin treatment would partly compensate the down-regulation of BRCA-1 by melatonin that is shown in Fig. 3B. The difference in BRCA-1 expression after melatonin treatment between the cell line MCF-7 Mel1a showing only a minute decrease in p53 expression and MCF-7, where a clear-cut decrease of the p53 expression was detected is too small to judge whether this effect of p53 on the BRCA-1 expression occurs in these cell lines.
In the colon carcinoma cell lines SW480 and HCT116 BRCA-1 has been shown to stimulate the expression of growth inhibitory genes like p21WAF and p27KIP1. While p21WAF is also a p53-regulated gene the observed up-regulation of p21WAF by BRCA-1 has been interpreted by the presence of an additional p53-independent mechanism [28]. The expression analysis of p21WAF in breast cancer cell lines shown in Fig. 5B revealed a slight down-regulation of p21WAF by melatonin in parallel to the down-regulation of BRCA-1. In the presence of melatonin BRCA-1 is down-regulated because of a lower binding of phosphorylated CREB to the promoter and in consequence p21WAF is also down-regulated.
The next estrogen dependent gene analyzed in this study was the tumor suppressor p53. Expression of p53 was inducible by estradiol and treatment of the breast cancer cells with melatonin repressed this increased expression of p53 in all four breast cancer cell lines tested. This finding is in contradiction to the results of Mediavilla et al. [20] who observed an about 30% increased expression of p53 and p21WAF in MCF-7 cells after treatment with melatonin. In contrast to our experimental design, these authors analyzed the effect of melatonin in a medium supplemented by 10% serum. These conditions were less defined as in our experiments because serum contains variable amounts of estradiol as well as melatonin. Nevertheless, at least in the MCF-7 cells the expression of p53 remains slightly higher after melatonin treatment than the expression level in nonstimulated control cells (Fig. 4B). The same is true for p21WAF expression. mRNA for p21WAF remains at a higher level than in the nonstimulated breast cancer cells.
In the cell lines MCU-1 and MCF-7Mel1a expressing higher amounts of MT1 the decrease of p53- and p21WAF-expression by melatonin treatment is higher than in the MCF-7p181 cells expressing less MT1. This quantitative difference clearly demonstrates the involvement of MT1 in the down-regulation of p53 and p21 by melatonin.
It has been reported, that the tumor suppressor p53 prevents the recruitment of ERα to the BRCA-1 promoter and represses transcription of BRCA-1 [18]. On the other hand, BRCA-1 stabilizes p53 and stimulates its transcriptional activity [29]. BRCA-1 interacts with c-myc, at least in yeast and represses c-myc mediated transcription [30]. Although a direct effect of E2 on the expression of p53 is not probable as there is no consensus ERE present in the promoter of the p53 gene an indirect activation of p53 is possible via an activation of c-myc [31]. The E2 activated transcriptional activity of the ER is repressed by a physical interaction of p53 with the ER at its responsive element in E2 dependent genes [32]. In conclusion, there seems to be a finely tuned dependence of the expression of BRCA-1, p53 and p21 that is influenced by melatonin via the membrane-bound receptor MT1.
Estradiol is a potent regulator of c-myc transcription and the P1 promoter of p53 contains a c-Myc responsive element CATGTG +70 base pairs from the transcription start site [19]. c-myc decreased after treatment of E2 stimulated cells with 10−9 m melatonin. This finding is not in complete contrast to the data of Molis et al. [21] who observed a 90% increase of c-myc mRNA after treatment of MCF-7 cells with 10−9 m melatonin in estrogen deficient medium. The c-myc expression following melatonin treatment after estradiol stimulation in Fig. 6B was still 55% above the level measured in the control cells deprived from estradiol for 48 hr.
In summary, the anti-estrogenic effect of melatonin is detectable by the expression of all four estrogen-responsive genes analyzed, namely, BRCA-1, p53, p21WAF and c-myc. The comparison of the four breast cancer cell lines revealed that the attenuation of the effect of estradiol on the expression of these for important genes clearly depends on the expression of MT1. In the two breast cancer cell lines with highest expression of MT1, namely MCF-7Mel1a and MCU-1 the increase of the four estrogen-responsive genes was most strongly depressed. There was no correlation between the expression of the nuclear receptor RZRα and the effects of melatonin on these genes.
Acknowledgments
This study was supported by the German Federal Ministry for Environment, Nature Conservation and Nuclear Safety, grant numbers StSch4219 and StSch4461. We thank Dipl. Ing. Hartmut Schimming, University of Ulm, Germany for the conception of the exposure chambers and supervision of the construction of the electromagnetic field incubators used for the experiments. We are grateful to Prof. Steven M. Hill, Tulane University, New Orleans for providing the cell line MCF-7Mel1a transfected with a vector carrying the MT1 gene. We thank Ms Hiltrud Schulz and Matthias Läsche for excellent assistance.




