Altered basal and postprandial plasma melatonin, gastrin, ghrelin, leptin and insulin in patients with liver cirrhosis and portal hypertension without and with oral administration of melatonin or tryptophan
Abstract
Abstract: This investigation was designed to assess the effects of oral administration of melatonin (10 mg) and tryptophan (Trp) (500 mg) on fasting and postprandial plasma levels of melatonin, gastrin, ghrelin, leptin and insulin in 10 healthy controls and in age-matched patients with liver cirrhosis (LC) and portal hypertension. Fasting plasma melatonin levels in LC patients were about five times higher (102 ± 15 pg/mL) than in healthy controls (22 ± 3 pg/mL). These levels significantly increased postprandially in LC patients, but significantly less so in controls. Treatment with melatonin or l-Trp resulted in a further significant rise in plasma melatonin, both under fasting and postprandial conditions, particularly in LC patients. Moreover, plasma gastrin, ghrelin, leptin and insulin levels under fasting and postprandial conditions were significantly higher in LC subjects than in healthy controls and they further rose significantly after oral application of melatonin or Trp. This study shows that: (a) patients with LC and portal hypertension exhibit significantly higher fasting and postprandial plasma melatonin levels than healthy subjects; (b) plasma ghrelin, both in LC and healthy controls reach the highest values under fasting conditions, but decline postprandially, especially after oral application of melatonin or Trp; and (c) plasma melatonin, gastrin, ghrelin and insulin levels are altered significantly in LC patients with portal hypertension compared with that in healthy controls possibly due to their portal systemic shunting and decreased liver degradation.
Introduction
Previous studies demonstrated that patients with liver cirrhosis show altered postprandial plasma levels of gut hormones, e.g. ghrelin, insulin and leptin presumably because of their impaired degradation in the liver and shunting of portal blood around the liver due to portal hypertension and formation of porto-systemic collaterals [1, 2]. Ghrelin and leptin are known to influence energy expenditure and energy intake in humans [3]. Leptin suppresses energy intake and stimulates energy expenditure, while ghrelin rises just before the meal to enhance appetite and food intake. Both leptin and ghrelin have been reported to be deranged in liver cirrhosis [4–11]. It has been suggested that increased insulin release induced by a meal may be responsible for the depression of postprandial plasma ghrelin [12–14] and acute increase in leptin levels observed after a meal in healthy subjects [15]. An inverse relation between leptin and ghrelin has been documented and leptin has been proposed to contribute to the suppression of ghrelin level in normal insulinemic subjects after a meal [16, 17].
Gastrointestinal tract (GIT) is also a major source of other enterohormones such as melatonin generated by entero-endocrine (EE) cells distributed throughout the entire GIT [18, 19] and gastrin, produced by G cells present mainly in antral mucosa and involved in the stimulation of gastric acid secretion [19, 20]. To date, little attention has been paid to changes in plasma levels of melatonin and gastrin in healthy subjects and patients with liver cirrhosis under basal and postprandial conditions and following administration of exogenous melatonin and l-tryptophan (Trp), the precursor of melatonin in the GIT mucosa [19]. It should be emphasized that the GIT mucosa shows pathways of melatonin biosynthesis similar to those in the pineal gland, but based on experiments in animals, it was calculated [17–19] that the total amounts of this indoleamine produced daily in GIT may be about 500 times greater than those secreted by pineal gland. It is of interest that this gut-derived melatonin, found in high concentrations in portal circulation, exhibits changes dependent upon the food intake rather than circadian light/night rhythm [19, 21–24]. Gastrointestinal melatonin released into the portal circulation seems to contribute to the maintenance of basal plasma levels of this indoleamine in pinealectomized animals, but most of the indole released by intestinal EE cells into the portal blood is efficiently taken up from the blood by the liver [25]. Here, melatonin is metabolized mainly to 6-sulfatoxymelatonin and released as the degradation product into the bile which may protect the duodenal unit (liver, biliary tree and pancreas) and the intestines from damaging substances.
Thus far, only a few studies have been performed in humans to determine the changes in plasma levels of melatonin when its liver uptake is impaired such as in patients with liver cirrhosis. These studies showed an elevation of plasma concentrations of this indoleamine, possibly due to its decreased metabolic clearance in cirrhotic liver, related to reduced liver blood flow, lower activity of 6-β-hydroxylase, impaired intrahepatic transport system and decreased elimination of melatonin metabolites in cirrhotic liver [21–24]. It is of interest that cirrhotic patients exhibit alterations of plasma melatonin rhythm accompanied by a prolonged rise in this hormone during daytime and its ‘out of phase’ secretion [23, 24]. They were found to show higher prevalence of sleep disturbance [26]. Several experimental studies demonstrated that melatonin is capable of ameliorating the liver damage induced experimentally by carbon tetrachloride [27], thioacethamide [28] or high-fat diet [29]. This protective action of melatonin against acute liver and gall bladder damage has been attributed to the inhibition of oxidative stress and the reduction in pro-inflammatory cytokine production by experimentally damaged liver [30] and gall bladder, particularly that the bile was found to contain very high concentration of melatonin [31].
Gastrin is another gut hormone that has been reported to be taken up from portal blood and partly metabolized by the liver [32, 33]. A high incidence of gastro-duodenal ulcers in patients with liver cirrhosis has been attributed to elevated concentrations of plasma gastrin [32] and progastrin [33] and portal hypertensive gastropathy [34] accompanied by impaired urinary gastrin output [35]. Furthermore, cirrhotic patients exhibit abnormally high sensitivity to gastric secretory stimulants such as endogenous or exogenous gastrin [36]. Liver, as some other organs such as kidneys, are responsible for the removal of circulating gastrin [37] but only few studies [21, 32, 33] have been undertaken to assess the changes in plasma gastrin in cirrhotic patients. In addition, plasma insulin and glucagon levels have been reported to increase in cirrhotic patients and this has been attributed to reduced hepatic extraction of these hormones leading to increased systemic delivery of these hormones through extrahepatic porto-systemic shunting secondary to spontaneous splanchnic collaterals [38–40].
The purpose of this study was to assess the plasma levels of melatonin, gastrin, ghrelin, leptin and insulin in healthy controls and in patients with liver cirrhosis and portal hypertension under fasting conditions and following a protein-rich meal without and with supplementation with exogenous melatonin or Trp.
Materials and methods
Patients
Ten consecutive male patients with liver cirrhosis attending the outpatient clinic of the Department of Gastroenterology of Lublin Medical University, Lublin and 10 age- and weight-matched male healthy volunteers (58–65 kg b.w.) were enrolled in the study. The study was approved by Ethics Research Committee of the University of Lublin and informed consent was obtained from each subject tested. The severity of liver cirrhosis was assessed according to the Child-Pugh score [41, 42] and mostly class B patients with portal hypertension and normal levels of creatinine were enrolled in the study. Patients with malignancy, infections, known gastrointestinal or renal diseases, significant cardiac or respiratory dysfunctions, insulin-dependent diabetes mellitus, untreated thyroid dysfunction and hepatic encephalopathy were excluded. Patients with alcoholic cirrhosis had been abstinent for over 6 months at inclusion. All patients had undergone endoscopy showing the presence of esophageal varices as an evidence of portal hypertension, but none had macroscopic evidence of gastric mucosal atrophy. None had peripheral edema and their body mass index (BMI) matched that of healthy weight stable volunteers, including mainly healthy professionals, acting as controls. Most of them participated in several previous studies as healthy volunteers before and none was taking any medications, none was obese and all denied alcohol consumption and had normal liver tests.
Experimental design
On the test day, cirrhotic patients and healthy volunteers were recruited in the morning after an overnight fast (10 hr) and were offered about 500 kcal test meal including rolls with 450 mL of milk and meat cheese sandwich, consisting of 40% of energy as carbohydrate, 30% of energy as fat and 30% of energy as protein. The patients were instructed to take either meal alone or (on separate day) similar meal but preceded 10 min by intake of melatonin (two tablets of 5 mg, LEL-AM, Zakroczyn, Poland) or Trp (one tablet of 500 mg, Ardeytropin; Ardeypharm, Herdecke, Germany). Blood samples for assay of plasma melatonin, gastrin, ghrelin, leptin and insulin melatonin were drawn from peripheral vein through indwelling cannula at baseline just before food intake and 15 and 30 min after the start of the test meal. The blood samples were collected in EDTA-coated polypropylene tubes and centrifuged at 1500 g for 20 min at 4°C, and supernatant clear plasma was then stored at −80°C until measurements of plasma hormone levels using specific radioimmunoassays.
Hormone measurements
Plasma melatonin levels were assessed using human melatonin RIA kit bought from DRG Diagnostics GmbH, Marburg, Germany. The detection limit for melatonin was 2.5 pg/mL and the intra- and inter-assay variations were 5% and 12% respectively. Plasma gastrin was determined using gastrin anti-serum No. 4562 (kindly supplied by Professor Jens Rehfeld from Copenhagen University, Denmark) and employed in final dilution of 1:500,000. The sensitivity of the amidated gastrins was about 5 pmol/L. The intra- and inter-assay variations were 5.4% and 7.5% respectively. This antiserum recognizes the C-terminal-amidated end of human gastrin-17 (G-17) and G-34 as described previously and was employed in our laboratory for about 20 yr [43]. The plasma ghrelin levels were assessed using commercially available human ghrelin RIA kit bought from Phoenix Pharmaceutical Inc, Burlingame, CA, USA, and plasma leptin levels using lepin human RIA kit purchased from Linco Research Inc., St Louis, MO, USA. The detection limit for ghrelin was 12 pg/mL and intra- and inter-assay variations were 10% and 14% respectively. The detection limit for leptin was 70 pg/mL and intra- and inter-assay variations were 3.5% and 5% respectively. Plasma insulin concentration was measured using RIA kit (INS-Irma) bought from Biosource Europe S.A., Nivelles, Belgium, and used in accordance with the manufacturer’s instruction. The detection limit was 1.5 μU/mL and the intra- and inter-assay variations were 4% and 5% respectively.
Statistical analyses
Data are expressed as mean ± S.E.M. Results were analyzed using the Student’s t-test. A value of P < 0.05 was considered statistically significant.
Results
Fasting and postprandial levels of plasma melatonin in control healthy subjects and in cirrhotic patients are presented in Fig. 1. Basal fasting levels of plasma melatonin in healthy controls are about five times lower (22 ± 3 pg/mL) than those recorded in fasting cirrhotic patients (102 ± 15 pg/mL); this difference is statistically significant. Following ingestion of melatonin or Trp, fasting plasma melatonin concentrations in healthy controls showed a small but significant rise (Fig. 1; upper panel). In cirrhotic patients, these values rose about twice over those recorded in fasting patients not receiving melatonin or Trp (Fig. 1; lower panel). Following a test meal, the plasma levels of melatonin in healthy controls did not change significantly, but they rose significantly over fasting levels in these subjects at 15 and 30 min after meal when melatonin or Trp was given orally. Cirrhotic patients showed significantly higher plasma melatonin under fasting and postprandial conditions, especially when melatonin or Trp was supplemented before the meal (Fig. 1; lower panel).
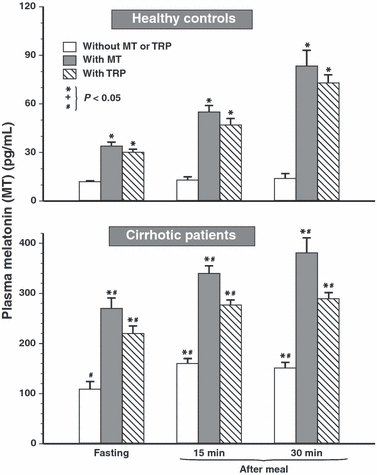
Plasma concentrations of melatonin in healthy controls and cirrhotic patients under fasting conditions and at 15 and 30 min postprandially in tests without and with oral administration of melatonin (10 mg) or l-tryptophan (Trp) (500 mg). Asterisk indicates (P < 0.05) significant increase above control value in tests under fasting or postprandial conditions when neither melatonin nor Trp was administered orally. Slash indicates significant increase above the values recorded in healthy controls.
Fasting plasma gastrin concentrations in healthy controls averaged 16 ± 1.5 pg/mL and at 15 and 30 min after meal they rose significantly to 51 ± 4.2 and 60 ± 3.7 pg/mL respectively. In cirrhotic patients, fasting gastrin levels averaged 76 ± 7.4 pg/mL and in tests with supplementation of melatonin or Trp plasma gastrin they showed further significant increments. After 15 and 30 min upon ingestion of the meal, plasma gastrin reached, respectively, 109 ± 8.3 and 104 ± 10 pg/mL, and these increments were significantly higher than those in fasting patients. In tests with meal combined with oral administration of melatonin or Trp in cirrhotic patients, plasma gastrin increased significantly above the levels recorded after a meal alone without pretreatment with melatonin or Trp in these patients (Fig. 2).
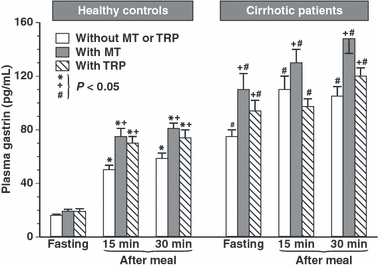
Plasma concentrations of gastrin in healthy controls and cirrhotic patients under fasting conditions and at 15 and 30 min postprandially in tests without and with oral administration of melatonin (10 mg) or l-tryptophan (Trp) (500 mg). Asterisk in healthy controls indicates significant (P < 0.05) increase above fasting values in tests without or with oral administration of melatonin or Trp. Cross indicates significant increase in plasma gastrin levels observed postprandially compared with postprandial gastrin levels in these subjects in tests without or with oral administration of melatonin or Trp (on the left). In cirrhotic patients, cross indicates significant increase in plasma fasting or postprandial gastrin levels above the values obtained in these patients not supplemented with oral melatonin or Trp. Slash in cirrhotic patients indicates significant increase in plasma gastrin levels when compared with those recorded in healthy controls (on the right).
Fasting plasma ghrelin levels in healthy controls (85 ± 6 pg/mL) were significantly higher than those recorded in these subjects at 15 and 30 min after a meal (50 ± 5.1 and 41 ± 3 pg/mL). Pretreatment with melatonin or Trp reduced significantly fasting and postprandial plasma ghrelin levels in these subjects (Fig. 3). In cirrhotic patients, fasting plasma ghrelin concentration was almost twice as high as in healthy controls (181 ± 19 pg/mL), and these levels were also significantly reduced at 15 and 30 min after food intake and further significantly attenuated when melatonin or Trp was applied intragastrically in these patients (Fig. 3).
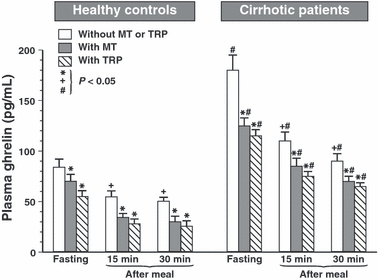
Plasma concentrations of ghrelin in fasting period and at 15 or 30 min after a meal in healthy controls and cirrhotic patients in tests without and with oral administration of melatonin (10 mg) or l-tryptophan (Trp) (500 mg). Asterisk indicates (P < 0.05) significant decrease below the control value observed in tests under fasting or postprandial conditions when neither melatonin nor Trp was administered orally. Cross indicates significant decrease in plasma ghrelin below that observed in healthy controls or cirrhotic patients when neither melatonin nor Trp was administered. Slash in cirrhotic patients indicates significant increase above the corresponding values recorded under fasting or post.
Plasma leptin concentrations in healthy subjects showed similar values under fasting (1.6 ± 0.2 ng/mL) and postprandial conditions after 15 min (1.4 ± 0.1 ng/mL) and after 30 min (1.5 ± 0.2 ng/mL), but, in cirrhotic patients, these values were several times higher both under fasting (10.5 ± 1.7 ng/mL) and postprandial (10.2 ± 1.6 ng/mL) conditions than in healthy subjects. Administration of melatonin or Trp caused a small but significant increase in fasting and postprandial levels of leptin in healthy subjects but reduced those levels observed after meal in cirrhotic patients when compared with the values obtained in these patients without administration of melatonin or Trp (Fig. 4).
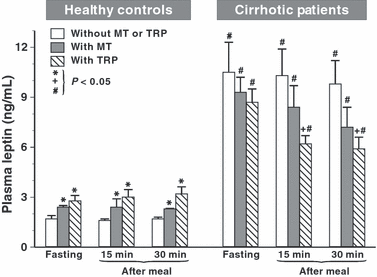
Plasma concentrations of leptin in healthy controls and cirrhotic patients under fasting conditions and at 15 and 30 min after a meal in tests without and with oral administration of melatonin (10 mg) or l-tryptophan (Trp) (500 mg). Asterisk in healthy controls indicates (P < 0.05) significant increase in plasma leptin above control values in tests under fasting or postprandial conditions when neither melatonin nor Trp was administered orally. Slash in cirrhotic patients indicates significant increase above the values recorded in healthy controls. Cross in cirrhotic patients indicates significant decrease below the control value when neither melatonin nor Trp was administered.
Fasting plasma insulin levels in healthy controls averaged 8.8 (±0.9 μU/mL and showed several fold rise after a meal (33.6 ± 6.2 μU/mL at 15 min and 57 ± 7.1 μU/mL at 30 min). In patients with liver cirrhosis both fasting and postprandial levels of plasma insulin were significantly higher than in healthy controls (Fig. 5). Administration of melatonin or Trp to healthy controls failed to affect fasting insulin levels but increased significantly postprandial rise (at 15 min) in plasma levels of insulin. This increase in plasma insulin levels by the administration of melatonin or Trp was especially marked under postprandial conditions in cirrhotic patients.
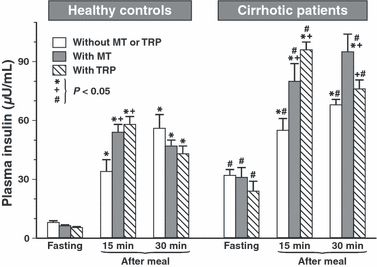
Plasma concentrations of insulin in healthy controls and cirrhotic patients under fasting conditions and at 15 and 30 min after a meal in tests without and with oral administration of melatonin (10 mg) or l-tryptophan (Trp) (500 mg). Asterisk in healthy controls and cirrhotic patients indicates significant (P < 0.05) increase above control value in tests under fasting conditions. Cross indicates significant increase observed in above the control postprandial value when neither melatonin nor Trp was administered orally. Slash indicates significant increase above the values recorded under corresponding fasting or postprandial conditions in healthy controls.
Discussion
This study was carried out on healthy volunteers and age-, weight- and gender-matched patients with liver cirrhosis and portal hypertension in an attempt to assess the role of the liver in the metabolism of major plasma hormones originating from the GIT and transported in the portal blood to the liver. We confirmed that the enterohormones, especially melatonin, gastrin, ghrelin, leptin and insulin, undergo some modifications in the liver. In the cirrhotic liver when mechanical obstruction to portal flow caused by fibrotic disruption of liver architecture and dynamic component due to active constriction of liver vascular smooth muscle, the porto-systemic shunts develop and up to 90% of portal blood may flow through numerous porto-systemic collaterals to the heart [44]. This results in the reduction in the liver uptake of hormones originating in the GIT and in the alteration of liver metabolism of these hormones.
It was found previously in experimental animals [18, 19] that GIT melatonin biosynthesized in large amounts from its precursor, Trp, in numerous entero-endocrine (EE) cells via the same biosynthetic pathways as in pinealocytes, is released into the portal blood and then almost completely taken up and metabolized by the liver [31]. Rather than being exclusively metabolized by the liver, portal melatonin may be shunted into the bile to protect the biliary tree from oxidative damage [31]. The aim of this study was to determine the importance of the liver in the degradation of melatonin in humans. As the withdrawal of portal blood in humans for measurement of plasma hormones is not technically possible, we used patients with liver cirrhosis with portal hypertension and porto-systemic shunts under basal and postprandial conditions without and with oral supplementation of melatonin or Trp to test the role of the liver in melatonin metabolism. We found that, indeed, the liver has an impressive influence on the uptake and metabolism of exogenous melatonin and that formed in GIT from the orally supplemented Trp in humans. As documented in experimental animals and humans [18–20], under fasting conditions, the melatonin is absorbed from the GIT after its oral administration or generated from the Trp given orally and is carried out in the portal blood to the liver in many times higher concentrations than in systemic circulation. This is supported by our present observations in cirrhotic patients with portal hypertension, in whom most of the portal blood is shunted by collaterals to systemic circulation, resulting in several fold higher plasma melatonin levels than in healthy controls. The oral supplementation of melatonin or its precursor, Trp, resulted in a significant rise in fasting and postprandial plasma melatonin in healthy subjects, indicating that although most of the portal melatonin is taken up by the liver and shunted into the bile, some of this indoleamine escapes liver uptake and reaches systemic circulation. However, markedly increased plasma melatonin concentrations were observed in cirrhotic patients given orally small dose (10 mg) of melatonin or its precursor (500 mg) probably due to port-systemic shunting of portal blood in these patients and/or decreased liver uptake of this indole caused by liver cirrhosis.
It should be emphasized that only few studies of the plasma pharmacokinetics of melatonin have been performed to explain the role of the liver in the metabolism of this idoleamine. Waldhauser et al. [45] reported that after ingestion of crystalline melatonin (80 mg) in gelatine capsule, the peak serum of melatonin level rose from 350 to 1000 times over that occurring physiologically at night-time with an elimination half-life of about 0.40 hr. Alghous et al. [46] used melatonin in smaller dose (2 mg) in a gelatin capsule, corn oil preparation or slow release pills given orally in three subjects and observed that peak plasma melatonin concentrations occurred within 1–2 hr following oral ingestion of these preparations. They noticed that the peak plasma hormone concentrations depended upon the nutritional status of the subjects.
It should be emphasized that these initial pharmacokinetic studies on humans assumed that the major source of plasma melatonin is the pineal gland without considering that the GIT may also contribute to the maintenance of plasma melatonin. Only Messner et al. [47] studied the distribution of plasma melatonin in human hepato-biliary-GIT, revealing high concentrations of melatonin in gastric and duodenal mucosa with large amounts of this indoleamine excreted in the bile and higher concentrations of this indole in the portal than systemic blood at any point of the circadian period.
The fact that melatonin may also originate from the GIT was first reported by Raikhlin and Kvetnoy [48], who discovered serotonin-rich EE cells producing melatonin and releasing of this indoleamine into the portal vein in rats. Thereafter, Huether et al. [49] showed that oral application of Trp in rats causes a rapid elevation of circulating melatonin reaching much higher levels than those recorded after parenteral administration of this amino acid. The quantitative determination of the melatonin originating from the GIT when compared with that produced by the pineal gland was carefully studied in pigs by Bubenik et al. [50], who found that the highest concentrations of melatonin in these animals can be recorded in portal blood after feeding and that this GIT-originated indoleamine in portal blood does not show any circadian rhythm.
We reported recently [51], that the rat gastric mucosa is expressing mRNA for two major enzymes involved in the biosynthesis of melatonin, i.e. arylalkylamine-N-acetyltransferase (AA-NAT), that converts serotonin to N-acetyl serotonin and hydroxyindole-O-methyltransferase (HIOMT), which transforms N-acetylserotonin to melatonin. The expression of mRNA for both NAT and HIOMT was significantly increased in rats supplemented with intragastrically applied melatonin or Trp and accompanied by enhanced healing phase of gastric ulcers, suggesting that locally generated melatonin and/or its metabolites [52, 53] in gastric mucosa may contribute to the healing of gastric lesions. This reinforces previous notion that GIT mucosa is capable of biosynthesizing melatonin from Trp through similar pathways as pinealocytes. Our present study compared the plasma levels of melatonin in systemic circulation in humans with intact healthy liver and in patients with liver cirrhosis and portal hypertension with obvious porto-systemic shunting of portal blood to the systemic circulation. In general, we confirmed previous observations made on pigs and rats [50, 51] that in humans the GIT is the major source of circulating melatonin and that it may originate from ingested Trp.
The major gastric hormone, responsible for the stimulation of gastric acid secretion via activation of the enterochromaffin-like (ECL) cells and histamine release is gastrin secreted by the G cells located predominantly in the antral mucosa [20]. We showed previously that viral hepatitis and liver cirrhosis are accompanied by an increase in plasma elevation of gastrin and progastrin [33]. This remains in agreement with other reports showing that liver cirrhosis results in the increase in plasma gastrin and higher incidence of gastric peptic ulcerations [32], possibly due to increased gastric acid secretion caused by enhanced sensitivity of parietal cells to excessive plasma gastrin [35, 36]. Our present report is in keeping with the above observations and demonstrates that plasma gastrin is increased in tests in healthy controls given exogenous melatonin or its precursor, Trp, confirming our previous finding that melatonin is an effective stimulant of gastrin release [51]. Liver cirrhosis is accompanied by a marked increase in basal and postprandial plasma gastrin when compared with that in healthy controls. This enhancement of plasma gastrin concentration in cirrhotic patients [32] could be attributed, at least in part, to the decrease in the degradation of this hormone by the damaged liver and perhaps also to the increased stimulation by melatonin of the G cells of the stomach to release gastrin.
Ghrelin and leptin are key hormones that influence energy intake and energy expenditure [3, 19]. Ghrelin has been shown to rise just before meal to stimulate the appetite and food intake, but then declines, whereas leptin release was reported to rise postprandially to suppress food intake [19]. Both appetite controlling peptides originate, at least in part from the stomach, and inverse relationship has been suggested between ghrelin and leptin with leptin suppressing ghrelin release [19]. According to our observations, plasma leptin in healthy subjects remains unaffected by food intake, but it reaches significantly higher levels just before the meal in cirrhotic patients and then declines gradually at 15 and 30 min after the meal, particularly in tests with oral supplementation of melatonin and Trp. By contrast, the plasma fasting ghrelin, that showed several fold higher levels in cirrhotic patients than in healthy controls, declined throughout the postprandial periods. It is of interest that following administration of exogenous melatonin or its precursor Trp, both fasting and postprandial ghrelin levels showed a significant reduction in both healthy controls and cirrhotic patients, suggesting that melatonin is capable of decreasing plasma levels of ghrelin in these patients. This confirms that melatonin exerts a potent inhibitory influence on the secretion of ghrelin both in healthy controls with reduced levels of this hormone and in cirrhotic patients with highly increased plasma ghrelin concentrations. The elevation in plasma ghrelin under fasting conditions in cirrhotic patients could be simple explained by the impairment of liver degradation of this orexigenic peptide by the cirrhotic liver, but the decrease in plasma ghrelin observed postprandially, particularly in cirrhotic patients might contribute to the reduction in appetite observed in cirrhotic patients. This decrease in plasma ghrelin could be attributed to the marked elevation of plasma leptin observed in melatonin- or Trp-treated healthy subjects and cirrhotic patients. An alternative explanation for the decrease in plasma ghrelin after a meal both in healthy subjects and cirrhotic patients could be attributed to increased plasma insulin levels observed in both healthy subjects and cirrhotic patients. Insulin has been reported to be essential for meal-induced ghrelin suppression [12–14, 16] and our results are in accordance with these findings.
In summary, the liver is the crucial organ for the degradation of numerous gut hormones, especially melatonin, gastrin, leptin, ghrelin and insulin, and this can be conveniently examined by comparing plasma levels of these hormones in healthy subjects with those in cirrhotic patients with portal hypertension and marked porto-systemic shunts.




