Melatonin ameliorates nonalcoholic fatty liver induced by high-fat diet in rats
Abstract
Abstract: Nonalcoholic fatty liver disease (NAFLD) is an increasingly recognized condition that may progress to end-stage liver disease, which ranges from simple steatosis to steatohepatitis, advanced fibrosis, and cirrhosis. Oxidative stress and lipid peroxidation are key pathophysiological mechanisms in NAFLD. We investigate the preventive effects of intraperitoneal administration of melatonin (2.5, 5, 10 mg/kg, daily, respectively) in NAFLD rats induced by high-fat diets for 12 wk. Liver damage was evaluated by serological analysis, serum and hepatic lipid assay as well as hematoxylin–eosin staining in liver sections. Oxidative stress and lipid peroxidation were assessed by measuring malondialdehyde (MDA) levels and superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) activities in liver. The results showed that high-fat diet induced oxidative stress with extensive liver steatosis in rats. Melatonin (5 or 10 mg/kg) was effective in reducing hepatic steatosis and inflammation with lowering serum alanine aminotransferase, aspartate aminotransferase, and levels liver total cholesterol and triglycerides in high-fat diet rats. Moreover, melatonin (2.5, 5, 10 mg/kg) increased SOD and GSH-Px activities and the 10 mg/kg dose of melatonin reduced MDA levels in liver. This study shows that melatonin exerts protective effects against fatty liver in rats induced by high-fat diet possibly through its antioxidant actions.
Introduction
Nonalcoholic fatty liver disease (NAFLD) is an increasingly recognized condition that may progress to end-stage liver disease. It is characterized by its association with obesity, diabetes, and hyperdyslipidemia. NAFLD refers to the wide spectrum of liver damage that ranges from simple steatosis to steatohepatitis, advanced fibrosis and cirrhosis [1–4]. To dates, no medications have been proved to directly reduce or reverse liver damage independent of weight loss, but such medications would be desirable [2]. Oxidative stress is implicated in the pathogenesis of NAFLD. Some studies have found that hepatic and plasma oxidative stress-related parameters were correlated with clinical and histological findings [5, 6]. These findings suggest that impaired antioxidant defense mechanisms may be an important factor in the pathogenesis of NAFLD. Treatment approaches that affect the antioxidant enzymes may be beneficial in patients with NAFLD.
Melatonin (N-acetyl-5-methoxytryptamine), a lipophilic indoleamine derived from tryptophan, is synthesized chiefly by the pineal gland but has recently been detected in many other tissues as well. It has a variety of important physiological and biochemical functions [7, 8]. It also has been shown to be a powerful antioxidant which scavenges reactive oxygen and reactive nitrogen species [7, 9]. The antioxidant properties of melatonin have been shown experimentally to protect against aging, cancer, liver injury, and atherosclerosis [10].
The aim of the present study was to evaluate the effects of melatonin on liver function, liver injury, lipid metabolism in serum and liver as well as oxidative stress in rats with nonalcoholic fatty liver induced by high-fat diet.
Material and methods
Animals
Male Wistar rats, weighing between 170 and 200 g at the beginning of the study, were purchased from Shanghai SLAC Laboratory Animal Co., China (SPF, Certificate No. 0006613). The animals were housed five per cage in rooms maintained temperature at 22–25°C and humidity at 50 ± 5% with 12 hr light–dark cycles and given food and water ad libitum. All animal studies were performed with the approval of the Animal Care and Use Committee of Anhui Medical University, China.
Experimental design and treatments
Fifty animals were given a normal diet for 1 wk to be adapted to vivarium conditions and then randomly divided into five groups: (i) control group: animals were treated with a standard laboratory rat diet; (ii) high-fat diet group: animals were treated with high-fat diet; (iii) MEL1 group: animals were treated with high-fat diet and injected intraperitoneally with melatonin (2.5 mg/kg); (iv) MEL2 group: animals were treated with high-fat diet and injected intraperitoneally with melatonin (5 mg/kg); (v) MEL3 group: animals were treated with high-fat diet and injected intraperitoneally with melatonin (10 mg/kg). The high-fat diet contained 87.7% standard diet (w/w), 10% pork fat (w/w), 2% cholesterol (w/w) and 0.3% bile salt (w/w). Melatonin (Sigma-Aldrich, St Louis, MO, USA) was dissolved in absolute ethanol and diluted further with 0.9% NaCl (w/v) with the final concentration of ethanol in 0.9% NaCl being ≤1% (v/v). The animals in control and high-fat diet groups were intraperitoneally injected with the same volume of vehicle (0.9% NaCl containing 1% ethanol). Melatonin or vehicle was administrated daily for 12 wk. Twenty-four hours after the last injection, rats were anesthetized by intraperitoneal injection of 3% butaylone (1 mL/kg) and killed. Blood was collected from the abdominal aorta, after which the livers were quickly excised from the animals. Blood was collected into tubes and centrifuged (1500 × g, 10 min, 4°C). Serum was aspirated and assayed as described below. The livers were quickly weighed and pieces were frozen in liquid nitrogen for the measurement of liver lipid, malondialdehyde (MDA), superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) status. Pieces of the remaining liver tissue were processed for histology. Liver index of rats is expressed as the percentage of wet liver weigh to body weigh (%).
Blood chemistries and hepatic lipid analyses
Alanine aminotransferase (ALT), aspartate aminotransferase (AST), triglyceride and total cholesterol levels in serum were assayed using an automatic biochemistry analyzer (CL-7300; SHIMADZU Corporation, Kyoto, Japan) with assay kits (Shanghai Kehua Bioengineering Co., Ltd, Shanghai, China). 0.5 g liver was homogenized and extracted with chloroform:methanol (2:1, v/v) [11]. Hepatic cholesterol and triglyceride levels were enzymatically assayed using kits provided by Nanjing Jiancheng Institute of Biotechnology, China. Protein concentration in liver was determined by the Bradford method with a commercial kit (Nanjing Jiancheng Institute of Biotechnology, Nanjing, China) using bovine serum albumin as a standard.
Measurement of MDA, SOD and GSH-Px levels in liver
Liver tissue was homogenized in Tris-HCl (5 mmol/L containing 2 mmol/L EDTA, pH 7.4). Homogenate was centrifuged (1000 × g, 10 min, 4°C). The supernatant was used immediately for the assays of MDA, SOD and GSH-Px. These were determined following the kit instructions (Nanjing Jiancheng Institute of Biotechnology). In brief, MDA in liver tissue was determined by the thiobarbituric acid method [12]. The assays for SOD and GSH-Px were based on their ability to inhibit the oxidation of oxyamine by the xanthine–xanthine oxidase system.
Histopathological examination of liver
One piece of liver tissue which was removed from the same part in left lobe of each rat was fixed in buffered 4% formaldehyde in phosphate-buffered solution and embedded in paraffin. Sections were cut at a thickness of 4–6 μm and stained with hematoxylin and eosin. Light microscopic examinations were performed in a blinded fashion by two pathologists and histological grade for hepatic steatosis was determined according to Brunt et al. [13] as follows: 0, no steatosis, normal liver; I, ≤25% of hepatocytes affected; II, 26–50% of hepatocytes affected; III, 51–75% of hepatocytes affected; IV, ≥76% of hepatocytes affected. Additionally, a histological grade for steatohepatitis based on the Brunt's grading system was assigned [13].
Statistical analysis
Date are expressed as mean ± S.E.M.; statistical analysis of the quantitative data for multiple comparisons between experimental groups were performed by one-way ANOVA with a least significant test (LSD). The frequency data were compared using the Ridit procedure. Values of P < 0.05 were considered significant.
Results
As shown in Table 1, the body weight in the control rats was not significantly different compared with the high-fat diet group at the end of the experiment, but body weight was reduced in the MEL3 rats. Wet liver weight and liver index rose significantly in high-fat diet rats, but were reduced in the MEL2 and MEL3 rats (Table 1).
| Groups | Body weight (g) | Wet liver weight(g) | Liver index (%) |
|---|---|---|---|
| Control | 363 ± 5.2 | 9.6 ± 0.16* | 2.7 ± 0.00** |
| High-fat diet | 373 ± 5.2 | 13.7 ± 0.51 | 3.7 ± 0.00 |
| MEL1 | 369 ± 6.3 | 13.0 ± 0.36 | 3.5 ± 0.00 |
| MEL2 | 351 ± 8.3* | 11.7 ± 0.38* | 3.4 ± 0.00* |
| MEL3 | 339 ± 6.4** | 11.1 ± 0.23* | 3.3 ± 0.00* |
- Results are mean ± S.E.M. of 10 rats. MEL1: melatonin (2.5 mg/kg); MEL2: melatonin (5 mg/kg); MEL3: melatonin (10 mg/kg). The rats that received melatonin also were fed a high-fat diet.
- *P < 0.05, **P < 0.01 versus high-fat diet group.
ALT and AST were measured in order to evaluate the liver function. The results showed that ALT and AST levels were significantly higher in high-fat diet rats and MEL1 rats than in the control, MEL2 and MEL3 animals (Fig. 1). The serum triglycerides and total cholesterol date are shown in Fig. 2. The level of total cholesterol in serum increased significantly in high-fat diet rats, but decreased in rats given melatonin, especially in MEL3 (10 mg/kg) group though there was no significant difference when compared with high-diet only rats (Fig. 2A). The level of triglycerides in serum was lower in high-fat diet than in control rats as well as after melatonin administration (Fig. 2B). The concentrations of cholesterol and triglycerides in liver tissue of high-fat diet rats rose remarkably compared with control group, while treatment with a dose of moderate or high melatonin (5 or 10 mg/kg) reduced significantly these increases (Fig. 3A,B).
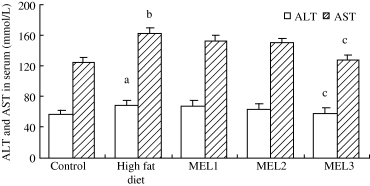
Effects of melatonin on serum alanine aminotransferase and aspartate aminotransferase in rats fed a high-fat diet. Results are mean ± S.E.M. of 10 rats. MEL1: melatonin (2.5 mg/kg); MEL2: melatonin (5 mg/kg); MEL3: melatonin (10 mg/kg). The rats that received melatonin also were as the high-fat diet. aP < 0.05, bP < 0.01 versus control group; cP < 0.05 versus high-fat diet group.
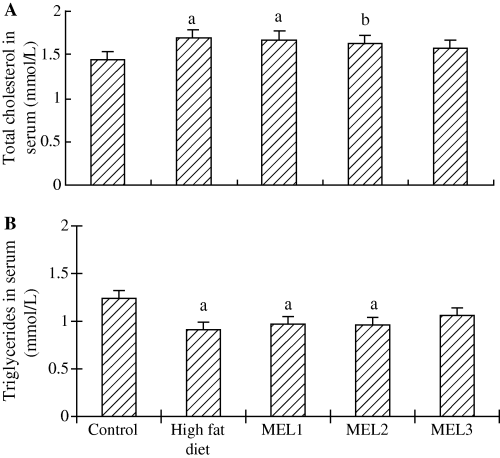
Effects of melatonin on serum total cholesterol and triglycerides in rats fed high-fat diet. Results are mean ± S.E.M. of 10 rats. MEL1: melatonin (2.5 mg/kg); MEL2: melatonin (5 mg/kg); MEL3: melatonin (10 mg/kg). The rats that received melatonin also were as the high-fat diet. aP < 0.01, bP < 0.05 versus control group.
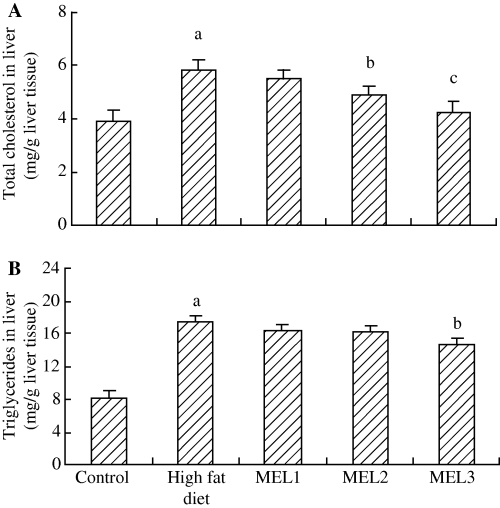
Effects of melatonin on liver total cholesterol and triglycerides in rats fed high-fat diet. Results are mean ± S.E.M. of 10 rats. MEL1: melatonin (2.5 mg/kg); MEL2: melatonin (5 mg/kg); MEL3: melatonin (10 mg/kg). The rats that received melatonin also were as the high-fat diet. aP < 0.01 versus control group; bP < 0.05, cP < 0.01 versus high-fat diet group.
Feeding a high-fat diet caused a significant increase in liver MDA level in model rats while liver SOD and GSH-Px activities decreased compared with the control group. Melatonin treatment significantly reversed these values (Fig. 4A–C).
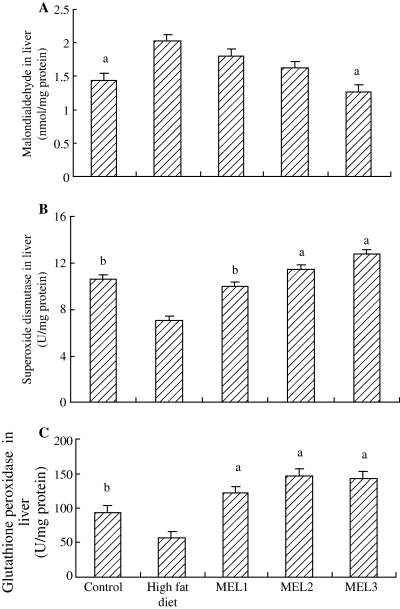
Effects of melatonin on liver malondialdehyde, superoxide dismutase and glutathione peroxidase in rats fed high-fat diet. Results are mean ± S.E.M. of 10 rats. MEL1: melatonin (2.5 mg/kg); MEL2: melatonin (5 mg/kg); MEL3: melatonin (10 mg/kg). The rats that received melatonin also were as the high-fat diet. aP < 0.01, bP < 0.05 versus high-fat diet group.
No histological abnormalities were observed in liver of control group. The hepatic parenchyma manifested normal hepatocytes arranged around the central vein. All rats in high-fat diet group had III–IV grade hepatic steatosis, and some of them showed steatosis and inflammation, which were significant improved by melatonin administration, especially in MEL2 (5 mg/kg) and MEL3 (10 mg/kg) rats (Table 2 and Fig. 5A–D).
| Groups | 0 | I | II | III | IV |
|---|---|---|---|---|---|
| Control | 10 | 0 | 0 | 0 | 0 |
| High-fat diet | 0 | 0 | 0 | 4 | 6 |
| MEL1 | 0 | 1 | 2 | 4 | 3 |
| MEL2 | 0 | 4 | 5 | 1 | 0* |
| MEL3 | 0 | 8 | 1 | 1 | 0* |
- Results are 10 fields. MEL1: melatonin (2.5 mg/kg); MEL2: melatonin (5 mg/kg); MEL3: melatonin (10 mg/kg). The rats that received melatonin also were fed a high-fat diet.
- *P < 0.001 versus high-fat diet group.
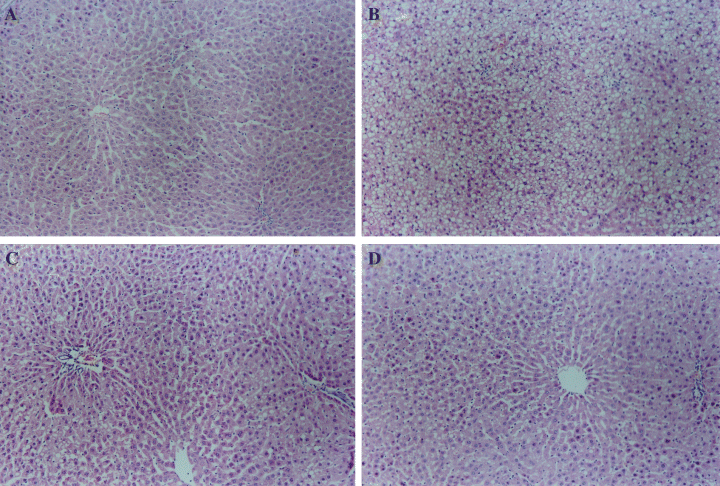
Effect of melatonin on nonalcoholic fatty liver disease in rats treated with high-fat diets. (A) control, (B) animals given with high-fat diet, (C) animals given with high-fat diet and injected intraperitoneally with melatonin 5.0 mg/kg (MEL2), (D) animals given with high-fat diet and injected intraperitoneally with melatonin 10.0 mg/kg (MEL3). Melatonin reversed significantly nonalcoholic steatohepatitis induced by high-fat diet (C and D). Magnification ×100.
Discussion
As occurs in people, NAFLD is strongly associated with obesity, diabetes, and hyperlipemia in experimental animals. Feeding a high-fat diet is commonly used to induce of nonalcoholic fatty liver in experimental animals. When animals are fed a high-fat diet, almost all species develop obesity. This has been demonstrated in nonhuman primates, pigs, dogs, cats, and rodents [14]. Lieber et al. [15] found that feeding male Sprague–Dawley rats a high-fat diet reproduced many of the features found in nonalcoholic steatohepatitis (NASH). These include fatty liver, inflammation, biochemical evidence of insulin resistance and oxidative stress, which are consistent with the current concept that the pathogenesis of NASH involves a two-hit hypothesis in which the initial metabolic disturbance causes steatosis, and the second pathogenic stimulus causes oxidative stress, lipid peroxidation, generation of reactive oxygen or nitrogen species, and a resultant steatohepatitis [16]. In the current study, we fed rats with high-fat diet plus cholesterol and bile salt for 12 wk; they developed significantly hepatic steatosis and steatohepatitis, as well as AST, ALT, total cholesterol elevations in serum and triglyceride and total cholesterol increases in liver. These findings demonstrated that we had successfully reproduced the model of NAFLD in rats.
In many published reports, melatonin shows marked protective effects against liver injuries in experimental animals induced by a variety of agents and processes, including treatment with thioacetamide [17], carbon tetrachloride [18, 19], dimethylnitrosamine [20], lipopolysaccharide [21] ochratoxin A [22], a heavy metal [23, 24] ischemia-reperfusion [25], bile duct ligation [26], a high-cholesterol diet [27], and pinealectomy [28]. The role of melatonin in reducing liver damage relates chiefly to its antioxidant properties, which are manifested in direct free radical scavenging [29, 30] and melotonin's regulation of gene transcription [31] for antioxidative enzymes. Our study showed that a moderate or high dose of melatonin (5 or 10 mg/kg) alleviated the much of the hepatic steatosis and inflammatory cell infiltration. These data indicate that melatonin prevents fatty liver induced by a high-fat diet. Previous research reports have shown mild to moderately elevated serum levels of AST and ALT are the most common and often the only laboratory abnormalities found in patients with NAFLD [32]. Tunez et al. [25] recently found that melatonin markedly suppressed the elevation of serum ALT and AST in rats treated with thioacetamide. Our results showed that a high dose of melatonin (10 mg/kg) reduced serum ALT and AST in NAFLD rats; these levels were not modified by low or moderate melatonin (2.5 or 5 mg/kg) treatment. These results also reveal that melatonin improve liver function.
Some studies have claimed melatonin has hypocholesterolemic effects [33–36]. Melatonin treatment reduced plasma, liver cholesterol levels as well as liver triglyceride levels and even aortic cholesterol levels in hypercholesterolemic mice [27]. The hypolipidemic effect of melatonin may be related to enhancement of the catabolism of cholesterol to bile acids [35], inhibition of cholesterol synthesis and LDL receptor activity [37] and actions directly on the adipose tissue through specific MT1 and MT2 receptors [38, 39]. In the current study, the moderate or high dose of melatonin (5 or 10 mg/kg) reduced high levels of cholesterol in liver of rats fed on high-fat diet while only a high dose reduced the content of hepatic triglycerides. These findings demonstrate that melatonin's effect on hepatic lipometabolism is dose dependent. However, melatonin did not cause a statistically significant change in serum lipid levels in rats given high-fat diets.
It has been proposed that oxidative stress and lipid peroxidation are key pathophysiological mechanisms in NAFLD. Oxidative stress results from an imbalance between pro-oxidant and antioxidant species that lead to oxidative damage to cellular macromolecules [40]. The predominant pro-oxidants in fatty liver are singlet oxygen, the superoxide anion radial, hydrogen peroxide, and the hydroxyl radical: these are collectively referred to as reactive oxygen species (ROS) [41], which derived from microsomal, mitochondrial, and/or other hepatocellular pro-oxidant pathways when antioxidant defenses are critically reduced. The oxidation of fatty acids is an important source of ROS in fatty liver [42]. ROS attack polyunsaturated fatty acids and initiate lipid peroxidation within the cell, which results in the formation of aldehyde by-products such as MDA and trans-4-hydroxy-2-nonenal. These molecules have the potential to diffuse from their site of origin to reach distant intracellular and extracellular targets, thereby amplifying the effects of oxidative stress [43]. Antioxidant enzymes, especially GSH-Px, reduce the levels of lipid peroxides as well as hydrogen peroxide and are important in preventing lipid peroxidation and maintaining the structure and function of biological membranes. SOD catalyses the dismutation of the superoxide anion radical to hydrogen peroxide and GSH-Px catalyses the oxidation of glutathione. In addition, certain antioxidants, such as vitamin E, betaine and acetylcysteine, have been reported to be effective in reducing serum transaminases and improving hepatic histopathology in patients with NAFLD [44–46]. Therefore, we investigated the protective role of melatonin against fatty liver induced by a high-fat diet by measuring MDA, SOD and GSH-Px in liver. The results revealed that melatonin (2.5, 5 or 10 mg/kg) markedly increased SOD and GSH-Px activities in liver of rats on a high-fat diet. At the same time, only the high dose of melatonin (10 mg/kg) reduced the concentration of MDA in rats given high-fat diets. These observations are in accordance with previous reports [21, 27]. The most important effects of melatonin are (a) the direct ability to scavenge a variety of toxic oxygen and nitrogen-based reactants, (b) stimulation of antioxidative enzymes, (c) increases the efficiency of the electron transport chain thereby limiting electron leakage and free radical generation, and (d) promoting ATP synthesis. Via these actions, melatonin preserves the integrity of the mitochondria and helps to maintain cell functions and survival [47].
In the present study, body weight of rats given a high-fat diet only was similar to of the control rats. However, body weights of melatonin-treated (5 or 10 mg/kg) rats was significantly decreased, while food (data not show) intake did not differ between control and high-fat diet rats. We also found that melatonin (5 or 10 mg/kg) markedly reduced the wet liver weight and liver index in rats treated with high-fat diets. Melatonin likely influenced body weight via reducing fat deposition in internal organs and intra-abdominal adiposity [48, 49].
In conclusion, melatonin administration in a dose-dependent manner improved the pathological abnormalities and decreased ALT, AST in serum as well as triglycerides, total cholesterol in the liver of rats given a high-fat diet. Also, hepatic MDA level was reduced while SOD and GSH-Px activities were increased in livers of rats given this diet and melatonin. These findings suggest that melatonin exert protective effects against fatty liver in rats induced by high-fat diets probably through its antioxidant actions.




