Role of calcium in the gating of isoproterenol-induced arylalkylamine N-acetyltransferase gene expression in the mouse pineal gland
Abstract
Abstract: Melatonin and its autonomic regulation serve important physiological functions. We recently demonstrated that stimulation of beta-adrenergic receptors only increases nighttime arylalkylamine N-acetyltransferase (Aa-Nat, the rate-limiting enzyme in melatonin synthesis) mRNA levels in mouse pineal gland in vitro, which suggests that pineal clocks may gate Aa-Nat gene expression. In the present study, our data reveal that cAMP analog increased Aa-Nat at any time of day but only in the presence of ionomycin. Using Fura-2AM in ratiometric calcium measurements, we show that isoproterenol stimulation increased intracellular free calcium levels at night, contrary to previous reports. Further, intra- or extracellular calcium depletion suppressed the isoproterenol-induced calcium responses as well as Aa-Nat gene expression. These results suggest calcium may be a critical factor in isoproterenol-induced Aa-Nat gene expression, which may be limited in the daytime. We also found that basal intracellular calcium levels were lower during the night and responses to isoproterenol and KCl depolarization were more robust. In addition, pineals of Cryptochrome mutant mice exhibited no significant difference between day and nighttime basal calcium or isoproterenol response. Together, these results suggest that basal calcium levels in the pineal may be controlled by the endogenous pineal clock, which may influence calcium dynamics, cellular homeostasis and sensitivity to external stimulation. Although the mechanism underlying Aa-Nat gene expression has been well studied, the role of calcium as a link between the pineal clock and Aa-Nat gene expression has been underestimated in rodent pineals.
Introduction
Melatonin, synthesized in the mammalian pineal gland, has shown involvement in physiological functions such as sleep–wake cycles, circadian rhythm modulation in the suprachiasmatic nucleus (SCN, the central circadian clock), drug addictions, regulation of cell survival, seasonal physiological changes, and those as antioxidant [1–5]. Recent study has revealed that arylalkylamine N-acetyltransferase (Aa-Nat) is the rate-limiting enzyme in melatonin synthesis during the daytime and in the early phase of the night [6]. Nocturnal elevations in melatonin and Aa-Nat mRNA levels are controlled by the SCN via the postganglionic–adrenergic mechanism [1, 7, 8]. At night, the SCN sends signals to release norepinephrine from the postganglionic sympathetic neurons, which activates the protein kinase A (PKA) signaling cascade via beta-adrenergic receptors. The activated PKA subsequently phosphorylates cAMP response element-binding protein, which activates Aa-Nat transcription via CRE in the promoter regions. Although activation of alpha-adrenergic receptors increases intracellular calcium, which modulates adenylyl cyclases activities, the impact on melatonin levels appears only moderate, as shown within the rat pineal gland where stimulation of the beta-adrenergic receptors and/or PKA signaling cascade is sufficient to increase Aa-Nat mRNA and melatonin levels.
In the past, rat pineal glands have been used in melatonin studies primarily due to mutations in Aa-Nat and/or hydroxyindole-O-methyltransferase (Hiomt) genes and consequent lack of melatonin synthesis in most commercially available mouse species [9]. However, recent studies have shown that regulation of Aa-Nat gene expression in the pineal of melatonin-less mouse is similar to that found in the melatonin-proficient mouse and rat pineal glands [10, 11]. As a series of genetic mouse models are available, and most of them are of the C57BL/6 background, it would be advantageous to use melatonin-less mouse pineal glands to study the regulation of Aa-Nat gene expression. Moreover, previous studies, including ours, have shown that the human, mouse, and hamster, but not rat, pineal glands gate Aa-Nat mRNA and melatonin responses to beta-adrenergic receptors stimulation [11–13]. In other words, stimulation with the beta-adrenergic receptor agonist isoproterenol increases Aa-Nat and melatonin levels at only limited times of day, suggesting the mouse pineal gland is a better model to study the gating of melatonin synthesis. The gating phenomenon has been reported in a variety of cell, tissue, and organ types, from transcriptional to behavioral levels [11, 14–16]. Such time of day-dependent changes in sensitivities to external stimulation are controlled by the circadian clocks, and represent an important aspect of chronobiological studies, e.g. chronotherapy and chronopharmacology. However, the molecular mechanisms underlying the gating phenomenon are not yet fully elucidated.
In the present study, we investigated the potential gating steps, which regulate time of day-dependent isoproterenol-induced Aa-Nat gene expression, and found that beta-adrenergic receptors stimulation increases calcium levels at night, but not during the day. Beta-adrenergic receptor mediated alterations in cellular calcium may be critical for the Aa-Nat gene expression, a mechanism that may have limited availability in the daytime.
Materials and methods
Animals and experimental procedures
Six-week-old male mice of the C57BL/6 and C3H strain and 6-wk-old male SD rats (Charles River Laboratories, Frederick, MD, USA) were adapted to a 12–12 hr (lights on, 07:00–19:00 hr) light–dark cycle for 1 wk before experiments. Cryptochrome1 and Cryptochrome2 double heterozygotes were obtained from Dr A. Sancar and Dr C.P. Selby, University of North Carolina, NC, USA. The breeding pairs were maintained at Morehouse School of Medicine to expand the colony. To identify the genotypes, genomic PCR was performed using DNA extracted from tail samples as directed by Dr A. Sancar and Dr C.P. Selby [17, 18]. In this study, young adult male and female Cryptochrome1 and Cryptochrome2 double homozygotes (Cry1−/−Cry2−/−) were used. Those mutant mice were transferred to constant darkness a day before start of the calcium imaging experiments.
The onset of light was defined as zeitgeber time (ZT) 0, and the onset of darkness as ZT12. Animals were allowed free access to food and water throughout the experiments. Animals were decapitated following an overdose of halothane anesthesia, and their pineal glands were removed. All the experimental procedures were approved by IACUC of the Atlanta University Center.
Pineal organ culture
Detailed experimental conditions were described in previous studies [11, 19]. Circadian time (CT) was defined as extended clock time (ZT) in culture conditions. In all the experiments, the pineal glands were placed on a mesh disk attached to a well of a 6-well plate. As it has been shown that phase of Period1-bioluminescence rhythms in the pineal gland is not significantly affected by timing when the pineal glands were dissected in light–dark and constant dark conditions [20], the pineal glands were removed and placed in the cultures at CT6 as described in previous study [11]. The tissue was incubated in BGJb medium (Invitrogen, Carlsbad, CA, USA) supplemented with antibiotics (penicillin 100 units/mL medium and streptomycin 100 μg/mL medium). Cultures were maintained at 36°C, and 95% oxygen: 5% carbon dioxide was continuously provided throughout the experiments. As Period1-luminescence showed circadian oscillations on the first, second and third day of culture [11], all the experiments were performed during this period from 36 hr (CT18) to 60 hr (CT18) after start of the cultures.
A series of chemicals were added to the pineal cultures at 36 hr (CT18), 42 hr (CT2), 48 hr (CT6), or 56 hr (CT14) after start of the cultures for 4 hr before harvest. Inhibitors were added to the culture medium 30 min before chemical stimulations. All the samples were immediately frozen using dry ice, and kept at −80°C until RNA extraction.
Real-time quantitative PCR
Detailed conditions were described previously [11, 19, 21, 22]. Each set of samples was simultaneously processed for RNA extraction, DNase I treatment, cDNA synthesis, and PCR reaction. Real-time quantitative PCR was carried out using an iCycler (BioRad, Hercules, CA, USA). For primers we used Gapdh, GenBank Accession Number X02231 (5′-AGACAGCCGCATCTTCTTGT-3′, 5′-TGATGGCAACAATGTCCACT-3′), and mouse Aa-Nat GenBank Accession Number AF004111 (5′-GTCGACTCCTATGAAACAGTCGT-3′, 5′-ATCTAAAGTCCTACAGTTCGGGA-3′).
Forty cycles of amplification was carried out following 15 s of denaturation at 95°C. Once the temperature reached 95°C, it was decreased to 60°C, maintained for 45 s, and raised to 72°C for 30 s. Fluorescence was measured at melting temperature after each cycle, rather at the end of 40 cycles of amplification, which allow comparison of fluorescence intensities among samples while their increase is within the linear range. Melting temperatures used were 86°C for Gapdh and 82°C for Aa-Nat.
Quantification of Aa-Nat and Gapdh cDNAs was accomplished by comparing the threshold cycles for amplification of the unknowns to those of four concentrations of Aa-Nat and Gapdh standards using the iCycler software (BioRad). After 40 cycles of reactions, the PCR products were run on an agarose gel to verify that a single amplicon of appropriate size was amplified. Aa-Nat mRNA levels were normalized using Gapdh mRNA levels. In each experimental set, maximum value was represented as 100, and each value was normalized relative to the maximum value. All the experiments were repeated at least four times.
Ratiometric measurement of calcium levels
The pineal glands were removed from C57BL/6 and C3H mice and rats at ZT2 and ZT10 for day and night recordings, respectively. Dissociated pinealocytes were obtained by mechanical dissociation using a fire-polished glass pipette. One pineal gland was used to obtain one dish. After pinealocytes were seeded in DMEM (Cellgro, Mediatech Inc., Herndon, VA, USA) containing 10 mm HEPES on Poly-D lysine coated glass bottom microwell dishes (MatTek Corp., Ashland, MA, USA), the cells were incubated at 36°C for at least 3 hr before Fura-2AM loading. We utilized a single wavelength calcium imaging technique to assess intracellular calcium dynamics in a pinealocyte in response to chemical stimulations. Fluorescence measurements were made from an area that was defined by a circular diaphragm in the conjugate image plane of the microscope.
The calcium indicator dye Fura-2AM (10 μm, Kd = 140 nm), introduced into the cell via a 30-min bath incubation, was excited at wavelengths of 340 and 380 nm, and the emitted light was collected at 510 nm (LAMBDA DG5 Mercury ARC Lamp, Sutter Instruments, Novato, CA, USA). Calcium concentrations were calculated from the 340 nm/380 nm values using a series of concentrations of calcium standards [23, 24]. Cell images were captured using a Cascade 16-bit CCD camera (Photometrics, Tucson, AZ, USA) and an 1 X 71 inverted microscope (Olympus Inc., Melville, NY, USA). The images were analyzed using METAFluor 6.1 imaging software (Molecular Devices Corp., Sunnyvale, CA, USA). Isoproterenol (1 μm) or KCl (30 mm) was added to the cultures at the indicated time using a pipette tip, which released the chemical to the entire dish. The inhibitors were added to the dishes 25 min before agonist stimulations. Recordings for the inhibitor alone and inhibitor plus agonist were obtained by independent experiments, as a longer recording leads to bleaching of the dye. For the calcium-free experiments, medium was replaced to calcium free-DMEM (Invitrogen) supplemented with 100 μm EGTA for 5 min before isoproterenol stimulation at night. Daytime recordings were performed between ZT6 and ZT10, and the nighttime results were obtained between ZT14 and ZT18. In case the chemical stimulations did not affect calcium dynamics, the pinealocytes were stimulated using 30 mm KCl for cell quality determination. Independent experiments were repeated at least three times.
Statistical analysis
Comparisons between or among different groups were performed using nonparametric statistics (ANOVA and t-test). ANOVA (Kruskal–Wallis) analysis was followed by multiple nonparametric comparisons tests.
Results
To examine whether the gating step is downstream or upstream of adenylyl cyclases, the pineal cultures were stimulated using the cAMP analog 8-(4-chlorophenylthio)-adenosine 3′,5′-cyclic monophosphate (8-pCPT-cAMP). If the gating step precedes adenylyl cyclase activation, 8-pCPT-cAMP stimulation should increase Aa-Nat mRNA levels similarly at any time of the day, as observed in the rat retina [21]. As our preliminary results suggested 8-pCPT-cAMP stimulation did not fully increase Aa-Nat mRNA at CT6, we first determined the dose-dependent effects of 8-pCPT-cAMP to obtain the optimal concentration to use. The pineal cultures were stimulated using 8-pCPT-cAMP at doses of 0, 0.01, 0.1, 0.5, and 1 mm for 4 hr, starting at CT6 (Fig. 1A). At 0.5 mm, 8-pCPT-cAMP significantly increased Aa-Nat at this time of the day. We further examined the time of day-dependent effects of 8-pCPT-cAMP (0.5 mm, Fig. 1B). Stimulation at CT2 did not significantly increase Aa-Nat mRNA levels, whereas at CT6 and CT14 the mRNA levels were significantly increased (CT6, P < 0.0100; CT14, P < 0.0010). Interestingly, Aa-Nat mRNA levels were significantly increased at CT18 (P < 0.0001), similar to those obtained after isoproterenol stimulation (P > 0.0700).
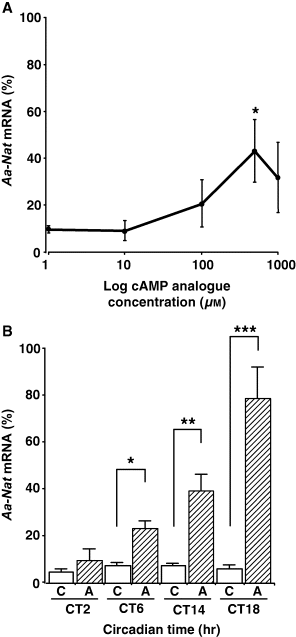
Effects of cAMP analog 8-(4-chlorophenylthio)-adenosine 3′,5′-cyclic monophosphate on Aa-Nat mRNA levels. (A) Dose-dependent effects of 8-pCPT-cAMP. The pineal cultures were stimulated using a series of 8-pCPT-cAMP concentrations at CT6. At a concentration of 0.5 mm, 8-pCPT-cAMP significantly increased Aa-Nat levels at CT6. (B) Phase-dependent effects of 8-pCPT-cAMP. The pineal cultures were stimulated using 8-pCPT-cAMP (0.5 mm) at CT2, CT6, CT14, and CT18 for 4 hr before harvest. Aa-Nat mRNA levels were significantly increased at CT6, CT14, and CT18. C, vehicle; A, 8-pCPT-cAMP. Values are means ± S.E.M. (n = 5–6 per group). *P < 0.0500; **P < 0.0050; ***P < 0.0005.
At CT6, stimulation of both PKA and protein kinase C using 8-pCPT-cAMP and phorbol 12-myristate 13-acetate did not fully increase Aa-Nat mRNA levels (data not shown). Therefore, we speculated that a lack of full Aa-Nat induction by 8-pCPT-cAMP might be due to the lack of activation or reduced availability of certain signaling cascade mediators under the alpha-adrenergic receptors, such as calcium. To test this hypothesis, we examined the effects of the calcium ionophore, ionomycin, which facilitates increases in intracellular calcium levels, on 8-pCPT-cAMP-induced Aa-Nat mRNA levels. As shown in Fig. 2A, 8-pCPT-cAMP and ionomycin (5 μm) stimulation similarly increased Aa-Nat mRNA levels at CT2 (P < 0.0100), CT6 (P < 0.0030), and CT18 (P < 0.0100). No significant difference was observed among the mRNA levels obtained at the three CTs tested (ANOVA, P > 0.5000). Also, there was no significant difference between the mRNA levels obtained at each time point and that obtained after isoproterenol stimulation at CT18 (all the cases, P > 0.4000). When the effect of ionomycin alone was tested, Aa-Nat mRNA levels were not significantly increased at any time of the day (Fig. 2B, all the CTs, P > 0.3000). However, the mRNA levels after ionomycin stimulation at CT18 were also not significantly different from those obtained after isoproterenol stimulation at CT18 (P > 0.1700).
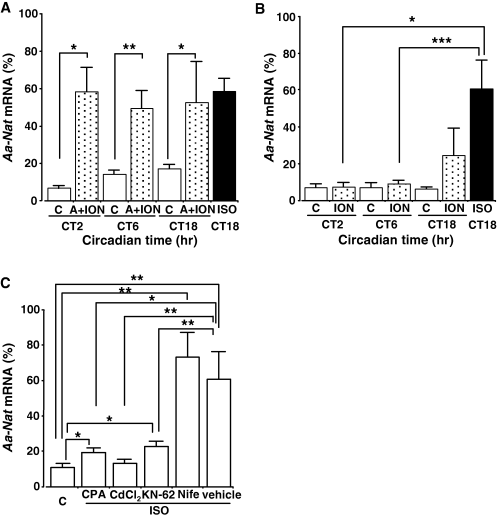
Aa-Nat gene expression in the pineal gland is regulated by cellular calcium. (A) Effects of 8-pCPT-cAMP (0.5 mm) and ionomycin (5 μm). Aa-Nat mRNA levels were similarly increased after stimulation with 8-pCPT-cAMP and ionomycin at CT2, CT6, and CT18. C, vehicle; A, 8-pCPT-cAMP; ION, ionomycin; ISO, isoproterenol. (B) Effect of ionomycin. Ionomycin did not significantly affect Aa-Nat mRNA levels at CT2 and CT6. At CT18, the mRNA levels were not statistically different between ionomycin and isoproterenol-treated samples. C, vehicle; ION, ionomycin; ISO, isoproterenol. (C) Effects of cyclopiazonic acid (CPA), CdCl2, KN-62, and nifedipine (Nife) on isoproterenol-induced Aa-Nat mRNA responses. The C57BL/6 pineal organ cultures were stimulated using isoproterenol (1 μm) in the presence of CPA (30 μm), CdCl2 (200 μm), KN-62 (30 μm), nifedipine (10 μm), or vehicle for 4 hr. Although isoproterenol stimulation significantly increased Aa-Nat in the presence of CPA or KN-62, those levels were significantly lower than those obtained by isoproterenol and vehicle stimulation. Nifedipine showed no significant effect on the isoproterenol-induced Aa-Nat levels. C, vehicle. Values are means ± S.E.M. (n = 5–6 per group). *P < 0.0500; **P < 0.0050; ***P < 0.0005.
To determine whether calcium originating from the intracellular calcium pools is necessary for isoproterenol-induced Aa-Nat at night, the pineal cultures were treated with an endoplasmic reticulum (ER) calcium ATPase inhibitor, cyclopiazonic acid (CPA, 30 μm) 30 min before isoproterenol stimulation (Fig. 2C). Aa-Nat mRNA levels were decreased close to the control levels, which were significantly higher than the vehicle-treated control (P < 0.0300), but significantly lower than isoproterenol-treated samples obtained at CT18 (P < 0.0100).
The effects of extracellular calcium depletion and/or reduced calcium entry on isoproterenol-induced Aa-Nat mRNA levels were further examined. The nonspecific calcium channel blocker, cadmium (CdCl2, 200 μm), was added to the pineal culture 30 min before isoproterenol stimulation at CT18, and resulted in an attenuation of the mRNA increase (Fig. 2C, versus vehicle, P > 0.5000; versus isoproterenol, P < 0.0030).
Suppression of Aa-Nat mRNA levels in the presence of the calcium/calmodulin protein kinase II (CaMKII) inhibitor KN-62 (30 μm, versus control, P < 0.0070; versus isoproterenol, P < 0.0050) further supports the idea that calcium is a necessary signal mediator in isoproterenol-induced Aa-Nat gene expression. Testing of the voltage-gated l-type calcium channel blocker, nifedipine (10 μm), showed no significant effect on isoproterenol-induced Aa-Nat mRNA levels (versus control, P < 0.0050).
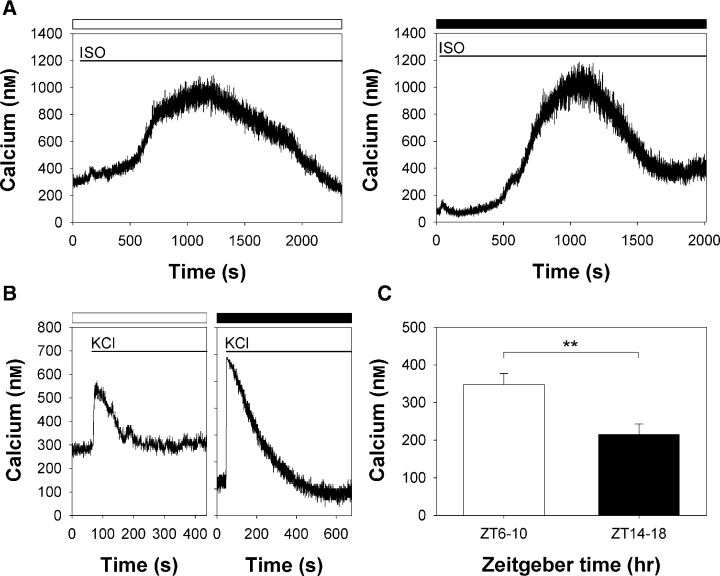
Based on the results above, we hypothesized that beta-adrenergic receptors stimulation does not increase Aa-Nat mRNA levels during the day, which might be due to lack of the calcium responses/sensitivity to isoproterenol stimulation. To test this hypothesis, we stimulated pinealocytes using isoproterenol and measured intracellular free calcium levels during the day and at night. As shown in Fig. 3A, calcium levels were not affected by isoproterenol stimulation during the day, which is consistent with previous observations (12 of 12 cells from 12 independent experiments). Stimulation with KCl (30 mm, Fig. 3C), however, acutely and transiently increased calcium levels, suggesting that cells were healthy, although the responses in the daytime (n = 6) were somewhat smaller than the ones observed at night (n = 3). By contrast, the bath application of isoproterenol at night resulted in robust calcium responses (Fig. 3A, 16 of 16 cells tested from 16 independent experiments). It took an average of 1049.69 ± 95.17 s (n = 16) to reach peak magnitude. Intracellular free calcium levels were changed from 157.82 ± 12.17 nm to 843.24 ± 39.10 nm (n = 16), which resulted in an average of 685.42 ± 34.07 nm increase in cellular calcium after isoproterenol stimulation. These day–night differential calcium responses to isoproterenol stimulation in C57BL/6 mouse pinealocytes were also observed in C3H mouse pinealocytes (Fig. 3B, both day and night, three of three cells each were recorded from three independent experiments).
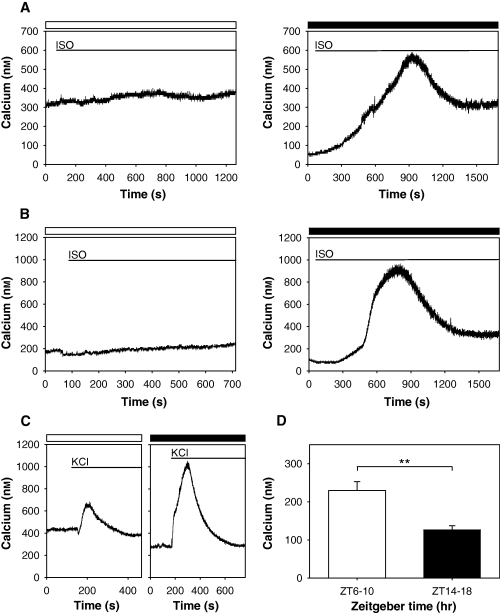
Isoproterenol induces differential calcium responses during the day and at night. Effects of isoproterenol on calcium responses during the day and at night in C57BL/6 (A) and C3H (B) pinealocytes. Bath application of isoproterenol (ISO, 1 μm) increased intracellular free calcium levels only at night. Daytime (open bars) and nighttime (filled bars) recordings were performed between ZT6 and ZT10, and ZT14 and ZT18, respectively. Typical calcium responses are shown from C57BL/6 (day, 12 of 12 cells from 12 independent experiments; night, 16 of 16 from 16 independent experiments) and C3H (day, three of three from three independent experiments; night, three of three from three independent experiments). (C) Although KCl stimulation lead to increases in free calcium levels both in the day (open bar) and night (filled bar), nighttime stimulation resulted in a more robust and long-lasting response in C57BL/6 pinealocytes (day, n = 6; night, n = 3). All the cells responded in a similar manner. The timing of bathing of each drug in (A), (B) and (C) is represented as a line. (D) Intracellular free calcium levels during the day (ZT6–10) were significantly higher than those at night (ZT14–18). Values are means ± S.E.M. (day, n = 24; night, n = 42). **P < 0.0050.
During the recordings of calcium dynamics, we found that intracellular free calcium basal levels differed significantly between daytime and nighttime. Calcium basal levels were significantly higher during the daytime than during the night (P < 0.0020, Fig. 3D).
As calcium responses to isoproterenol stimulation showed day–night differences, we examined whether such time of day-dependent calcium responses persisted in pinealocytes of Cryptochrome double knockout mice whose functional circadian clocks have been abolished. The mutant mice were kept in constant darkness for a day prior to experimentation as circadian locomotor activity rhythms in Cryptochrome double knockout mice have been reported to be abolished immediately after transferring animals to constant conditions [17, 18]. Isoproterenol stimulation caused robust and sustained calcium responses, even during the subjective day (Fig. 4A). Such responses were also observed in the subjective night. Intracellular free calcium levels in the subjective daytime (112.53 ± 57.41 nm, n = 4) were not significantly different from those observed in the subjective night (113.72 ± 27.63 nm, n = 3) (Fig. 4B, P > 0.9000).
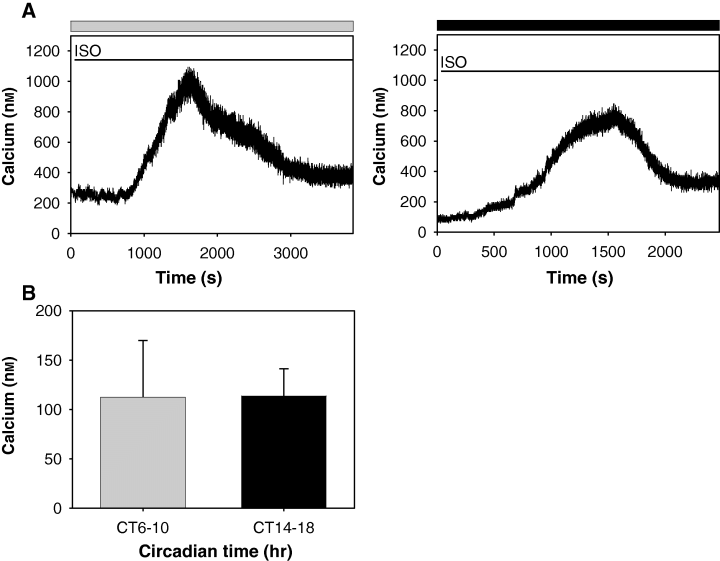
Day–night differential calcium dynamics are abolished in pinealocytes of Cryptochrome double knockout mice. (A) Representative traces to demonstrate calcium responses to isoproterenol (ISO) stimulation were similarly observed in the subjective day (gray bar) and subjective night (dark bar) in pinealocytes of Cryptochrome double knockout mice (Cry1−/−Cry2−/−). Two pinealocytes each from two mice were tested in the subjective day and night, respectively. The timing of bathing of isoproterenol is represented as a line. (B) Intracellular free basal calcium levels were not significantly different between the subjective day and subjective night in pinealocytes of Cry1−/−Cry2−/− mice (subjective day, n = 4; subjective night, n = 3).
To assess the specificity of isoproterenol on beta-adrenergic receptors-mediated induction of intracellular free calcium, the effect of an alpha-adrenergic receptor antagonist prazosin (10 μm) was examined (Fig. 5A). When prazosin was added to the medium prior to isoproterenol, calcium responses were still observed, which were similar to those observed after isoproterenol stimulation alone (five of five cells tested).

Regulation of isoproterenol-induced intracellular free calcium levels. (A) Effect of an alpha-adrenergic receptor antagonist prazosin on isoproterenol-induced calcium responses. Prazosin (PRAZ, 10 μm) was added to the culture medium 25 min prior to isoproterenol (ISO) stimulation at night. Prazosin did not abolish the beta-adrenergic receptors-mediated calcium responses (n = 5). (B) Effects of cyclopiazonic acid (CPA) on isoproterenol-induced calcium responses at night. CPA (5 μm) stimulation resulted in an initial transient increase in intracellular calcium, which recovered partially and remained elevated above baseline concentrations throughout the recording period (left panel, n = 6). When isoproterenol was added after 25 min CPA preincubation, the calcium responses were abolished (right panel, n = 8). (C) Isoproterenol-induced calcium responses were abolished when pinealocytes were incubated in calcium-free medium and EGTA (100 μm) for 5 min prior to isoproterenol stimulation in the night (n = 8). (D) Pretreatment of cells using KCl entailed calcium responses following isoproterenol stimulation in the day. Prior to isoproterenol stimulation, pinealocytes were stimulated using KCl (30 mm), which resulted in a brief transient calcium response. Subsequent isoproterenol stimulation resulted in a robust and sustained elevation in calcium similar to the nighttime levels (n = 7). Open and filled bars are representative of daytime and nighttime records respectively. The timing of bathing of each drug is represented as a line.
As CPA significantly decreased isoproterenol-induced Aa-Nat mRNA at CT18, we tested whether or not isoproterenol-stimulated calcium increases originated in CPA-sensitive calcium stores. CPA (5 μm) stimulation resulted in an initial transient increase in intracellular calcium, which recovered partially and remained elevated above baseline concentrations throughout the recording period (Fig. 5B, left panel, six of six cells tested). When isoproterenol was added after 25 min CPA preincubation, the calcium responses were abolished (Fig. 5B, right panel, eight of eight cells examined). We further examined whether extracellular calcium was necessary for isoproterenol-induced calcium, and found that isoproterenol failed to increase intracellular calcium levels in the presence of calcium-free medium and 100 μm EGTA (Fig. 5C, 10 of 10 cells tested). When calcium-free medium was replaced with regular DMEM after isoproterenol stimulation, cells were responsive to KCl stimulation, suggesting that cells were still survived (data not shown, four of four cells examined). Fig. 5D shows an example of isoproterenol-triggered calcium responses recorded after using cell depolarization to charge the ER stores with releasable calcium. Even during the daytime, isoproterenol increased intracellular free calcium levels after KCl (30 mm) stimulation (seven of seven cells examined).
Finally, we determined whether isoproterenol increases intracellular free calcium, and if so, whether the calcium increases are similarly observed between daytime and nighttime in rat pinealocytes in which beta-adrenergic receptors stimulation augments melatonin and Aa-Nat mRNA levels at any time of the day. Isoproterenol (1 μm) stimulation increased calcium levels in both the daytime and nighttime; however, calcium responses were more robust at nighttime than during the day (Fig. 6A). It took the same amount of time after isoproterenol stimulation to reach peak magnitude during the day (1246.54 ± 217.76 s, n = 6) and night (1334.77 ± 272.45 s, n = 6). The peak calcium levels during the daytime and nighttime did not show significant differences; however, as basal free calcium levels were significantly different between daytime and nighttime (Fig. 6C, P < 0.0030), amplitudes of calcium responses observed at night (980.56 ± 153.63 nm, n = 6) were significantly bigger than those obtained during the daytime (467.26 ± 116.95 nm, n = 6, P < 0.0300). Calcium responses to KCl stimulation at night (n = 3) also showed bigger amplitudes than those found in the daytime (n = 3, Fig. 6B).
Calcium responses in rat pinealocytes. Day–night differential calcium responses after isoproterenol (1 μm) stimulation (A) and KCl (30 mm) stimulation (B) were examined in rat pinealocytes. Calcium responses during the daytime (open bars) after isoproterenol (ISO) and KCl stimulation were as robust as those obtained at night (filled bars) (day: ISO, n = 6, KCl, n = 3; night: ISO, n = 6, KCl, n = 3). The timing of bathing of each drug in (A) and (B) is represented as a line. (C) Intracellular free calcium levels were recorded from rat pinealocytes prepared in the day (ZT6–10) and night (ZT14–18). As seen in mouse pinealocytes, intracellular free calcium levels in the daytime were significantly higher than those found at night. Values are mean ± S.E.M. (day, n = 19; night, n = 26). **P < 0.0030. See details in Fig. 3 legend.
Discussion
Previously, we reported that isoproterenol-induced increases of Aa-Nat mRNA levels in the mouse pineal gland depends on the time of day [11]. In the present study, to identify a potential regulatory step, we first tested whether the gating occurred above or downstream from adenylyl cyclases using a cAMP analog. Our present results suggest that cAMP stimulation alone is not sufficient to fully increase Aa-Nat mRNA levels during the daytime (Fig. 1), thereby suggesting that gating is regulated at a point downstream from the cAMP stimulation, e.g. PKA, and/or signaling cascades outside the cAMP-PKA cascade. Our data further suggest activation of both cAMP and calcium cascades are likely necessary to fully increase Aa-Nat mRNA levels after isoproterenol stimulation (1, 2). It has been shown that increased free calcium activates calcium sensitive adenylyl cyclases, which results in the enhancement of cyclic nucleotides and melatonin levels [21, 25–29]. However, the present observations in mouse taken together with previous hamster results, suggest that the action of calcium is unlikely only via calcium-sensitive adenylyl cyclases, but potentially further downstream in the cascades.
Our data also suggest that calcium entry from the extracellular compartment as well as release from internal stores are critical for isoproterenol-induced Aa-Nat gene expression, which is mediated by CaMKII (Fig. 2C). Although we may be able to neglect the involvement of nifedipine-sensitive calcium channels, other voltage-gated and store-operated calcium channels, transient receptor potential cation channels, and cyclic nucleotide-gated calcium channels within pineal cell membranes may be involved in the regulation of isoproterenol-induced changes in calcium dynamics. It is interesting to note that nifedipine-insensitive, voltage-gated 40-pS cationic channels (ILOT) have been identified in the chicken pineal, which may play a role in the regulation of melatonin synthesis [30, 31]. However, no information currently exists regarding ILOT expression in mammals.
In the present study, all the chemicals were bath applied to dishes to mimic in vivo conditions, i.e. norepinephrine levels are high throughout the night in the pineal [32] and, we found that unlike results shown in the rat and mouse pineal glands [33, 34], activation of the beta-adrenergic receptors might have been able to affect calcium dynamics at night (Fig. 3A,B). As prazosin did not affect isoproterenol-induced calcium increases (Fig. 5A), the effects of isoproterenol are unlikely mediated by prazosin-sensitive adrenergic receptors. Our results also show that isoproterenol stimulation affected calcium dynamics in both melatonin-deficient and -proficient mouse pinealocytes (Fig. 3A,B). Therefore, such calcium responses are unlikely artifacts caused by the presence or absence of melatonin. However, it should be mentioned that time courses of the calcium responses differed between the two mouse strains: the responses were faster and more robust in the C3H mouse pinealocytes. We also found that the same origins of calcium are likely involved in both isoproterenol-induced calcium responses and Aa-Nat gene expression. It is also likely that these may be inter-dependent, in that the filling of internal calcium stores may be dependent on entry from the extracellular compartment (2, 5).
Isoproterenol-induced calcium responses differ from the quick calcium responses after phenylephrine stimulation (Fig. 3A,B). Rather, it resembled that observed after norepinephrine stimulation in rat pinealocytes [35]. Such isoproterenol-induced increases in intracellular free calcium levels might not have been seen in previous studies for the following reasons: (i) only acute and fast calcium responses were determined; (ii) calcium recordings were probably done only in the daytime; and (iii) 24-hr-old dissociated cells were used in which circadian rhythm generation may have been disturbed.
From the data obtained in the first part of the current study, it could be concluded that the time of day-dependent isoproterenol-induced Aa-Nat gene expression is controlled by mechanisms located downstream from the PKA, where activation may be controlled by calcium and CaMKII. Unlike previous studies, stimulation of beta-adrenergic receptors modulates calcium dynamics in the time of day-dependent mechanisms. At this moment, it is still unclear whether PKA and calcium cascades merge at certain steps, or whether they independently control the expression of the Aa-Nat gene.
How are such day–night differential calcium responses to isoproterenol stimulation controlled? We can assume that circadian clocks may control such day–night differential calcium responses. Indeed, we observed that day–night differential calcium responses to isoproterenol in Cryptochrome double knockout mouse pinealocytes disappeared after a day in constant darkness (Fig. 4), while day–night differential calcium responses were maintained in wild type mouse pinealocytes in the same conditions (data not shown). How do circadian clocks control day–night differential calcium responses? One can argue that intracellular free calcium levels as well as calcium in intracellular calcium pools can affect calcium responses [36]. We therefore explored potential mechanisms by which calcium dynamics are controlled differently during the day and at night from the above two viewpoints. As shown in Fig. 3D, intracellular free calcium levels significantly differ between day and night, which supports the first argument and it further suggests that free calcium levels may affect sensitivity to external stimulation-driven calcium responses. This may be applicable to both the G protein-coupled receptor-intracellular signaling cascades-dependent and -independent calcium responses, since such day–night differential calcium responses were also observed after KCl stimulation (Fig. 3C). Cryptochrome double mutant data further indicate that day–night differential calcium basal levels may be driven by circadian clocks (Fig. 4B). Taken together, it suggests that intrinsic mechanisms may control gated calcium responses.
For the second argument, we present indirect evidence for support. Conditioning, which primes calcium into the intracellular pools using KCl, results in robust calcium responses after isoproterenol stimulation, even during the day (Fig. 5D), suggesting that calcium responses may be affected by calcium levels in intracellular calcium pools. Further investigations will provide direct evidence whether calcium levels in the ER change throughout the day.
In the last part of the study, we measured calcium dynamics in rat pinealocytes, in which Aa-Nat mRNA and melatonin increases after isoproterenol stimulation can be seen at any time of day [22, 37]. As expected, isoproterenol stimulation significantly increased intracellular free calcium levels with somewhat smaller amplitudes during the day (Fig. 6A). Such day–night differential responses were also observed after KCl stimulation, and intracellular free calcium levels differed between day and night (Fig. 6B,C). As Aa-Nat mRNA levels are fully increased even in the middle of the day after isoproterenol stimulation, the calcium increase observed during the day is likely sufficient for the gene expression. It has been shown that melatonin synthesis in the rat pineal is affected by calcium, depending on the time of day [38, 39]; therefore, the rat pineal clock may modulate melatonin synthesis beyond expression of the Aa-Nat gene. As rat pineals possess a circadian clock [40] similar to that seen in the mouse [8], it would be of interest to understand species-dependent diversity of circadian clock functions in melatonin synthesis.
In the latter part of the present study, our results suggest that day–night differential calcium responses to isoproterenol stimulation appeared to be controlled by the circadian clock, which may employ multiple mechanisms, such as changes in the basal calcium levels and availability of intracellular calcium.
Although regulations of melatonin synthesis in the rodent pineal glands have been well studied in the past, it appears to be more complex than previously recognized. There is an apparent missing link between the pineal circadian clock and melatonin synthesis. In the past, the role of calcium has been underestimated in rodent pineal glands, because of a moderate role identified for alpha-adrenergic receptors in melatonin synthesis. However, in previous studies, it was suggested that calcium is employed by the SCN clock to control output circadian events [41, 42]. Furthermore, in the chicken pineal gland, calcium also appears to be involved in regulation of melatonin synthesis, but not circadian rhythm generation itself [43]. These observations suggest that calcium may be the potential link between the circadian clock and melatonin synthesis.
Acknowledgements
The authors would like to thank Dr P.R. MacLeish, Dr Y. Ishida, and Dr J. Russell for invaluable suggestions. Cryptochrome double heterozygotes mice were generous gifts of Dr A. Sancar and Dr C.P. Selby at University of North Carolina School of Medicine, NC, USA. This work was supported by the grants from the NINDS NS034194 and NS38963, and from the CBN under the STC Program of the National Science Foundation under agreement No. IBN-9876754. The iCycler is part of the KECK genomic core facility within the MSM Neuroscience Institute. This investigation was conducted in a facility constructed with support from Research Facility Improvement Grant 1 C06 RR07571 from the National Center for Research Resources, National Institutes of Health. E.I. is supported by the JSPS.




