Neuroprotective effects of melatonin against anoxia/aglycemia stress, as assessed by synaptic potentials and superoxide production in rat hippocampal slices
Abstract
Abstract: Melatonin, which plays an important role in circadian rhythm regulation, is highly potent endogenous free radical scavenger and antioxidant. To clarify the neuroprotective effects of melatonin as a free radical scavenger, we recorded changes in synaptic potentials and monitored the generation of superoxide (O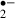 ) (using hydroethidine) in the CA1 pyramidal layers of rat hippocampal slices exposed to anoxia/aglycemia (‘ischemic’) stress. Synaptic responses evoked by stimulation of Schaffer collateral/commissural afferents were suppressed by ischemic stress. When the duration of the stress was 8 min, the suppression was reversible, irrespective of the presence or absence of melatonin treatment, while the amount of O
) (using hydroethidine) in the CA1 pyramidal layers of rat hippocampal slices exposed to anoxia/aglycemia (‘ischemic’) stress. Synaptic responses evoked by stimulation of Schaffer collateral/commissural afferents were suppressed by ischemic stress. When the duration of the stress was 8 min, the suppression was reversible, irrespective of the presence or absence of melatonin treatment, while the amount of O generated was reduced by the presence of melatonin. When stress duration was 12 min, the suppression of synaptic responses lasted more than 90 min, but melatonin significantly improved the recovery. The amount of O
generated was reduced by the presence of melatonin. When stress duration was 12 min, the suppression of synaptic responses lasted more than 90 min, but melatonin significantly improved the recovery. The amount of O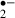 generated in the ‘recirculation’ phase after a 12 min ischemic stress was less in the ischemic alone group than in the melatonin-treated group. This probably reflects that the number of viable cells with the ability to generate O
generated in the ‘recirculation’ phase after a 12 min ischemic stress was less in the ischemic alone group than in the melatonin-treated group. This probably reflects that the number of viable cells with the ability to generate O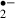 had been reduced by the more severe ischemic stress. Other radical scavengers (ascorbic acid and α-tocopherol) had similar effects. These results show that melatonin has the potential to protect the functions of neurons against an ischemic insult by reducing O
had been reduced by the more severe ischemic stress. Other radical scavengers (ascorbic acid and α-tocopherol) had similar effects. These results show that melatonin has the potential to protect the functions of neurons against an ischemic insult by reducing O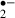 generation.
generation.
Introduction
Reactive oxygen radicals are considered to be causally involved in the brain injuries that follow events such as cerebral ischemia and reperfusion [1]. Ischemic insult induces neural hyperactivity due to an elevation of extracellular excitatory amino acid levels, followed by an excessive intracellular Ca2+ accumulation, triggering the process of neuronal death [2, 3]. Intracellular Ca2+-overload initiates a number of deleterious processes, including the generation of free radicals such as reactive oxygen species (ROS) [4, 5]. ROS are scavenged by superoxide dismutase (SOD), glutathione peroxidase (GSHPx), catalase and the low molecular weight antioxidants (LMWA). Animals overexpressing SOD show a significant reduction in hippocampal CA1 neuronal cell death after global ischemia [6, 7]. Moreover, increased cell death and edema of the CA1 region have been observed after cerebral ischemia in SOD-deficient mice [8, 9].
Melatonin, a secretory product synthesized primarily in the pineal gland, is released into the blood stream and cerebrospinal fluid (CSF). It behaves as a hormone, a tissue factor, an autocoid, etc. [10] and has several important functions, including circadian rhythm regulation, immune enhancement, sleep induction, seasonal reproductive regulation, and light-dark signal transduction [11]. Recently, it has been found that melatonin is also an effective free radical scavenger and antioxidant [12, 13]. Indeed, melatonin reduces the molecular damage and tissue destruction caused by transient ischemia and reoxygenation [14–16].
Although many findings have been made regarding the chemical mechanisms underlying the interactions between melatonin and free radicals, and the ability of melatonin to protect neurons against several stresses (stroke [17], kainic acid stimulation [18, 19], etc.), little is known about how the radical-scavenging ability of melatonin protects neuronal function. To obtain a more comprehensive view of the neuroprotective function of melatonin as a radical scavenger, we performed the present study to monitor synaptic potentials and O production in rat hippocampal slices exposed to anoxia/aglycemia (‘ischemic’) stress in the presence or absence of melatonin.
production in rat hippocampal slices exposed to anoxia/aglycemia (‘ischemic’) stress in the presence or absence of melatonin.
Materials and methods
All experiments conformed to guidelines on the ethical use of animals for research in Hamamatsu University School of Medicine, and all efforts were made to minimize both the number of animals used and any suffering caused.
Slice preparation
Wistar rats 5–6 wk old were deeply anesthetized with ether, and the brain removed and placed in ice-cold oxygenated, modified artificial cerebrospinal fluid (ACSF). The solution contained the following (in mm): 220 sucrose, 2.5 KCl, 1.25 NaH2PO4, 10 MgSO4, 0.5 CaCl2, 26 NaHCO3, and 30 glucose. Transverse hippocampal slices (400 μm) were cut on a vibrating blade microtome (LEICA VT 1000 S, Nussloch, Germany), then allowed to recover for at least 60 min in standard ACSF consisting of (in mm): 126 NaCl, 2.5 KCl, 1.25 NaH2PO4, 2 MgSO4, 2 CaCl2, 26 NaHCO3, and 10 glucose. This recovery took place in a tightly sealed box filled with 95% O2–5% CO2 at a pressure of 50–100 kPa at room temperature.
Individual slices were transferred to the recording chamber, and perfused with oxygenated standard ACSF at 28–29°C, at a rate of 1–2 mL/min. Slices were subjected to ischemic stress by perfusing them with glucose-free ACSF equilibrated with 95% N2–5% CO2. There was about a 1-min delay to replace the medium in the bath.
Electrophysiological recordings
Extracellular recordings were made from the stratum radiatum in the CA1 region, using pipettes filled with standard ACSF (resistance ∼3 MΩ). Field potentials were evoked by stimulating Schaffer collateral/commissural afferents via a bipolar tungsten electrode placed in the stratum radiatum in the CA1 area. Such stimulation (a square pulse of 100 μs duration) was delivered every 30 s before and after the ischemic stress, and every 10 s during the stress itself. The intensity was initially set to 3–7 V, to elicit one half of the maximum response. For a more detailed observation of the time course of the suppression of field potentials by the stress, the intensity was increased to 5–15 V so as to induce the maximum response during the ischemic stress. After exposure to the ischemic stress (i.e. ‘recirculation’ phase), the stimulus intensity was returned to the initial value.
As our index of the synaptic response, we measured the presynaptic fiber volley (FV) and field excitatory postsynaptic potentials (fEPSPs) in area CA1 of pyramidal neurons. To evaluate the responses, ‘transmission ratio’ was calculated by dividing the amplitude of the fEPSPs by that of the FV. Before and after the ischemic stress, each value for transmission ratio was calculated by averaging four consecutive trials (i.e. every 2 min). During the stress itself, the ratio was calculated for each trial. The percentage transmission ratio, which was used to express changes in synaptic responses, was obtained by dividing each transmission ratio value by the baseline transmission ratio value (the mean for the 10 min before the ischemic stress), and multiplying by 100.
Recovery from the ischemic stress was assessed by calculating values for the percentage recovery of the transmission ratio (calculated by dividing averaged values obtained from 80 to 90 min after the stress by that obtained before the stress, and multiplying by 100).
Monitoring of superoxide anion (O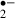 ) production
) production
O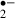 production was evaluated in real-time by the oxidized hydroethidine (HEt) method [20]. Briefly, to monitor O
production was evaluated in real-time by the oxidized hydroethidine (HEt) method [20]. Briefly, to monitor O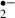 production we applied microfluorimetry to the oxidation of HEt (Molecular Probes, Eugene, OR, USA) to ethidium (Et). HEt (3.2 μm) was dissolved either in standard or glucose-free ACSF containing 0.1% dimethylsulfoxide (DMSO). The fluorescence of Et excited at 470 nm was filtered via a band-pass filter (610–670 nm) for detection. Images were obtained using a 10× objective lens (Fluor; NA 0.3; Nikon, Tokyo, Japan). The average fluorescence intensity of the Et was monitored in the pyramidal cell layer and stratum radiatum of the CA1 region every 30 s with the aid of an image processor and data-analysis software (ARGUS/HiSCA; Hamamatsu Photonics, Hamamatsu, Japan). Each value was divided by the initial fluorescence value for the purposes of normalization, and the slope of the fluorescence change for 5 min before the stress was taken as the baseline slope. Thus, an increase in the slope indicates an increases in the rate of generation of O
production we applied microfluorimetry to the oxidation of HEt (Molecular Probes, Eugene, OR, USA) to ethidium (Et). HEt (3.2 μm) was dissolved either in standard or glucose-free ACSF containing 0.1% dimethylsulfoxide (DMSO). The fluorescence of Et excited at 470 nm was filtered via a band-pass filter (610–670 nm) for detection. Images were obtained using a 10× objective lens (Fluor; NA 0.3; Nikon, Tokyo, Japan). The average fluorescence intensity of the Et was monitored in the pyramidal cell layer and stratum radiatum of the CA1 region every 30 s with the aid of an image processor and data-analysis software (ARGUS/HiSCA; Hamamatsu Photonics, Hamamatsu, Japan). Each value was divided by the initial fluorescence value for the purposes of normalization, and the slope of the fluorescence change for 5 min before the stress was taken as the baseline slope. Thus, an increase in the slope indicates an increases in the rate of generation of O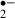 . To help us assess the effect of melatonin, the maximum slope of the fluorescence change for 1 min during the ischemic stress was divided by the baseline slope (giving ‘the relative slope value for the ischemic stress’), and the maximum slope of the fluorescence change for 3 min during the recirculation period was divided by the baseline slope (giving ‘the relative slope value for the recirculation’).
. To help us assess the effect of melatonin, the maximum slope of the fluorescence change for 1 min during the ischemic stress was divided by the baseline slope (giving ‘the relative slope value for the ischemic stress’), and the maximum slope of the fluorescence change for 3 min during the recirculation period was divided by the baseline slope (giving ‘the relative slope value for the recirculation’).
Drug applications
Melatonin and other drugs were applied from the beginning of experiments unless otherwise noted. Melatonin and α-tocopherol were dissolved in DMSO. The final concentration of DMSO in ACSF was adjusted to <0.1%. This concentration of DMSO had no effects on the recorded parameters.
As control experiments, slices exposed to ischemic stress only with DMSO (0.1%), instead of treating with melatonin or other radical scavengers.
Statistical analysis
For statistical comparisons, a paired/unpaired t-test or a one-way analysis of variance (ANOVA) followed by Fisher's PLSD test was used. P values of <0.05 were considered statistically significant.
Results
In slices subjected to ischemic stress for 8 min, the FV persisted during the stress whereas the fEPSPs were suppressed. The fEPSPs recovered gradually after the stress and returned to the preischemic level (Fig. 1A). The suppression of fEPSPs by ischemic stress in slices treated with melatonin (100 μm) was comparable with that observed in control (i.e. melatonin untreated) slices (Fig. 1B). Thus, the percentage transmission ratio was reversibly decreased by an 8 min stress (Fig. 1C,D), and no difference in the time course of suppression and recovery was observed between the control (n = 7, Fig. 1C) and melatonin-treated groups (n = 7, Fig. 1D).

Effects of an 8 min ischemic stress on field potentials. Time course of changes in the synaptic responses evoked by electrical stimulation during ischemic stress and recirculation (A: control, B: melatonin). The period of anoxia/aglycemia (ischemic stress) is indicated by the bar above each plots. Filled circles: FV; open circles: fEPSPs. Insets (a–d) show field responses obtained at the times indicated in (A) and (B). Scale bars: 500 μV, 10 ms. Averaged (n = 7) time-course data for transmission ratio (fEPSPs/FV) are shown for experiments involving 8 min ischemic stress: control (C) and melatonin (D). Error bars: SE. The suppression of the response and the recovery phase after the ischemic stress were similar between the melatonin-treated and control groups.
When the duration of the ischemic stress was 12 min rather than 8 min, FV was impaired, but it recovered soon after the stress (Fig. 2A). On the other hand, the suppression of fEPSPs by the stress was irreversible (i.e. no recovery to the preischemic level within 90 min). However, in slices treated with melatonin (100 μm), the fEPSP suppression was reversible (Fig. 2B). The percentage transmission ratio was decreased by a 12 min stress, and did not recover to the preischemic level in the control group (n = 6, Fig. 2C, Fig. 3, Control). However, the recovery was significantly improved in the melatonin-treated group (n = 6, Fig. 2D, Fig. 3, Mel, 100 μm). In slices without ischemic stress but with the same stimulation protocol, no significant change in the synaptic responses was observed throughout experiments with [n = 5, Fig. 3, Isch(-)/Mel] or without melatonin [n = 6, Fig. 3, Isch(-)].
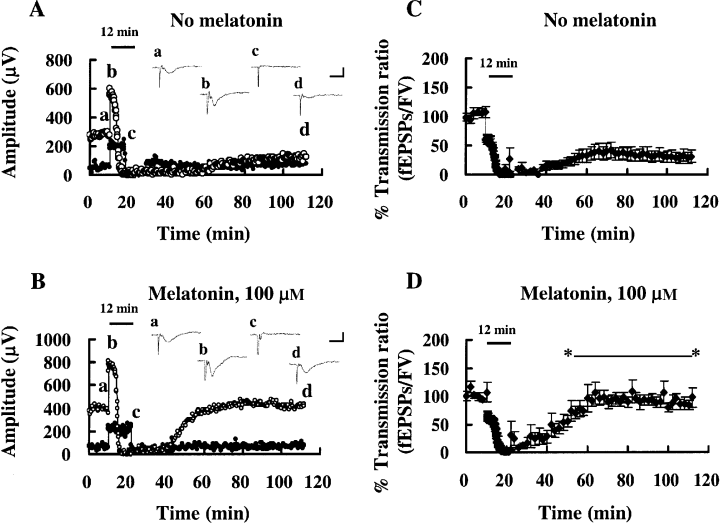
Effects of a 12 min ischemic stress on field potentials. Insets (a–d) show field responses obtained at the times indicated in (A) (control) and (B) (melatonin). Scale bars: 500 μV, 10 ms. Bar over each plot: period of anoxia/aglycemia (ischemic stress). Filled circles: FV; open circles: fEPSPs. Averaged (n = 6) time-course data for transmission ratio (fEPSPs/FV) are shown for experiments involving 12 min ischemic stress: control (C) and melatonin (D). Error bars: SE. Melatonin facilitated the recovery from 12 min ischemic stress: the transmission ratio was significantly larger in melatonin-treated slices than in the control group (t-test, P < 0.05) during the recirculation period, as indicated by *—*.
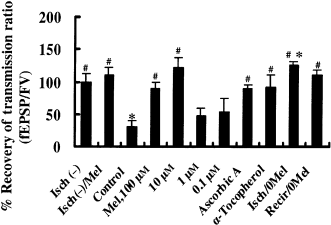
Recovery from a 12 min ischemic stress under various conditions. Isch(-): slices not treated with melatonin, and not exposed to ischemic stress. Isch(-)/Mel: Slices treated with melatonin (100 μm) throughout the experiment without ischemic stress. Control: slices exposed to ischemic stress, but with DMSO (0.1%) added as a control for melatonin. Mel (0.1–100 μm): slices treated with melatonin at the indicated concentration and exposed to ischemic stress. Ascorbic acid and α-tocopherol: as above, but with ascorbic acid (2 mm) or α-tocopherol (31 μm) instead of melatonin. Isch/0Mel and Recir/0Mel: melatonin removed either during or after the period of stress, respectively. #P < 0.01: compared with control (one-way ANOVA, Fisher's PLSD). *P < 0.01: compared with baseline transmission ratio (paired t-test). Note that effects similar to those of melatonin (10, 100 μm) were obtained with other radical scavengers, ascorbic acid (2 mm) and α-tocopherol (31 μm). Six slices were tested in each experiment except in Isch(-)/Mel (n = 5). Error bars: SE.
The protective effects of melatonin at concentrations of 0.1, 1.0, and 10 μm were tested in another series of experiments (Fig. 3). The 10 μm concentration of melatonin had comparable protective features to those seen at 100 μm. Other antioxidants [i.e. ascorbic acid (2 mm) and α-tocopherol (31 μm)] also had protective effects on the recovery of fEPSPs from a 12 min ischemic stress (Fig. 3). The time after the onset of an ischemic stress at which fEPSP had reached one half its maximum amplitude was not altered by melatonin or by the other antioxidants (Table 1).
| n | Mean ± S.E. (min) | |
|---|---|---|
| Control | 13 | 4.4 ± 0.3 |
| Melatonin (μm) | ||
| 100 | 13 | 4.2 ± 0.2 |
| 10 | 6 | 4.0 ± 0.4 |
| 1 | 6 | 4.5 ± 0.3 |
| 0.1 | 6 | 3.7 ± 0.2 |
| Ascorbic acid (2 mm) | 6 | 3.8 ± 0.4 |
| α -Tocopherol (31 μm) | 6 | 4.4 ± 0.4 |
- Values are ‘50% Max Resp’, indicating the time after the onset of a ischemic stress at which fEPSP had decreased to one half of its maximum amplitude.
To determine the effective period for melatonin application, melatonin (10 μm) was removed from the ACSF either during the ischemic stress itself or during the recirculation period (3, 4). Our results indicate that melatonin was required either during the stress or during the recirculation to exert a protective effect. When melatonin was removed during the ischemic stress, the synaptic response was actually potentiated after the stress. The value obtained for the percentage recovery of the transmission ratio in slices that were deprived of melatonin during the stress itself was significantly greater than the baseline transmission ratio (paired t-test, P < 0.01; n = 6; Fig. 3, Isch/0Mel, Fig. 4A).
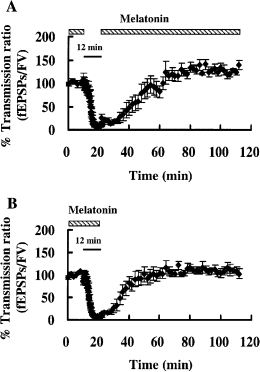
Differential effects of melatonin removal during (A) and after (B) a 12 min ischemic stress. The periods of ischemic stress (bars) and melatonin (10 μm) application (shadowed bars) are indicated over the plots. In those slices in which melatonin was removed during the stress itself, the transmission ratio was significantly larger than the baseline transmission ratio before the stress (paired t-test, P < 0.01; see also Fig. 3, Isch/0Mel). When melatonin was removed during the recirculation period, the transmission ratio was comparable with that in slices in which melatonin was present throughout the recording period.
When slices were perfused with ACSF containing HEt, the fluorescence increased gradually, with the strongest fluorescence in the pyramidal cell layer (Fig. 5A). When slices were exposed to an 8 or a 12 min ischemic stress, the slope of the fluorescence change became steeper than the baseline slope at the beginning of the ischemic stress. However, as the ischemic stress continued the slope became flat or even negative. After the ischemic stress, the slope became steeper again, and this persisted for over 10 min (Fig. 5B,D). Table 2 shows relative slope values for the fluorescence change during the ischemic stress itself and during the recirculation period. The increase in relative slope value during the ischemic stress was significantly reduced by melatonin (n = 12). Melatonin also reduced the increase in the relative slope value during the recirculation period (n = 5) when the duration of the ischemic stress was 8 min. When the duration of the ischemic stress was prolonged to 12 min, the relative slope value during the recirculation tended to be smaller in the control group (n = 7) than that in the 8 min group (although the p value was 0.11). In contrast, in the melatonin-treated group the relative slope value during the recirculation after a 12 min ischemic stress was significantly larger than that after an 8 min ischemic stress. The former was almost the same as that obtained for the recirculation after an 8 min ischemia in the control group. Ascorbic acid (2 mm) had comparable effects to those of melatonin for the recirculation after a 12 min ischemic stress (n = 4). The baseline slope was not affected by either melatonin or ascorbic acid.
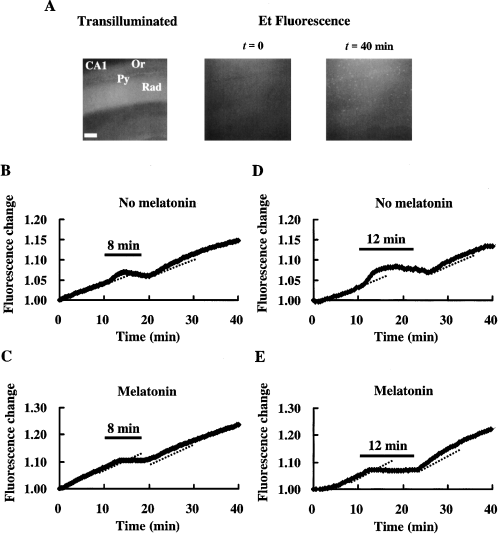
O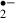 generation monitored by the oxidation of HEt to Et. (A) Transilluminated image (left) and fluorescence images (middle and right) of the CA1 region of a hippocampal slice, stained with Et (from the experiment shown in C). O
generation monitored by the oxidation of HEt to Et. (A) Transilluminated image (left) and fluorescence images (middle and right) of the CA1 region of a hippocampal slice, stained with Et (from the experiment shown in C). O generation due to an 8 min (B: control, C: melatonin, 10 μm) or a 12 min ischemic stress (D: control, E: melatonin, 10 μm). The changes of slope in B–E indicate changes in the rate of O
generation due to an 8 min (B: control, C: melatonin, 10 μm) or a 12 min ischemic stress (D: control, E: melatonin, 10 μm). The changes of slope in B–E indicate changes in the rate of O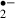 generation. The period of ischemic stress is indicated by the bar over each plot. Dotted lines indicate the basal rate of O
generation. The period of ischemic stress is indicated by the bar over each plot. Dotted lines indicate the basal rate of O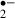 generation (estimated by linear regression) in the last 5 min before exposure to ischemic stress. Py: pyramidal cell layer, Or: stratum oriens, Rad: stratum radiatum. Scale bar: 200 μm.
generation (estimated by linear regression) in the last 5 min before exposure to ischemic stress. Py: pyramidal cell layer, Or: stratum oriens, Rad: stratum radiatum. Scale bar: 200 μm.
| Ischemic stress (mean ± S.E.) | Recirculation (mean ± S.E.) | ||
|---|---|---|---|
| 8 min | 12 min | ||
| Melatonin (10 μm) | 1.52 ± 0.18*,#(n = 12) | 1.14 ± 0.09#(n = 5) | 1.67 ± 0.17**,#,&(n = 7) |
| Ascorbic acid (2 mm) | 1.15 ± 0.20 (n = 4) | 1.82 ± 0.24*(n = 4) | |
| Control | 2.54 ± 0.41**(n = 12) | 1.59 ± 0.21*(n = 5) | 1.29 ± 0.11*(n = 7) |
- The slope in the 5 min immediately before the ischemic stress was defined as the baseline slope. The maximum slopes during the periods of ischemic stress and recirculation following the stress were divided by the baseline slope to give relative values.
- * , * *Comparisons with baseline slope (paired t-test).
- # Comparison with control (unpaired t-test).
- &Comparison with recirculation after 8 min ischemic stress (unpaired t-test).
- * ,#,& P < 0.05; **P < 0.01.
Discussion
The present study has demonstrated an acute neuroprotective effect of melatonin against the consequences of ischemic stress that may be due to its action as a radical scavenger. Field EPSPs were monitored electrophysiologically to evaluate neural damage, and the oxidized HEt method [20] was used to monitor the generation of O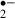 in rat hippocampal slices under ischemic stress.
in rat hippocampal slices under ischemic stress.
The main sites of O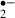 generation are the ubiquinone and NADH dehydrogenase located within mitochondria [21]. Electrons are transferred to form ubisemiquinone, which reacts with O2 to form O
generation are the ubiquinone and NADH dehydrogenase located within mitochondria [21]. Electrons are transferred to form ubisemiquinone, which reacts with O2 to form O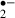 [22]. It has been known that approximately 2–5% of the electron flow in isolated brain mitochondria produces superoxide anion radicals and hydrogen peroxide (H2O2) [23]. These constantly produced ROS are scavenged by SOD, GSHPx, catalase and LMWA endogenously. The generation of O
[22]. It has been known that approximately 2–5% of the electron flow in isolated brain mitochondria produces superoxide anion radicals and hydrogen peroxide (H2O2) [23]. These constantly produced ROS are scavenged by SOD, GSHPx, catalase and LMWA endogenously. The generation of O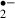 transiently increases at the beginning of glucose-oxygen deprivation or ischemic stress. An insufficient energy state for mitochondria might cause the generation of a large amount of O
transiently increases at the beginning of glucose-oxygen deprivation or ischemic stress. An insufficient energy state for mitochondria might cause the generation of a large amount of O . In this study, melatonin and ascorbic acid almost eliminated O
. In this study, melatonin and ascorbic acid almost eliminated O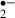 generation during these periods. As oxygen (the source of O
generation during these periods. As oxygen (the source of O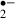 ) was unavailable, generation of O
) was unavailable, generation of O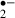 was not observed in the middle of the ischemic period.
was not observed in the middle of the ischemic period.
It is noteworthy that the amount of O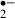 production occurring during an 8 min ischemic stress and recirculation in the control group was more than that in the melatonin-treated group, although the suppression of synaptic responses was reversible, irrespective of whether we did or did not give melatonin treatment. On the other hand, when ischemic stress was allowed to continue for 12 min, the suppression of synaptic responses was irreversible in the control group, but not in the melatonin-treated group. As the amount of O
production occurring during an 8 min ischemic stress and recirculation in the control group was more than that in the melatonin-treated group, although the suppression of synaptic responses was reversible, irrespective of whether we did or did not give melatonin treatment. On the other hand, when ischemic stress was allowed to continue for 12 min, the suppression of synaptic responses was irreversible in the control group, but not in the melatonin-treated group. As the amount of O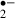 generated in the recirculation phase after a 12 min ischemic stress was smaller in the control group than in the melatonin-treated group, this reflects that the number of viable cells with the ability to generate O
generated in the recirculation phase after a 12 min ischemic stress was smaller in the control group than in the melatonin-treated group, this reflects that the number of viable cells with the ability to generate O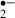 might have been reduced by the more severe ischemic stress (i.e. for 12 min). In other words, melatonin might have protected neurons against ischemic damage in the 12 min ischemic stress group as the O
might have been reduced by the more severe ischemic stress (i.e. for 12 min). In other words, melatonin might have protected neurons against ischemic damage in the 12 min ischemic stress group as the O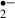 production was equivalent to that after an 8 min stress in the control group. Namely, melatonin reduced the damage of neurons in 12 min stress to that in 8 min stress.
production was equivalent to that after an 8 min stress in the control group. Namely, melatonin reduced the damage of neurons in 12 min stress to that in 8 min stress.
A reduction in cell death in hippocampal CA1 neurons after global ischemia has been demonstrated in the SOD-overexpressing animals [6, 7]. Moreover, increased cell death in the CA1 region has been observed after cerebral ischemia in SOD-deficient mice [8, 9]. Although the mechanisms responsible for the delayed neuronal cell death among hippocampal CA1 pyramidal neurons several days after transient forebrain ischemia are not yet clear [24, 25], the participation of free radicals, including ROS, may be suggested. As neurons in the control group were exposed to more O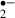 than those in the melatonin-treated group, the damage in the control group would be expected to be more severe than that in the melatonin-treated group. Indeed, a previous study suggested that melatonin protects CA1 neurons against delayed neuronal cell death in vivo [14]. This protective action of melatonin against delayed neural cell death may be attributable to its radical-scavenging action, as indicated by the present data. This study observed acute effect of melatonin on the ischemic stress and did not show whether melatonin rescued the slice from the delayed neural cell death. Observation of a long-term cultured slices suffered the ischemic stress is necessary to conform the effect of melatonin on the delayed neuronal cell death as the next step.
than those in the melatonin-treated group, the damage in the control group would be expected to be more severe than that in the melatonin-treated group. Indeed, a previous study suggested that melatonin protects CA1 neurons against delayed neuronal cell death in vivo [14]. This protective action of melatonin against delayed neural cell death may be attributable to its radical-scavenging action, as indicated by the present data. This study observed acute effect of melatonin on the ischemic stress and did not show whether melatonin rescued the slice from the delayed neural cell death. Observation of a long-term cultured slices suffered the ischemic stress is necessary to conform the effect of melatonin on the delayed neuronal cell death as the next step.
O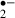 is produced in response to NMDA-receptor activation [20], which plays an important role in the induction of the long-term potentiation (LTP) in hippocampal area CA1. Indeed, transgenic mice that overexpress extracellular SOD show an impairment of LTP [26]. Perfusion with 100 μm melatonin blocks LTP induction [27], and it is possible that the block is caused by O
is produced in response to NMDA-receptor activation [20], which plays an important role in the induction of the long-term potentiation (LTP) in hippocampal area CA1. Indeed, transgenic mice that overexpress extracellular SOD show an impairment of LTP [26]. Perfusion with 100 μm melatonin blocks LTP induction [27], and it is possible that the block is caused by O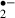 scavenging by melatonin. Interestingly, induction of anoxic LTP has been reported in CA1 neurons [28, 29]. When melatonin was removed during the ischemic stress in the present study, the transmission ratio for fEPSPs during the recirculation was potentiated. This result may be consistent with the unmasking of anoxic LTP by the removal of a scavenger for O
scavenging by melatonin. Interestingly, induction of anoxic LTP has been reported in CA1 neurons [28, 29]. When melatonin was removed during the ischemic stress in the present study, the transmission ratio for fEPSPs during the recirculation was potentiated. This result may be consistent with the unmasking of anoxic LTP by the removal of a scavenger for O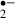 , the latter being essential for anoxic LTP induction.
, the latter being essential for anoxic LTP induction.
As melatonin did not change the time course of fEPSP suppression, it does not participate in the mechanism that neurons lose ability to respond to electric stimuli by ischemic stress, but must have a potential to protect neurons from ischemic insult without affecting the metabolism of neuron itself.
In this study, the effective concentration of melatonin needed for protection against ischemic insult was over 1 μm. In vitro studies have indicated that the radical-scavenging ability of melatonin occurs within the μm range [30, 31]. In humans, the serum concentration of melatonin is between 50 pm (daytime) and 500 pm (nighttime) [32]. On the other hand, Skinner and Malpaux reported that as melatonin is released directly into the third ventricle from the pineal gland, the melatonin levels in the third ventricle during the night were about 20-fold higher than that in plasma [33]. Furthermore, concentrations of melatonin in various subcellular compartments of neurons are predicted to be higher than those in CSF [34, 35]. Thus, the melatonin concentration in CSF and subcellular compartments of neurons could be at a level at which it effectively scavenges radicals. It is also reported that pinealectomized animals suffer from greater neurodegeneration by brain ischemia than the control [15, 36]. It is therefore suggested that melatonin is an antioxidant substance in the brain under physiological conditions.
Although various studies have shown that melatonin is a broad-spectrum antioxidant, its scavenging ability for O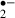 is generally considered to be weak [37, 38]. However, our results showed that 10 μm of melatonin suppressed O
is generally considered to be weak [37, 38]. However, our results showed that 10 μm of melatonin suppressed O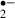 generation both during and after ischemic stress as effectively as 2 mm ascorbic acid. Melatonin may first donate an electron to the hydroxyl radical, with the loss of an electron from the melatonin molecule leading to the formation of a melatonyl radical cation [39, 40]. This relatively stable radical secondarily scavenges superoxide anion radicals to form a final product, N1-acetyl-N2-formyl-5-methoxykynuramine [41]. Another metabolite deriving from melatonin, N1-acetyl-5-methoxykynuramine is also a radical scavenger [42]. These melatonin reactions occurring in slices would reduce O
generation both during and after ischemic stress as effectively as 2 mm ascorbic acid. Melatonin may first donate an electron to the hydroxyl radical, with the loss of an electron from the melatonin molecule leading to the formation of a melatonyl radical cation [39, 40]. This relatively stable radical secondarily scavenges superoxide anion radicals to form a final product, N1-acetyl-N2-formyl-5-methoxykynuramine [41]. Another metabolite deriving from melatonin, N1-acetyl-5-methoxykynuramine is also a radical scavenger [42]. These melatonin reactions occurring in slices would reduce O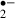 generation. Furthermore, melatonin stimulates antioxidative enzyme activity and increases cellular mRNA levels for these enzymes [43]. Other radical scavengers, ascorbic acid and α-tocopherol, had effects on synaptic responses similar to that of melatonin. Thus, the mechanism by which melatonin suppresses the generation of O
generation. Furthermore, melatonin stimulates antioxidative enzyme activity and increases cellular mRNA levels for these enzymes [43]. Other radical scavengers, ascorbic acid and α-tocopherol, had effects on synaptic responses similar to that of melatonin. Thus, the mechanism by which melatonin suppresses the generation of O and protects neurons from ischemic damage may be its direct and indirect radical scavenging action.
and protects neurons from ischemic damage may be its direct and indirect radical scavenging action.
This study showed that melatonin protects the functions of neurons against ischemic insult by reducing O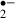 generation. This is, as far as we know, the first report of melatonin function as a radical scavenger through real-time monitoring of O
generation. This is, as far as we know, the first report of melatonin function as a radical scavenger through real-time monitoring of O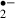 generation. We proposed the physiological proof of melatonin function as a radical scavenger in this study.
generation. We proposed the physiological proof of melatonin function as a radical scavenger in this study.
Acknowledgment
This work was partly supported by a Grant-in-Aid from the Ministry of Education, Science, Culture, Sports and Technology, Japan (A.F.).




