Why do melanomas get so dark?
Abstract
Abstract: Cutaneous malignant melanomas often exhibit pigmented regions that are darker than the surrounding skin. While melanoma cells are the original source of the melanin, keratinocytes and melanophages also contribute to the tumor colour because they contain melanin obtained from melanoma cells. However, little is known of the origin of darkly pigmented melanoma cells or of the molecular pathways regulating their melanin production. Here we discuss observations that dark melanoma cells emerge from within populations of melanoma in situ and that, in addition to producing abundant dark pigment, they appear to be undergoing autophagy. Moreover, autophagy appears to be a common trait of invasive melanoma cells in the dermis. The underlying cause of this phenomenon may stem from aberrant production of glycosylation structures known as β1,6-branched oligosaccharides. Our studies of dark cutaneous melanomas were prompted by analyses of experimental mouse macrophage-melanoma hybrids fused in the laboratory. Like melanoma cells in cutaneous malignant melanoma, experimental hybrids also displayed abundant dark pigment and autophagy, and had high levels of β1,6-branched oligosaccharides. Whether or not darkly pigmented malignant melanoma cells originate from fusion with macrophages in vivo remains to be determined. In any event, pigmentation in melanoma, long considered as a secondary aspect of the malignancy, may be a visible warning that the cells have gained competence for invasion and metastasis.
Introduction
The incidence of melanoma in humans is, in general, inversely proportional to skin melanin content. Thus fair-skinned individuals are more likely to develop melanoma than are those with dark skin (reviewed in 1). Yet many melanomas and some nevi exhibit regions that are markedly darker than the normal colour of the patient’s skin. Indeed, a diagnostic feature of primary cutaneous melanoma is the presence of variegated pigmentation with darkly pigmented areas (Fig. 1).
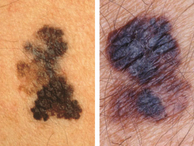
Cutaneous malignant melanomas demonstrating obvious asymmetry, irregular borders, and variegation in colour with prominent darkly pigmented areas. Photographs provided by Prof. Jean Bologna, Yale School of Medicine.
In our recent studies of dark pigmentation in melanoma, we have noted that while melanoma cells are the original source of the tumor melanin, there are actually three melanin-containing cell types that contribute to the dark colour: melanoma cells, keratinocytes and melanophages (Fig. 2). Moreover, the melanin in dark areas of melanoma often appears brown-black in colour, consistent with being eumelanin, and thus qualitatively different from the gold-red pheomelanin, characteristic of fair-skinned individuals (1). Here we describe our recent observations regarding cellular and molecular pathways that accompany dark pigmentation in malignant melanoma. We also discuss the remarkable similarity between the darkly pigmented phenotype in malignant melanoma and the phenotype expressed by metastatic macrophage-melanoma hybrids created by fusion in the laboratory (2–5).
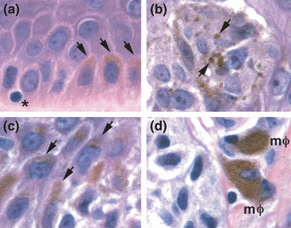
Images from a single histologic section of cutaneous malignant melanoma. The section was stained with hematoxylin and eosin. Using the PhotoshopTM program, the unprocessed images were co-joined into a single flattened layer and then enhanced simultaneously with tools for contrast, brightness, and sharpening. Magnification is identical for all images. (a) Normal epidermis containing a melanocyte (asterisk) and basal layer keratinocytes with apical (supranuclear) melanin (arrows). (b) A nest of melanoma cells containing coarse melanin (arrows) in a region of florid MIS. (c) Epidermal keratinocytes with supranuclear melanin (arrows) adjacent to an area of florid MIS (not shown). (d) Melanophages (mφ) adjacent to invasive melanoma cells in the dermis.
Melanization of normal skin
Normal skin of fair-skinned individuals contains melanocytes with relatively low melanin content in the basal layer of the epidermis. The melanin in melanocytes is produced and stored in melanosomes, which are sub-cellular organelles that normally exist individually in the cytoplasm (1). Melanocytes donate melanosomes to surrounding keratinocytes where, in fair-skinned individuals, they are packaged into membrane-bound clusters and distributed in the cytoplasm above the keratinocyte nucleus, offering protection from UV radiation (Fig. 2a) (6). Melanin in keratinocytes generally has a gold/red colour (1). This colour derives from pheomelanin, the major melanin species in fair-skinned individuals (1). Production of pheomelanin is often a reflection of low responses to the hormone melanocortin-1 (MC1, also known as melanocyte stimulating hormone, MSH). This is due to impaired function of the MC1 receptor (MC1R), a genetic hallmark of fair-skinned individuals (1).
Melanization in dark melanoma cells
We recently made an observation that darkly pigmented melanoma cells arise as a separate population of cells from within early melanoma in situ (MIS) (2). By light microscopic examination it is apparent that in the dark regions of melanoma tumors, melanoma cells produce more melanin per cell than do normal melanocytes in adjacent skin (Fig. 2b). Melanoma melanin often appears brown/black compared to the gold/red in normal skin, suggesting that dark melanoma cells produce more eumelanin. Though not yet proven chemically, this is supported by optical scanning studies of cutaneous malignant melanomas where the spectrum in dark tumors tended to show a eumelanin-type profile (7,8). It should be noted that increased melanin synthesis and the production of eumelanin are both signs of a functional MC1/MC1R system (1). It is tempting to speculate that dark melanoma cells may express an MC1/MC1R system which is activated differently from that in normal melanocytes, perhaps through aberrant glycosylation or other post-translational modifications. This is of particular interest because the MC1/MC1R system is thought to play a role in melanoma progression, at least in part through its activation of the proto-oncogene cMet, whose signalling pathway is a key regulator of metastasis in melanoma and many other cancers (9–13). It is also possible that cyclic AMP pathways that induce melanization are activated down-stream of the MC1/MC1R system in dark melanoma cells.
Autophagy and coarse melanin
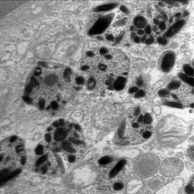
In the dark regions of melanomas, melanosomes are packaged into autophagosomes of heterogeneous sizes, long known to dermatopathologists as ‘coarse melanin’ (Fig. 3, cf. Fig. 2b) (2,3). This suggests that these melanoma cells may be undergoing autophagy, a process of “self eating” wherein a cell’s cytoplasmic components are engulfed and digested as an energy source in times of oxygen and nutrient deprivation, as is the case in wounds and tumors. Autophagosomes, by enveloping defective melanosomes, may also protect the cells from leakage of the toxic oxidative by-products of melanization. While autophagy is associated with cancer cell death pathways, paradoxically it is also a predictor of worse patient outcome in a variety of cancers (reviewed in 2,4).
Aberrant glycosylation and dark melanoma cells
Another feature in the emergence of dark melanoma cells is the production of glycosylation structures known as β1,6-branched oligosaccharides (2,3,14). We have observed that these structures are not produced by normal melanocytes or by cells of early melanoma in situ but are a characteristic of florid MIS, as well as invasive and metastatic tumors (2,3,14) (Fig. 4). The rate-limiting enzyme in the production of these structures is β1,6-N-acetylglucosaminyltransferase V (GnT-V; Mgat-5; E.C.2.4.1.155), a Golgi complex enzyme that is normally expressed in macrophages and other migratory leucocytes. GnT-V and β1,6-branched oligosaccharides are associated with poor outcome in melanoma and carcinoma of the breast, colon, lung, GI tract, and endometrium (reviewed in 4,5). It is thus of great interest that the emerging dark, autophagic melanoma cells in MIS as well as cells invading the dermis also expressed β1,6-branched oligosaccharides. Moreover, these sugars were likely conjugated to autophagosomal proteins since they co-localized with autophagosomes in pathology specimens (2,3). Therefore, aberrant expression of β1,6-branched oligosaccharides is associated with increased melanin production, autophagy and high invasive potential in melanoma cells. It should be noted that β1,6-branched oligosaccharides are widely expressed in both pigmented and non-pigmented melanomas and their expression is independent of pigment levels (3).

(a) A case of invasive malignant melanoma stained with H&E. (b) A sequential section of the same tumor stained with the biotinylated lectin LPHA (leucocytic phytohemagglutinin) that is specific for β1,6-branched oligosaccharides.
The origin of dark melanoma cells
Where do dark melanoma cells come from? We observed that these darkly pigmented, autophagic cells seem to emerge from within populations of weakly melanized, non-autophagic cells of early MIS and subsequently form nests in the epidermis (2). These dark autophagic cells in MIS appear to be the source of invasive cells in the dermis although some lose pigment in the process. We surveyed 12 cases and noted that autophagsome-bearing cells were the major if not the only cell type in the invasive dermal melanoma cells. Nonetheless, 8 of the 12 cases showed marked reductions in the pigmentation of invasive cells and the presence of autophagosomes therefore appeared to be independent of the level of pigment. It was recently suggested that loss of pigment in the invasive cells might represent an immune escape mechanism for melanoma (14).
Tumor-associated keratinocytes
Keratinocytes in the vicinity of melanomas also contribute to their dark colour. Keratinocytes do not produce melanin but rather receive it from melanocytes in a process involving attachment of filapodial extensions of melanocytic dendrites to keratinocytes, with subsequent transfer of melanosomes (5,15,16). As with melanoma cells, melanin in tumor-associated keratinocytes often appears to be of a darker, brown/black colour as opposed to a lighter colour in keratinocytes of normal adjacent skin (Fig. 2c). In addition, while melanin in keratinocytes of normal skin is distributed evenly in fine, homogenous granules, the melanin in tumor-associated keratinocytes shows heterogeneous granularity as seen in melanoma cells (Fig. 5). In 10 out of 12 cases of malignant melanoma surveyed, keratinocytes around the MIS showed darker melanin with heterogeneous granularity compared to keratinocytes in adjacent normal skin (Lazova and Pawelek unpublished). These findings suggest that melanoma cells can transfer their dark autophagosomal melanosomes to surrounding keratinocytes. While melanin transfer to keratinocytes by melanoma cells has been described in cell culture, to the best our knowledge this has not as yet been demonstrated in vivo (17).
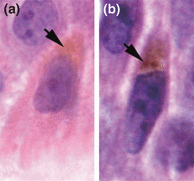
Melanin in epidermal keratinocytes from a case of malignant melanoma in situ. Images were photographed from the same section and processed together with the use of the PhotoshopTM program as described in the legend to Fig. 2. (a) A keratinocyte in normal skin adjacent to the tumor. (b) A keratinocyte associated with a region of dark melanoma cells. The arrows show melanin in the apical, supranuclear region of the cells.
Tumor-associated melanophages
Tumor-associated melanophages further contribute to the dark colour of melanoma tumors. Melanophages acquire melanin through ingestion of melanized melanoma cells. Figure 2d depicts dark tumor-associated melanophages in the dermis. Like melanoma cells, melanophages contain autophagosomes laden with partially degraded melanosomes (2).
Lessons from macrophage-melanoma hybrids
The above features of dark malignant melanoma cells were also characteristic of highly metastatic experimental hybrids created by fusion in culture between mouse melanoma cells and mouse or human macrophages. These hybrids were generated in order to study a long-standing theory that metastasis is a result of such fusion in vivo (4,5). Indeed, analysis of a large panel of hybrid cell lines revealed that about half of them showed significantly increased metastasis when implanted in mice, and increased chemotactic migration in culture, a well-known capability of metastatic cells. Surprisingly, most of the metastatic hybrids were also highly pigmented, with increased expression of MC1R and strong responses to MC1. In addition, melanosomes in the metastatic hybrids were packaged within autophagosomes, suggesting that the cells were undergoing autophagy (18). We established a long list of molecules expressed by macrophage-melanoma hybrids that were also characteristic of metastatic melanoma cells and normal macrophages alike (4,5). Central to the metastatic phenotype of hybrids was GnT-V, which, through addition of β1,6-branched oligosaccharides to several glycoproteins, caused multiple phenotypic changes, including increased chemotaxis, melanogenesis, and possibly autophagy (19,20). This was illustrated in experiments where inhibition of β1,6-branched oligosaccharide production virtually eliminated melanin synthesis (Fig. 6) and markedly decreased chemotactic motility in hybrids (19). This implied that the metastatic and melanogenic phenotypes in hybrids were each dependent on β1,6-branched oligosaccharides.
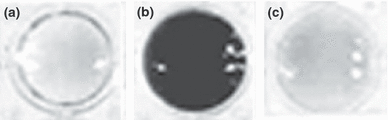
Suppression of melanogenesis in experimental macrophage-melanoma hybrid 48 through inhibition of GnT-V and β1,6-branched oligosaccharide production (from Ref. 18). β1,6-branched oligosaccharide production was inhibited by transfection of a gene for the glycosyltransferase GnT-III (β1,4-N-acetylglucosaminyltransferase III) a competitive inhibitor of GnT-V. Shown are centrifuged cell pellets from three cell lines. (a) Weakly pigmented and weakly metastatic parental melanoma cells. (b) Strongly pigmented and highly metastatic macrophage-melanoma hybrid 48. (c) Hybrid 48 following transfection of GnT-III.
It is not yet known whether β1,6-branched oligosaccharides regulate pigmentation in human malignant melanoma, however, the remarkable phenotypic similarities between naturally occurring malignant melanoma and experimental hybrids, especially high expression of β1,6-branched oligosaccharides, makes this a likely possibility. Indeed, a role for glycosylation in pigmentation has been demonstrated by several laboratories (e.g. 21) Finally, the many similarities between dark melanoma cells and metastatic hybrids, including the apparent emergence of dark melanoma cells from within MIS, are consistent with macrophage-melanoma cell fusion as a possible mechanism. While fusion has been demonstrated in animal tumors, including melanoma, analogous proof of fusion in human melanoma still awaits analysis at the genetic level.
In summary, while the melanin in dark regions of melanoma is originally produced by melanoma cells, some of this melanin is acquired by tumor-associated keratinocytes and melanophages and these cells also contribute to the dark regions of tumors. Another contributing factor to the dark colour is that melanoma melanin may have higher eumelanin versus pheomelanin content compared to that in adjacent normal skin of fair-skinned individuals. This suggests the possibility of an activated MC1-MC1R system in these cells. Dark melanoma cells produce coarse melanin – autophagosomes filled with melanosomes – and thus appear to be undergoing autophagy. Autophagosomes continue to be expressed by invasive melanoma cells in the dermis, even though cellular pigment production is reduced in many cases. Associated with the emergence of dark, autophagic cells and invasion into the dermis is the aberrant production of β1,6-branched oligosaccharides – structures that are highly associated with invasion and metastasis in a number of cancers (4,5). Laboratory studies with macrophage-melanoma hybrids reveal remarkable similarities to dark malignant melanoma cells (4,5) and point to the possibility that macrophage fusion with melanoma cells is the underlying mechanism, although further research will be necessary to determine its validity.
A tentative answer to the question ‘Why do melanomas get so dark?’ is that this phenomenon is triggered, albeit through unknown mechanisms, by the aberrant expression of β1,6-branched oligosaccharides. These structures are produced by dark melanoma cells that appear to emerge from within pre-existing populations of melanoma cells in situ. Thus, pigmentation in melanoma, long considered as a secondary aspect of the malignancy, may be a visible warning that the cells have gained competence for invasion and metastasis.
Acknowledgments
We thank Vincent Klump for his excellent technical assistance and Dr. Jean Bolognia for providing the photographs for Fig. 1. Supported in part by a gift from the Amway Corporation.




