The human skin/chick chorioallantoic membrane model accurately predicts the potency of cosmetic allergens
Abstract
Abstract: The current standard method for predicting contact allergenicity is the murine local lymph node assay (LLNA). Public objection to the use of animals in testing of cosmetics makes the development of a system that does not use sentient animals highly desirable. The chorioallantoic membrane (CAM) of the chick egg has been extensively used for the growth of normal and transformed mammalian tissues. The CAM is not innervated, and embryos are sacrificed before the development of pain perception. The aim of this study was to determine whether the sensitization phase of contact dermatitis to known cosmetic allergens can be quantified using CAM-engrafted human skin and how these results compare with published EC3 data obtained with the LLNA. We studied six common molecules used in allergen testing and quantified migration of epidermal Langerhans cells (LC) as a measure of their allergic potency. All agents with known allergic potential induced statistically significant migration of LC. The data obtained correlated well with published data for these allergens generated using the LLNA test. The human-skin CAM model therefore has great potential as an inexpensive, non-radioactive, in vivo alternative to the LLNA, which does not require the use of sentient animals. In addition, this system has the advantage of testing the allergic response of human, rather than animal skin.
Introduction
Reports of allergic contact dermatitis to cosmetics are constantly rising, in spite of significant efforts by the cosmetic industry (1). The prevalence of allergic contact dermatitis to cosmetics is estimated to be between 10% and 15% among patients who have been referred to contact allergy clinics (2–7), compared to only 5% in the 1980s. Trattner (8) reported that 32% of patients with suspicion of cosmetic allergies reacted to at least one of the allergens of the European Standard and Cosmetic Series. Allergenic potential is not an all-or-none phenomenon and can be quantified, including a threshold for induction. The murine local lymph node assay (LLNA) (9,10) is considered the ‘gold standard’ in estimating allergenic potency of a chemical. The stimulation index (SI) expresses the ratio of the proliferative responses of draining lymph nodes induced by topical exposure to test chemicals compared to treatment with the control vehicle. The estimated concentration of a chemical required to induce an SI of 3 relative to controls is known as the EC3 value, expressing relative allergic potency. EC3 values have been used to classify contact allergens according to their relative potency using four levels: extreme = EC3 value <0.1%; strong = EC3 between 0.1% and 1%; moderate = 1EC3 from 1% to 10% and weak = EC3 between 10% and 100% (11).
Much effort has been put into improving the LLNA, such as reducing the number of animals required (12) and its modification to utilize non-radioactive tracers (13). However, the major global trend of reducing the use of sentient animals in scientific research in general, and in cosmetic testing in particular, renders the LLNA increasingly problematic. Moreover, murine skin and the murine immune response are obviously an imperfect model for human skin and the human immune response. Thus, human organotypic skin explant culture (HOESC) has been studied as an alternative for testing contact allergenicity (14,15). A great deal of data have been collected measuring Langerhans cell (LC) migration out of the epidermis using this model, suggesting that it is at least as good in predictive ability as the LLNA (16). However, viability of intact skin in vitro is limited to a few days, and longer term effects of potential allergens on the skin are less amenable to study using this system. In addition, the skin is not maintained in vitro in a state similar to that in situ, as it is not nourished with a blood supply. In contrast, human skin transplanted to severe combined immunodeficient (SCID) mice provides a near in situ state, which can be used as a long-term model for testing human skin. However, the SCID model requires a month for engraftment, is expensive, and like the LLNA, requires sentient animals (17,18).
An alternative potential experimental system for study of intact human skin is transplantation to the chicken chorioallantoic membrane (CAM), a method used for over 100 years for medium-term maintenance of mammalian tissues.
Goodpasture et al. (19) were the first to engraft human skin to the chick CAM. They observed that human skin engrafts on the chick CAM within a few days, the blood vessels of the CAM fusing with those of the skin. The blood supply keeps the skin viable and healthy for up to 9 days. The developing chick embryo is naturally ‘immunocompromised’, and evidence of an innate rejection response usually appears only in grafts of more than 8 days in duration, if at all (in preparation).
In a more recent study, Kunzi-Rapp et al. (20) transplanted human skin to the CAM and then assayed a number of molecular markers after 5 days. They found CD1a-expressing LC in the epidermis, suggesting to us that this system could be used for testing for allergenicity. We recently tested this possibility using the human skin/CAM system by treating grafted skin with the strong sensitizer dinitrofluorobenzene (DNFB). DNFB treatment elicited a dose-dependent migration of LC out of the epidermis (21). The human skin-CAM system was then used to test the sensitizing ability of a number of potential antigens for antimelanoma immunization and it was found that there was a good correlation between elicitation of an antibody response in rodents, and the level of migration of LC from the treated skin for several experimental molecules (21). These encouraging results led us to examine whether this human skin–CAM system could be compared quantitatively with the established LLNA assay for testing sensitization potential. The aim of the present study was to compare the strength of the immune response to selected cosmetic allergens on CAM-engrafted human skin versus EC3 data from the published literature.
Materials and methods
Skin grafting
Fertilized eggs obtained from a local supplier were incubated vertically at 37 °C at high humidity for 7–9 days postfertilization. Normal human breast skin was obtained from women undergoing mastectomy or cosmetic surgery after obtaining their informed consent and IRB approval. The skin reached our laboratory within 2 h of removal from the operating theatre. Subcutaneous tissue was removed and skin samples of about 5 mm thickness were prepared using a 6-mm biopsy punch.
Skin fragments were maintained in sterile phosphate buffered saline at 15 °C until the eggs were prepared for accepting the grafts. Eggs were opened under sterile conditions and the shell membrane deflected to expose the CAM. The CAM was gently damaged to induce angiogenesis and release growth factors, thus yielding better engraftment of the skin. The skin was then placed on the CAM with the dermis in contact with the chick tissue. The opening in the shell was then closed with cellotape and the eggs incubated for 48–72 h to permit engraftment.
Cutaneous application of allergens
The viability of the engrafted skin fragments was determined by the normal colouration and texture of the skin indicating vascularization by the host and by the typical distribution of CAM blood vessels radiating out from the graft (20). A 5-mm diameter polyethylene ring cut from a pipettor tip was then placed on the skin fragment. Fifteen microlitres of each allergen dissolved in Vaseline was placed within the ring. The opening in the shell was then resealed and the egg incubated overnight. The allergens tested and the concentrations used are listed in Table 1. The concentrations selected for the allergens are the standard concentrations used clinically in patch tests. All experiments included as a positive control the highly allergenic compound, DNFB (22), at a concentration of 0.1% in acetone/olive oil 4:1. Negative controls included Vaseline-only treated skin fragments and skin that was fixed immediately upon receipt without grafting. At least four skin samples were used for each allergen tested in each experiment, and each allergen was tested on the skin obtained from three different patients. All samples in an individual experiment were obtained from the same patient and up to five allergens were tested on each patient’s skin.
| Chemical | Potency category | EC3 |
|---|---|---|
| Dinitrofluorobenzene 0.1% | Extreme | 0.03 |
| Para-phenylelediamine 1% | Extreme | 0.06 |
| Isoeugenol 2% | Moderate | 1.3 |
| Cinnamic aldehyde 1% | Moderate | 2 |
| Eugenol 2% | Weak | 13 |
| Propylene glycol 5% | Weak | >50 |
| Tween-80 5% | Weak | >50 |
Histology and immunohistochemical staining
After an overnight incubation with the allergens, the skin fragments were cut out together with the attached CAM, fixed in 4% buffered paraformaldehyde and embedded in paraffin. Transverse 6 μm sections were made and stained with haematoxylin and eosin to confirm viability and quality of histological preparation. Sections were then stained with a monoclonal antibody to CD-1a (Santa-Cruz Biotechnology Inc., Santa Cruz, CA, USA). Immunostained LC were detected with peroxidase-coupled secondary antibodies and observed following incubation with the chromogen AEC (21). Slides were then coverslipped in glycerin–gelatin.
Quantitative analysis
Langerhans cells were counted in the epidermis of two sections from each skin fragment using a 40x objective. The sections analysed had a gap between them of 12–18 μm (two to three sections) to eliminate the possibility of counting the same LC cell more than once. All LC with a stained soma and at least one stained process were counted, except for those cells within 200–400 μm of the cut edges of the graft, where the skin often became submerged within the CAM. After counting, photomicrographs were taken using a 10× objective with a Scion Color Firewire camera (Scion Corp., Fredrickberg, VA, USA). The pictures were assembled into a photomontage and the area of the epidermis measured using imagej (National Institutes of Health, Bethesda, MD, USA) software. The epidermal LC counts were then divided by the area yielding values of LC/mm2 epidermis, which was shown by others to be a reproducible measure for LC migration (23). Counts from the two sections from each of the four skin fragments were averaged together for each treatment. Statistical significance between groups was determined using a two-tailed t-test in Microsoft Excel. spss statistical software (SPSS Inc., Chicago, IL, USA) was used to determine the best curve for fitting results obtained here to the published values for the allergens tested using the LLNA.
Results
We have previously shown that the strong sensitizer DNFB elicits LC migration from human skin transplanted to the chicken CAM in a dose-dependent by staining sections with anti-CD1a and counting LC remaining in the epidermis (21). The grafting of the skin to the CAM for 2–3 days did not by itself induce the migration of LC, as no statistically significant difference in the number of epidermal LC was observed between skin fixed immediately upon receipt, compared with that grafted for 2 days and treated topically for 1 additional day with the solvent for DNFB, acetone/olive oil (21).
To evaluate whether the human skin-CAM system for testing allergens has the potential to be used for predicting allergenicity quantitatively, we tested here whether the migration of LC from the epidermis correlates to the known allergenicity of compounds used in clinical testing. Six standard chemicals at doses used in sensitization testing were dissolved in Vaseline and applied to CAM-grafted human skin and the number of LC remaining in the epidermis were counted after 1 day of incubation. Vaseline applied to the skin served as a negative control (Fig. 1a) and DNFB, a strong sensitizer, served as a positive control (Fig. 1b).
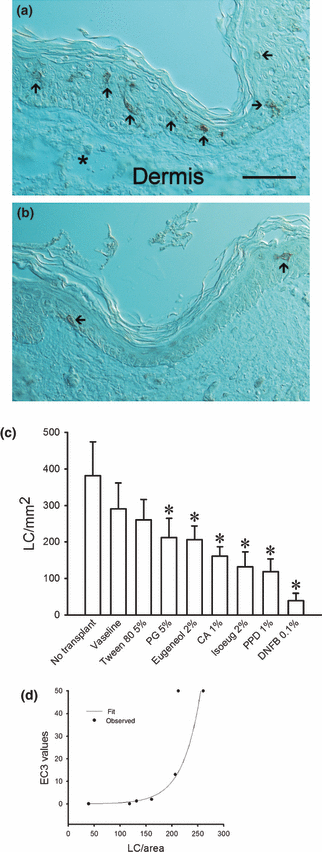
(a and b) Cross-sections of human skin grafted to the chick chorioallantoic membrane (CAM) and stained with anti-CD1a to reveal Langerhans cells (LC). The sections were photographed with Nomarski interference optics to reveal the general structure of the skin. The numerous red–brown stained LC are indicated by arrows. (a) A skin fragment treated overnight with Vaseline, the solvent used for all of the tested allergens. An asterisk marks a large blood vessel containing leucocytes in the dermis. In (b), a fragment treated with DNFB is shown. Two LC can be seen. Scale bar = 50 mm. (c) Migration of LC out of human epidermis grafted to the chick CAM in response to standard allergens. As has been shown in other systems, application of the carrier, Vaseline, elicits a small baseline migration of LC from the epidermis (see text). All materials tested except Tween-80, induced a statistically significant (P < 0.001, asterisks) increase in migration of LC when compared to the Vaseline carrier controls. PG, propylene glycol; CA, cinnamic aldehyde; Isoeug, isoeugenol; PPD, paraphenylene diamine; DNFB, dinitrofluorobenzene. (d) Comparison of results obtained from skin-CAM assay results with published LLNA EC3 data using an exponential regression model. The model shows a very good correlation between the assays. LC/area = number of immunopositive LC/mm2.
Vaseline application overnight resulted in a migration of approximately 1/3 of LC out of the epidermis compared to CAM-grafted, but non-treated controls. A non-significant decline in LC count induced by Vaseline application has been attributed to the weight of the ointment non-specifically stimulating the dendritic cells (24). All applied chemicals, except Tween-80, caused a statistically significant migration (P < 0.001) of LC from the epidermis when compared to the values obtained for the Vaseline carrier alone (Fig. 1c).
We then compared these results obtained with the human skin-CAM model with those appearing in the literature obtained using the LLNA test on mice. A curve estimation procedure was used to generate regression model parameters using the spss statistical software package. Visual inspection of the data revealed a better fit with an exponential compared to a linear model (Fig. 1d). The coefficient used was 0.003 exp(0.0417 cells/area). The F-test of the model fit was 37.939 (P < 0.002), with an R2 value of 0.884, indicating a very good correlation between the model and the published EC3 values. When the analysis was repeated excluding the weak sensitizers propylene glycol and Tween-80 (outlying values), it yielded a similar coefficient (not shown).
Discussion
The use of cosmetics is on the rise, in part, because of the declining age of users (25, http://www.colipa.com). It is not surprising, then, that the prevalence of cosmetic allergic dermatitis has been steadily increasing as well. The allergenic response is characterized by induction and elicitation phases. LC play a pivotal role in the induction phase. Upon topical exposure to contact sensitizers, up to 40% of the local LC become activated (26). Interactions between cytokines released from LC and keratinocytes facilitate migration of LC to the draining lymph nodes. As it is impractical to determine potency of contact sensitizers in absolute terms, relative measures are in common use, such as the EC3. EC3 values generated by the widely used and validated LLNA have been shown to be reproducible between different laboratories (27,28).
The LLNA has several advantages as a predictive assay for sensitization: (i) it is rapid and relatively inexpensive; (ii) it correlates well with clinical data and (iii) allergen potency can be predicted by interpolation from dose–response curves. On the other hand, there are significant problems with LLNA including differences between human and murine skin, the requirement for radiochemicals and the current societal objections to use of experimental animals in testing of cosmetics (29).
It is therefore desirable to find an alternative to the LLNA (and its predecessor, the guinea pig maximization test). Although several attempts have been made to develop cell culture systems for testing allergenicity, the complexity of the relationships among LC, keratinocytes and dermal blood vessels requires a system that closely approximates in vivo conditions. The human skin-CAM model, although first proposed more than 60 years ago, was only revisited using modern laboratory skin processing techniques a few years ago (20). However, until only recently (21), no practical use of the system was made.
The human skin-CAM model has several major desirable properties: (i) the skin used is human and not murine; (ii) there are no ethical issues in using excess skin from surgical procedures that is otherwise disposed of; (iii) large amounts of human skin are discarded in the ever-increasing number of cosmetic operations providing a reasonable source of tissue for testing; (iv) there is no pain to the non-sentient chick embryo, both because the CAM is not innervated and because the experiment is completed well before pain centres of the brain develop in the embryo; (v) the human skin is bathed by blood and remains viable for more than a week and (vi) the technique is very economical, as the cost of fertile chick eggs is negligible (<1$) and there are no animal facility costs.
Measurement of the reduction in the number of epidermal dendritic cells as an assay for allergenic potential of a chemical is well established (i.e. 14,15). We investigated the effect on LC migration of application of six common allergens used in clinical testing to human skin grafted to the chick CAM. Our results reveal a good correlation between published LLNA data and the CAM model. A recent two-site study using the HOSEC system showed that the in vitro system also gave predictive results similar to the LLNA and the previously standard guinea pig-based test, the GPMT and thus is an attractive alternative to animal-based methods. Our present study shows that in addition to general prediction of sensitization, our numerical values for LC migration correlate well with published LLNA values suggesting that the skin-CAM system has the potential to be used to predict not only likelihood of allergenicity, but, by interpolation, the level of allergenicity of potential sensitizers.
A significant advantage of the skin-CAM system compared to the HOSEC system is that the skin is maintained in a more physiological manner on the CAM than in culture, being bathed by circulating blood, rather than being partially submersed in culture medium. This is reflected by the much superior preservation of skin histology beyond 3 days in culture (manuscript in preparation). In addition, the ability to maintain skin in a near physiological state for more than 1 week with the skin-CAM system allows the medium-term investigation of other aspects of skin biology in response to potential sensitizers including keratinocyte proliferation, and changes in levels of elastin, dermal blood vessels and myofibroblasts. Another advantage of using the skin-CAM system compared to HOSEC is the presence of a rudimentary immune system in the chick embryos. This potentially extends the model to use for study of additional aspects of the allergenic responses, as it may permit following the migration of the LC after they leave the skin to determine whether they specifically migrate to the host immune organs such as the spleen and bone marrow.
In summary, the skin-CAM system is a very promising alternative to current animal-based sensitization testing systems such as LLNA and GPMT. Clearly, more studies are required to compare its utility and predictive ability with the more fully studied HOSEC system, and for either or both of these systems to be validated for routine testing in the cosmetics industry. With additional testing, this system has the potential to provide a humane and economical in vivo model system for cosmetic allergenicity testing, with the added advantage that the tests are performed on human skin rather than on animal skin. Finally, the skin-CAM model has the potential to be utilized to become a more general dermatological research tool, for example, for the investigation of molecular mechanisms of activation of LC and dendritic cells in intact human skin, as opposed to in vitro (30).
Acknowledgements
This research was supported by grants from the Horowitz Foundation and the Israel Ministry of Industry and Commerce to RSG (‘NOFAR’). We are indebted to Prof. A. Ingber of the Department of Dermatology, Hadassah-Hebrew University Medical Center for providing the sabbatical leave to enable DS to participate in the experimental work and for providing the allergens used in the study. Thanks to Dr Shoshana Frankenburg for critical reading of the manuscript. We are indebted to Chaya Morgenstern for essential technical and administrative help.




