Rituximab responsive immune thrombocytopenic purpura in an adult with underlying autoimmune lymphoproliferative syndrome due to a splice-site mutation (IVS7+2 T>C) affecting the Fas gene
Abstract
A 36 yr-old man of Israeli descent with a history of childhood splenectomy for severe thrombocytopenia and a family history of autoimmune lymphoproliferative syndrome (ALPS), presented with severe immune thrombocytopenic purpura refractory to standard therapy. He was found to possess a heterozygous mutation in the Fas gene (also termed TNFRSF6, CD95, Apo-1) affecting the donor splice site of intron 7 (IVS7+2 T>C). This frameshift mutation truncates the cytoplasmic domain of the Fas death receptor, resulting in circulating CD4/8 double negative T lymphocytes, lymphadenopathy and autoimmune complications typical of ALPS. Administration of Rituximab in this patient was associated with a durable hematologic response (currently more than 12 months). This report highlights the need to consider rare inherited causes of thrombocytopenia in adults with a family history of immune cytopenia(s) and the effective use of anti-CD20 monoclonal antibody in patients unresponsive to immunosuppression and splenectomy.
Case report
Immune thrombocytopenic purpura (ITP) in adults often has an infectious, autoimmune, malignant or idiopathic etiology. Steroid refractoriness occurs in approximately 9% of patients with ITP (1). A rare inherited cause of ITP secondary to a mutation encoding the Fas gene is presented, with ongoing complete hematologic response 12 months after anti-CD20 monoclonal antibody therapy.
A 36 yr-old man of Israeli descent presented with recent-onset blood blisters in the mouth and a florid petechial leg rash. Automated analysis of the peripheral blood showed severe isolated thrombocytopenia (8 × 109/L), with plentiful megakaryocytes present on bone marrow examination, consistent with a peripheral cause for thrombocytopenia. The patient was not on any medications and tests for immune and infective causes of thrombocytopenia were negative (HIV, Hepatitis B and C, EBV, CMV, ANA, ENA, ANCA, rheumatoid factor, anti-cardiolipin antibody, lupus anticoagulant, disseminated intravascular coagulation screen). The patient had a history of severe thrombocytopenia in childhood necessitating splenectomy which resulted in a sustained clinical remission until the current presentation. Importantly, there was a family history of autoimmune lymphoproliferative syndrome (ALPS) affecting the patient’s niece. The patient did not respond promptly to high-dose prednisolone (100 mg daily), intravenous immunoglobulin and azathioprine (Fig. 1C), and then developed severe anemia (Hb 64 g/L) 18 d after initial presentation. Immune hemolytic anemia was confirmed by the presence of spherocytosis on the peripheral blood film, positive direct Coombs’ test (IgG), reduced haptoglobin (0.1 g/L; range 0.3–2), raised lactate dehydrogenase (LDH) (351 U/L; range 140–270) and reticulocytosis (200 × 109/L; range 50–100). CT imaging of the thorax and abdomen identified enlargement of a pelvic lymph node (Fig. 1A), which was weakly fluorodeoxyglucose (FDG)-avid on positron emission tomography (PET) scanning. Due to the presence of refractory thrombocytopenia, a diagnostic biopsy was not undertaken. In the peripheral blood, polyclonal hypergammagloblinemia (IgG 24 g/L; range 9–16) was present, and in addition, a small population of CD4/8 double negative T cells (Fig. 2A). Four doses of Rituximab (375 mg/m2) given at weekly intervals resulted in brisk normalization of the platelet count (Fig. 1C), a normal reticulocyte count (85 × 109/L) 1 month later and complete resolution of the previous radiological abnormalities on a follow-up CT scan (Fig. 1B). On review 12 months after initial presentation, the patient remains in hematological remission. The patient’s niece, who had a long history of refractory ITP, was known to have a heterozygous Fas mutation (also termed TNFRSF6, CD95, Apo-1) affecting the donor splice site of intron 7 (IVS7+2 T>C; personal communication Frédéric Rieux-Laucat). This previously published frameshift mutation leads to the addition of four intron bases to the RNA sequence, resulting in a premature stop codon and truncation of the cytoplasmic death domain of Fas (2). The mutant Fas protein has dramatically reduced death receptor function. DNA from the peripheral blood of the patient confirmed the presence of a heterozygous IVS7+2 T>C lesion (Fig. 2B).
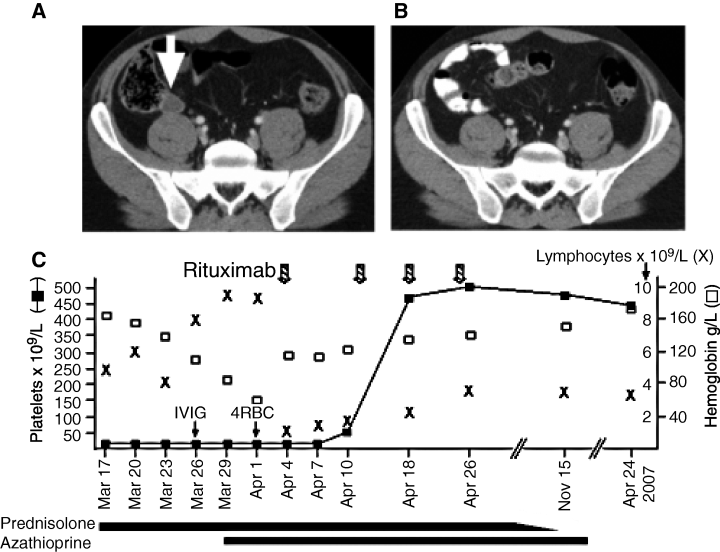
CT scan of the pelvis at diagnosis showing an enlarged lymph node (A; white arrow) which is no longer present (B) one month after completing Rituximab. (C) Line graph showing the platelet count (), hemoglobin (□) and lymphocyte count (×) over time. Interventions with prednisolone, azathioprine, packed red cell transfusion (4RBC), intravenous immunoglobulin (IVIG) and rituximab are shown.
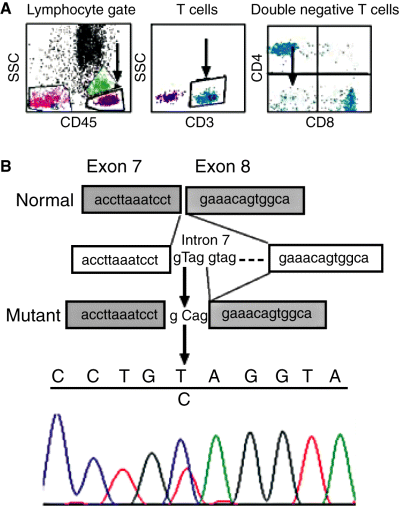
(A) Flow cytometric analysis (BD Facscalibur®, BD Biosciences, Franklin Lakes, NJ, USA) showing 8.4% of T lymphocytes were CD4/CD8 double negative. (B) Exon 7 of the Fas gene and the flanking intronic sequence was amplified by PCR from genomic DNA (Primers 5′tggccacttttaagtttcactg and 5′aaaaggaagtaacaaaaagccaaa). The 292 bp product was directly sequenced with an ABI3130xl genetic analyzer using the PCR primers as per the BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA) protocol. The presence of the heterozygous splice donor site mutation IVS7+2 T>C (arrow) was confirmed by repeat analysis.
Programmed cell death, or apoptosis, by activation of death receptors is a key mechanism for eliminating autoreactive lymphocytes. Functional failure of the Fas (located on chromosome 10q24.1) is associated with ALPS Ia, an autosomal dominant inherited disorder usually manifesting in infancy with lymphadenopathy, splenomegaly, autoimmune cytopenias and persistence of CD4/8- ‘double negative’ T cells (3). At least 69 unrelated families with Fas mutations have been recorded on the National Human Genome Research Institute database, with most mutations occurring in exon 9, the intracellular death domain of Fas (4). Rarely, the ALPS phenotype occurs with defects of Fas ligand (ALPS type Ib), caspase-8 or caspase-10 (ALPS Type II) (5). The reduced capacity to eliminate lymphocytes in ALPS is associated with an increased risk of Hodgkin (relative risk 51) and non-Hodgkin lymphoma (relative risk 14) (6). In ALPS patients with widespread lymphadenopathy and suspected lymphoma, PET scanning has been useful to guide diagnostic biopsies (7). The most effective treatment in symptomatic ITP is splenectomy, which results in long-term remission in 66% of patients (8). In ALPS patients failing splenectomy, limited published experience suggests a role for Rituximab (9) and mycophenolate mofetil (10) in such cases.
Although the Fas IVS 7+2 (T→C) mutation has been previously described by Kasahara et al. (2), limited clinical information on these rare disorders is available in the published literature. In the current case, the rapid increase in platelets was preceded by marked lymphocyte depletion induced by Rituximab (Fig. 1C), strikingly similar to a previous case report (9). However, in contrast to the previously reported case, our patient has not relapsed 12 months after Rituximab treatment and has not required additional therapy to maintain a platelet response (Fig. 1C). Although the rapid platelet response following Rituximab appears premature, this is well described in ITP (11). Intravenous immunoglobulin therapy counters immune thrombocytopenia by blocking macrophage Fc receptor clearance of opsonised platelets. In addition, Rituximab also eliminates B cells, making it an ideal drug for B-cell driven autoimmune diseases. This latter mechanism was likely to be dominant in our patient as he had previously failed intravenous immunoglobulin therapy. Although ALPS is typically associated with expansion of double negative T cells, Fas defects also prevent elimination of autoreactive germinal center B cells (12), predisposing the patient to autoimmune disease and rarely, B-cell non-Hodgkin lymphoma (6).
Although it is possible the weakly FDG avid pelvic node could harbor lymphoma, a percutaneous or laparoscopic biopsy was considered too hazardous in a patient with refractory thrombocytopenia. Of benefit to this patient, Rituximab is highly effective against low grade B-cell non-Hodgkin lymphoma (13), enhancing its utility in this patient.
In conclusion, this report highlights a rare inherited cause of immune thrombocytopenia in an adult with a family history of autoimmune cytopenia(s). Gene scanning confirmed a Fas mutation in the patient and his affected niece. Finally, the effective use of anti-CD20 monoclonal antibody therapy to treat the autoimmune complications of this disorder was remarkable and prevented a protracted course of immunosuppressive therapy in a patient lacking a spleen.
Acknowledgements
Frédéric Rieux-Laucat, Dalia Waldman, Ingrid Cutter, and Jacqueline Keyt.




