The natural history of human papillomavirus infections of the mucosal epithelia
Invited review
Abstract
Chow LT, Broker TR, Steinberg BM. The natural history of human papillomavirus infections of the mucosal epithelia. APMIS 2010; 118: 422–449.
Human papillomaviruses (HPVs), members of a very large family of small DNA viruses, cause both benign papillomas and malignant tumors. While most research on these viruses over the past 30 years has focused on their oncogenic properties in the genital tract, they also play an important role in diseases of the upper aerodigestive tract. Rapidly accelerating advances in knowledge have increased our understanding of the biology of these viruses and this knowledge, in turn, is being applied to new approaches to prevent, diagnose, and treat HPV-induced diseases. In this introductory article, we provide an overview of the structure and life cycle of the mucosal HPVs and their interactions with their target tissues and cells. Finally, we provide our thoughts about treatments for HPV-induced diseases, present and future.
Part I. Introduction and overview
Human papillomaviruses (HPVs) are most commonly known for their benign and neoplastic diseases of the anogenital tract. These viruses are also the causative agents of laryngeal papillomas and other hyperproliferative epithelial lesions of the airway mucosa, collectively known as recurrent respiratory papillomatosis (RRP; see the article by Larson and Derkay for a detailed description of this disease). HPVs can also infect other sites in the head and neck (H&N) region such as the conjunctiva of the eyes, ear canals, nasal sinuses, and oral cavity, especially the oropharynx and tonsils. Although not as prevalent as genital tract infections, HPV infections and lesions of the airway tract are among the most difficult to investigate, to understand, to prevent, and to bring under therapeutic control.
For biomedical research into papillomaviruses and their associated diseases to lead to effective public health measures, both viruses and host responses to infection must be understood in considerable molecular detail. This introductory overview considers many attributes of HPVs including some of the similarities and differences of HPV genotypes, disease manifestations and the natural history of infections as determined from analyses of patient specimens. We also describe the genetic and functional organization of the viral genome, the regulation of viral transcription and RNA processing, the mechanisms of viral DNA replication and long-term maintenance, the properties of the viral proteins, the nature of virus–host interactions in support of viral DNA amplification, and virion morphogenesis. Then we summarize the pathogenic consequences across the spectrum of benign lesions, neoplastic progression, and ultimately cancers, and the clinical management of infections. We necessarily consider in detail the biology of the host tissue for all papillomaviruses – the epithelium.
One of the most challenging aspects of HPV research has been that few, if any, infectious viral particles can be isolated from patient specimens. Unlike many other DNA viruses, HPVs could not be propagated in any submerged cell culture system. As a consequence of major difficulties in establishing experimental systems to recapitulate the complete infection cycle, early investigations relied heavily on cloning and sequencing of various HPV types from natural infections. Viral RNA transcripts recovered from a small number of benign patient lesions were characterized to determine their basic organization and major splicing patterns. From representative HPV genotypes, DNA sequences corresponding to the predominant mRNA exons were then used as probes to examine patient specimens from different anatomic sites and across the entire spectrum of lesions. What became clear from these analyses was that the papillomavirus reproductive cycle absolutely depends on complete squamous differentiation of the host epithelium and that squamous and glandular carcinomas do not support the productive program (1–3). Elevated levels of viral DNA and mRNA are restricted to the mid- and upper cell strata, while the capsid antigen is detected in only a small fraction of superficial keratinocytes. Viral activity is distinctly increased in lesions from patients with immunosuppressive disorders. In high-grade dysplasias and cancers, the viral genome is often integrated and only a subset of the viral genes is consistently expressed. Concurrently, the encoded viral proteins and their functions were identified using a variety of in situ and in vitro assays. Only when this portrait of viral activities and virus–host interactions in natural infections had emerged could development begin to establish appropriate experimental model systems that recapitulate real infections or selected elements of those infections.
HPV taxonomy and pathobiology
Papillomaviruses are a very ancient family of pathogens and are known to infect the epithelial tissues of amphibians, reptiles, birds, and mammals. These viruses appear to have co-evolved with their hosts. Almost all viruses are strictly specific to their natural host and do not infect even closely related species.
Human papillomaviruses are highly prevalent and are medically important pathogens. Infections may remain subclinical or they may be active and induce benign, hyperproliferative lesions of the epithelia, variously called warts, papillomas, or condylomata, according to the anatomic sites of infection. Well over 120 different genotypes of HPVs have been isolated from lesions, sequenced and phylogenetically characterized (4). Each genotype is characterized as being more than 10% different from all others in their DNA sequences. Closely related types (approximately 80–90% identical) are classified as members of the same species, and they tend to share such important biological properties as tissue tropism, disease manifestation, and pathogenicity.
The α-papillomavirus group of HPV types (comprised of 15 species distinguished to date) infects the anogenital tract, upper aerodigestive tract, and other H&N mucosa. These viruses have received the preponderance of research and clinical attention because they can be sexually transmitted and cause significant diseases. These mucosotropic HPVs are further classified into non-oncogenic or low-risk (LR) types, such as HPV-6 and HPV-11 (species 10), and potentially oncogenic or high-risk (HR) viruses, including HPV-16 (species 9), HPV-18 (species 7), HPV-51 (species 5), and HPV-53 (species 6). Within each of these species, there are other, closely related types. A small subset of the lesions induced by the oncogenic types can progress to high-grade dysplasias and cancers, notably cervical, vaginal, penile, anal, tonsillar, and oropharyngeal cancers (5). In contrast, the non-oncogenic or LR HPV-6 and HPV-11 are rarely found in genital cancers and are only associated with pulmonary cancers in a small fraction of patients with RRP (6).
Part II. Keratinocytes and the squamous epithelia
The natural host tissue for the complete infection cycle of all HPVs is the squamous epithelium, either the dry external cutaneous skin or moist mucosal epithelial lining of all body openings. Most viral types are predominantly trophic for one or the other of these tissue types, but certain genotypes can infect and reproduce (or at least persist) in both. The epithelium is a large and complex organ and is comprised primarily of tightly interlocked sheets of keratinocytes supported by an underlying dermis consisting of fibroblasts and extracellular matrices that together provide mechanical stability, flexibility, and the physical, chemical, and biological barriers to the external body surfaces and many of the internal surfaces. The dermis and epidermis working in concert present an immunologic defence to infection via various infiltrating immune cells and circulating antibodies.
The proximal epithelia lining each of the body openings are stratified squamous structures (described in detail below), while the more internal epithelia are typically comprised of columnar epithelia, some of which are ciliated, as in part of the respiratory tract. A band of rapidly cycling and dividing keratinocytes called the metaplastic or transformation zone establishes a squamo-columnar junction at each body opening. These anatomic regions are located in or near the larynx, nasal sinuses, urethra, uterine cervix, and anal/rectal junction. Each such metaplastic zone is particularly susceptible to papillomavirus infection and supports various degrees of reproduction. If the infecting virus is a HR genotype, the metaplastic tissues can, in certain cases and at a low frequency, undergo neoplastic progression to dysplasias of increasing severity and to carcinomas.
Squamous epithelia and dynamics
The squamous epithelium is a multilayered structure in which each stratum has a particular profile of gene expression, protein forms, and cellular architecture that continuously changes as keratinocyte differentiation proceeds (7). As such, the strata can be very well identified histologically and by in situ probing with antibodies for various biomarker proteins, with nucleic acid probes to individual mRNA species, and via metabolic labeling of replicating host cell DNA. The basal keratinocytes are in contact with and responsible for laying down the basement membrane network of the extracellular matrix that separates the dermis from the epidermis. Contact with the basement membrane gives basal keratinocytes special properties including cell cycling, lateral motility during wound healing, and asymmetical cell division and vertical polarity once wound closure has occurred and contact inhibition sets in. The integrity of the basement membrane is a critical barrier to invasion of keratinocytes into the dermis and beyond. However, inappropriate up-regulation of various matrix metalloproteinases in the transformed keratinocytes comprising carcinomas in situ can lead to the proteolytic breakdown of the basement membrane and to tumor cell invasion.
The continuous process of turnover and replacement of skin cells is governed by the limited number of cell divisions accorded to any cell, the so-called ‘Hayflick limit’ of about 60–120 divisions before onset of senescence, a consequence of erosion of the telomeres of chromosomes upon every round of replication until they are too short to avoid chromosome instability. How then do epithelial keratinocytes manage to keep up with the need for constant turnover and refreshment? The basal keratinocytes in most squamous epithelia are quiescent and do not cycle into the S phase and divide very often. Rather, the stratum that completes a full cell cycle every day or two is the next layer up, the parabasal keratinocytes or transit amplifying (TA) cells. The TA cells are committed to differentiation but maintain the ability to divide. This is evidenced by the presence of the proliferating cell nuclear antigen (PCNA) and pRB, the tumor suppressor which controls G1 to S transition in cycling cells, and the ability to incorporate labeled nucleosides into newly replicated cellular chromosomes. Conversely, basal cells lack PCNA, while being often high in p130 protein, which is related to pRB and known to be abundant in quiescent cells, keeping them from entering the cell cycle (8–10). There are additional deeper reservoirs, the stem cells that are among the basal cells or localized to the bulge of the hair follicles. Thus, a multi-tiered system of reserve cells, sporadically active basal cells, and highly active parabasal cells can ensure a healthy epithelium for a full lifetime. This division of activity, function, and lineage longevity might account for the temporal variability observed in persistence of HPV infections, regression of active lesions, and possible reappearance of papillomas, a concept that remains to be proven or established experimentally. Already it is fully supported by clinical observations of new cervical lesions in middle-aged and elderly patients who are immunosuppressed and by studies that have reported the presence of HPV DNA in the biopsies of RRP patients who have been in remission for a number of years (11, 12).
Only after several months of dividing do the parabasal cells reach their limit and undergo terminal differentiation, whereupon the underlying basal cell will divide once to replace it. The basal or parabasal keratinocytes divide asymmetrically; one daughter cell remains in place as a reservoir, while the other is pushed upward toward the cell surface. The upward moving daughters of TA cells permanently withdraw from the cell cycle and will not replicate their DNA again under normal conditions. These cells, the cells above them, and those to follow from below together establish a full thickness epithelium of differentiating cells, with each stratum contributing specialized functions as a consequence of the particular suites of genes that are turned on or off (Fig. 1). As examples, involucrin is expressed in all suprabasal cells, whereas the keratin pairs expressed in the basal stratum (e.g. K5 and K14) are replaced with higher molecular weight keratins in the parabasal and spinous strata (e.g. K1 and K10 in cutaneous skin or K4 and K13 in mucosal epithelium) that contribute increased mechanical stability to the skin. Near the surface, the cutaneous epithelium assumes a new, histologically identifiable feature as a result of having keratohyalin granules comprised of proteins such as (pro)filaggin and loricrin that help cross-link the intracellular keratin network under the direction of epithelial transglutaminases. These upper layers are also responsible for the considerable amount of lipid synthesis that helps establish the bidirectional waterproofing to the superficial strata. External cutaneous skin exposed to the drying effects of air undergoes a final process of maturation and programmed death and the cells convert from a ‘chemical reducing state’ characteristic of most anabolically active cells to an ‘oxidizing state’ associated with cell death. The nucleic acids are degraded, the nucleus disappears, and the residual fibrous proteins and outer membrane shell form a stratum corneum of highly cross-linked cell envelopes that pile up and slough off in response to mechanical abrasion. In contrast, the fully differentiated keratinocytes comprising the uppermost layers of the squamous mucosa maintain a moist surface and do not develop a stratum corneum, but the superficial cells, nonetheless, slough off as they die.
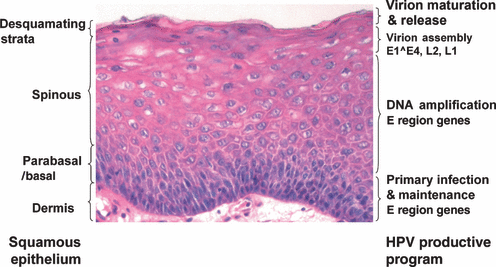
The HPV productive program in a mucosal squamous epithelium. A hematoxylin and eosin-stained tissue section of a laryngeal papilloma. The cellular differentiation profile and viral productive program are indicated on the left and right sides, respectively. Photograph provided by Dr. Hsu-Kun Wang from the Chow/Broker laboratory.
HPV can infect the squamous epithelium when basal or parabasal cells are exposed by wounding. Viral early genes are active during wound healing. In turn, the expression of viral early proteins E6, E7, and E5 may prolong the duration of cell proliferation, thereby expanding the infected cell population. Accordingly, warts, papillomas, and condylomata are often sequelae to a scratch, cut, abrasion, or microbial infection.
Metaplastic zone – the squamo-columnar junction
The dynamic cycling and cell division of the keratinocytes comprising the metaplastic zone contribute to the more external squamous epithelium and to the more internal columnar epithelium. As these keratinocytes do not have a protective overlay of non-cycling cells, they can be directly infected by papillomaviruses and other pathogens, a vulnerability that helps account for the particular susceptibility of the squamo-columnar junction to viral infection. If the metaplastic zone of the cervix becomes infected with HPV, its cell division, lateral migration, and glandular differentiation can provide a conduit for HPV to be conveyed into endocervical glandular mucosa. The columnar epithelium of the endocervical mucosa is just a single cell thick with polarized orientation, and it often forms an invaginated glandular network. This secretory tissue produces mucus that bathes the columnar epithelium exocervix and more external squamous epithelia. However, the columnar epithelium is not a supportive host for the HPV reproductive program as it does not establish the differentiation gradient characteristic of squamous epithelia. Rather, columnar endocervical tissue infected with HR HPVs can develop into carcinoma in situ (CIS) and cancers. In the airway, LR HPVs may infect cells at a laryngeal squamo-columnar junction and migrate into the ciliated columnar epithelium of the trachea, but there is also good evidence that they can directly infect the columnar epithelium. Without the appropriate squamous host factors to support virus expression, these tracheal infections remain latent, with no evidence of disease (12).
Part III. Papillomavirus genome organization, RNA transcription, and productive DNA amplification
Synopsis
Papillomavirus genome organization and functions have been perfected over millions of years of evolution and selection and are exquisitely sophisticated and interconnected. Each element of the biology of HPV raises fascinating questions and provides an intriguing example of diversion of cellular control in normal epithelia. HPV is the object of investigation and equally the informant concerning many of the fundamental attributes of vertebrate cell biology.
The genome organization is conserved among human and animal papillomaviruses, but with some instructive differences (for a review, see 13) (Fig. 2). The double-stranded circular DNA, which ranges from 7600 to nearly 8000 base pairs in length, replicates as multi-copy extrachromosomal plasmids in the nucleus of infected keratinocytes. The genome has (i) an upstream regulatory region (URR) or long control region 400–700 base pairs in length that does not encode proteins, (ii) six or more E (early) region open reading frames (ORFs), and (iii) two L (late) region ORFs (see Fig. 1). The URR contains the origin of DNA replication, early promoters, and binding sites for core transcription factors and various enhancer and repressor regulatory proteins (for a review, see 14). All transcription takes place in the same direction [left to right (5′–3′) on the conventional linear map or clockwise on the circular map] using multiple promoters. The E region and L region are both followed by a poly-A addition site.
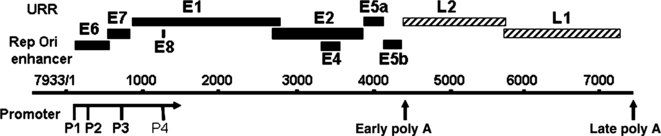
Genomic organization of HPV-11. The genome contains a non-coding upstream regulatory region (URR), also called the long control region (LCR), an Early (E) region and a late (L) region. The open reading frames (ORF) are denoted as boxes. The three major promoters (P1, P2, P3), a minor promoter (P4), and two poly-adenylation sites are indicated. Alternative splicing of the primary RNA transcripts, coupled with the utilization of alternative promoters and poly-A sites, allows the translation of viral proteins as the first or the second ORF in the messenger RNAs. This genomic organization is highly conserved except for the absence of P2 in high-risk HPV genotypes, where the E7 protein is translated from mRNAs with one of two alternative intragenic splices in the E6-coding region.
Low copy maintenance replication of HPV DNA takes place in the dividing basal and parabasal keratinocytes. Elevated viral RNA transcription and DNA amplification to high copy numbers occur primarily in differentiating mid- to upper spinous cells, whereas the capsid protein is only detected in relatively few superficial cells (1, 3, 4). As these differentiated cells have withdrawn from the cell cycle and no longer produce the enzymes and substrates that are essential for DNA replication, the virus must reactivate deoxyribonucleotide triphosphate (dNTP) synthesis and the production of the host DNA replication machinery to replicate or amplify its DNA. The E region proteins are devoted to this ultimate goal. Briefly, E1 and E2 proteins are directly involved in viral DNA replication, whereas E6, E7, and most likely E5, the three viral oncoproteins, condition the differentiated cells to support viral DNA amplification by inactivating major tumor suppressor proteins and activating signal transduction. The viral oncoproteins also modulate host immune surveillance to establish persistence. L1 and L2 assemble the newly replicated viral DNA into progeny virions in the superficial strata. The regulation of the promoters, mRNA processing, and a more complete description of the functions of each viral protein will be discussed.
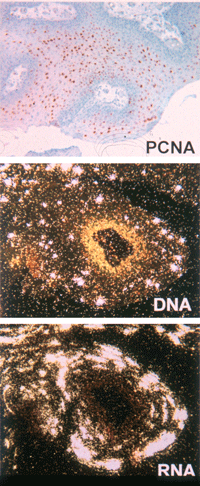
HPV-11 gene expression and replication in an unusually active laryngeal papilloma. The tissues were fixed in 10% buffered formalin and embedded in paraffin. Serial 4 μm sections were subjected to in situ analyses. Top panel: immunohistochemistry revealing the induction of proliferating cell nuclear antigen (PCNA) (reddish stain) in the differentiated strata. Middle panel: in situ hybridization with 35S-labeled sense-strand RNA probes demonstrates that viral DNA amplification occurs in the differentiated strata. Bottom panel: in situ hybridization with 35S-labeled antisense-strand RNA probes shows that elevated viral RNA expression occurs in the differentiated strata. In situ analyses were performed by Ms. Martha R. Hayes from the Chow/Broker laboratory.
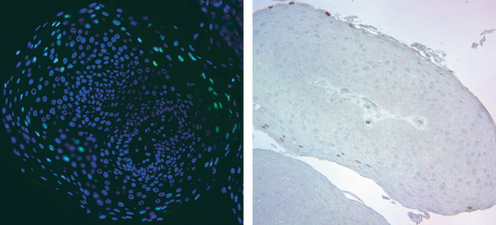
HPV-11 DNA amplification and capsid antigen detection. Sections of a formalin-fixed laryngeal papilloma were subjected to in situ analyses. Left panel: fluorescence in situ hybridization (FISH) with nick-translated DNA probes (green) to detect amplified viral DNA in the differentiated strata. Nuclear DNA is stained blue with DAPI. Right panel: L1 antigen detection by immunohistochemistry (reddish stain) in the same papilloma from a different area. Most regions were negative for L1. In situ analyses were performed by Dr. Hsu-Kun Wang and Ms. Eun Young Kho from the Chow/Broker laboratory.
The genome organization and viral protein functions
All HPV types have two major promoters. P1 is located immediately upstream (5′) of the E6 gene, and P3 is located within the E7 gene. There is also a universally conserved P4 promoter immediately upstream of the tiny E8 ORF. Rare RNA species have 5′ ends in the URR or in other coding regions, suggestive of additional minor promoters. The viruses rely heavily on alternative mRNA splicing to access the various ORFs, and some bi- or poly-cistronic mRNAs can or may encode more than one protein. One feature that distinguishes HR and LR viruses is the presence of E6 intragenic splices from one splice donor to one or two alternative splice acceptors. The splice leads to a translational frame shift and premature termination of E6 translation. Truncation of E6 protein is thought to allow more efficient translation initiation of the downstream E7 ORF in the same mRNA (15). However, mutation of the splice donor did not abolish E7 translation (9). It was not established whether a cryptic splice donor was used in the latter case. The LR HPVs such as HPV-11 or HPV-6 have a dedicated P2 promoter located in the E6 gene to transcribe the E7 mRNA (16). The critical elements that control the P1 promoter are located at the 3′ end of the URR, overlapping the origin of replication (ori). The ori consists of a cluster of (usually three) E2 protein-binding sites (E2BS), flanking an AT-rich region containing an array of E1 protein-binding sites (E1BS). These features are illustrated in Fig. 2.
In this article, we briefly review the functions of the viral proteins. A more detailed discussion of the E6 and E7 proteins, with comparisons between the LR and HR HPVs, is provided in the article by Pim and Banks (this issue).
E6 – Numerous reports have described the biological activities of the HR HPV E6 protein (about 150 amino acids in length) in proliferating primary human keratinocytes (PHKs) and in cell lines (reviewed by 17). The best-known property of the HR HPV E6 is its ability to degrade the major tumor suppressor protein, p53, which monitors and guards the integrity of the genome by inducing genes to effect cell cycle arrest, DNA repair or, alternatively, senescence or apoptosis (see article by Pim and Banks). The HR HPV E6 proteins also destabilize a number of PDZ domain-containing host proteins that regulate cell polarity and signal transduction, including hDLG, hScribble, and MAGI (reviewed by 18). In addition, the E6 protein can modulate G protein signaling by degrading GAP proteins (19) and can transactivate the catalytic subunit of the telomerase gene (hTert) (20). These properties are undoubtedly important for oncogenesis driven by the HR HPVs. In vitro, E6 alone can immortalize human mammary cells. In collaboration with the HR HPV E7 protein, which destabilizes the pRB family of pocket proteins, E6 immortalizes primary human foreskin or cervical keratinocytes (5, 21). However, the function of the E6 protein in the productive phase of the viral infection is not understood because significant E6 or E7 mutants cannot be stably maintained in transfected PHKs (22–25). As the LR HPV E6 proteins do not share the same properties, a common ability between the HR and LR HPV E6 is most likely to be involved. In this regard, both HR and LR HPV E6 can repress p53-dependent transcription regulatory functions by inhibiting p300 acetylation of p53 (26–28 and references therein). Our recent experiments in organotypic cultures of PHKs (see Part IV) harboring an HPV-18 mutant genome unable to encode a full-length E6 protein demonstrate that the mutant is severely affected in viral DNA amplification. High levels of p53, which is known to be induced by E7 protein activity, accumulate in numerous cells (29), implicating p53 in suppressing viral DNA amplification. This observation is consistent with transient replication assays where ectopic p53 represses amplification of human and bovine papillomavirus origin-containing plasmids by ectopically expressed homologous E1 and E2 replication proteins (30–32). However, these observations do not rule out the possible roles of other E6-targeted host proteins. The mechanisms of this aspect of E6 function remain to be investigated.
E7 – The maintenance mode of HPV DNA replication takes place in basal cells that cycle periodically and in the TA keratinocytes that cycle daily. Viral DNA amplification occurs only in a subset of post-mitotic differentiated keratinocytes (reviewed by 13). As viral DNA replication depends heavily on the cellular DNA replication machinery, the virus must promote the reestablishment of such a permissive milieu. This is the function of the E7 protein (of about 98 amino acids in length). Both HR and LR HPV E7 proteins can promote S-phase reentry by differentiated keratinocytes in a squamous epithelium developed in vitro (9, 33–35). Interestingly, unlike the cycling cells where the major tumor suppressor pRB (retinoblastoma susceptibility protein) controls the cell cycle entry, in the stratified squamous epithelia, including the larynx, cervix, and foreskin, the pocket protein p130, which is related to pRB, is primarily responsible for maintaining the homeostasis of differentiated cells, preventing them from reentering the S phase. The E7 protein destabilizes p130 (10, 36) and promotes S-phase reentry. The HR HPV E7 proteins can additionally destabilize pRB. If E7 is inadvertently over-expressed in undifferentiated cycling cells, E7 bypasses the growth stimuli normally needed for activating cyclin-dependent kinases (cdks) cdk4 or cdk6 by D type cyclins to phosphorylate and to inactivate pRB in order to promote S-phase entry. Moreover, ectopic expression of HR HPV E6 and E7 can each destabilize the chromosomes. The ability to destabilize two major tumor suppressors, p53 and pRB, as well as other cellular proteins, largely accounts for the oncogenic potential of the HR HPVs (reviewed by 21; see the article by Pim and Banks).
E1 and E2 proteins, the replication origin, and the mechanisms of viral DNA replication – To support PV DNA replication, the virus encodes two proteins: the dimeric E2 origin-binding protein and the E1 replicative DNA helicase, which assembles into a dihexameric complex (reviewed by 37). All other replication enzymes and proteins are supplied by the host cells. E1 is the only enzyme encoded by papillomaviruses, making it difficult to identify selective inhibitors of HPV replication. E2 is also required for proper plasmid partitioning in dividing cells to establish persistence (38–42 and references therein). By binding to the E2BS at the origin, which overlaps the P1 (E6) promoter, the E2 protein can also regulate transcription (see HPV-associated cancers).
The E1 and E2 mRNAs are derived from the same primary transcripts. The E1 protein is translated from a very low-abundance message (43). In the HR HPV, the E1 mRNA is initiated from the E6 promoter and E6 intragenic splicing appears to be important for the translation of both the E1 and E2 proteins (44). E1 is alteratively encoded by an unspliced messenger RNA that extends from the P3 promoter through the early poly-A site. The E2 protein cannot be translated from this transcript, as the 3′ end of the E1 ORF substantially overlaps the 5′ end of the E2 ORF. Rather, the full-length E2 protein is translated from a spliced mRNA that joins alternative splice donors in the E1 ORF to an acceptor about 100 bases upstream of the E2 ORF. This E1 intragenic splice leads to a frameshift and premature termination of the E1 peptide, thereby increasing the distance to the E2 initiation codon to allow efficient translation reinitiation (43, 45).
The three closely spaced E2 protein-binding sites in the replication origin (ori) bind three dimers of E2 (thus six monomers of E2). Together, these induce a toroidal loop in the supercoiled DNA (46). The extra twist in the DNA creates torsional stress that is relieved by denaturation of the AT-rich sequence in the origin, likely facilitating recruitment and loading of the E1 proteins to form a dihexamer, which occurs with the aid of heat shock proteins Hsp70 and Hsp40 (47). The E1 dihexamer is a very active bidirectional helicase on supercoiled DNA substrates when analyzed in the presence of the single-stranded DNA binding protein, topoisomerase I, and an ATP regenerating system (47). E1 recruits DNA polymerase α/primase and the single-stranded DNA-binding protein RPA to initiate replication. E1 is required throughout initiation and elongation, whereas E2 is only required for the initial recruitment of the E1 complex to the ori (reviewed by 48).
As E1 is such a potent helicase, its activity must be controlled so as not to unwind viral DNA without concomitant viral DNA replication. Efficient nuclear import requires the presence of a bipartite nuclear localization sequence (NLS) that is activated by phosphorylation of serine residues by mitogen-activated protein kinases (MAPKs) (Erk1/2 and Jnk). However, the default position of E1 is in the cytoplasm, attributable to a dominant nuclear export sequence (NES). The NES is only inactivated by cdks recruited by an adjacent cyclin-binding site. Most of these regulatory motifs are located within a span about 45 amino acids situated near the amino terminus, while the MAPK-binding motifs are located near the carboxyl terminus (49, 50). Thus, the unwinding of the viral DNA is tightly coupled to the cell cycle.
Alternative RNA splicing or alternative promoter usage also generates transcripts that can encode E1M^E2C (joining together the amino-terminal portion of E1 and the C-terminal portion of E2), E1Ma^E4 (51), or E2C (also called E8^E2C). The 5′ exon spanning the short E8 ORF contains the initiation codon for the E2C product (52–54). Of the many forms of E2-related proteins, only the full-length E2 protein can support viral ori-dependent replication, as its amino terminus interacts with the E1 protein (55). Competition between full-length E2 protein and E2-related proteins that contain the E2C dimerization and DNA-binding domain can modulate viral replication and transcription (51, 54, 56). In addition, the E8 domain can also recruit co-repressors and is indeed a potent transcription repressor (54, 57).
As will be discussed in Part VI of this introduction, in a newly developed model system in which organotypic cultures of PHKs harboring HPV-18 are used to study the reproductive program, the HPV-18 genome amplifies to a high copy number in the mid- and upper spinous cells. High titers of infectious virions are reproducibly generated. Detailed in situ analyses of the squamous epithelium demonstrated that viral DNA amplification lags behind the S phase when host DNA replication occurs and initiates in cells with high levels of cytoplasmic cyclin B, a signature of G2 arrest. Concomitant with viral DNA amplification, the E7 activity is lost, as evidenced by the reappearance of p130, disappearance of E7-induced PCNA, and inability to reenter into another round of the S phase. The program then switches to late gene expression. The progeny DNA is packaged into virions in the superficial cells, while particle maturation takes place in the stratum corneum where the oxidizing environment allows the disulfide cross-linking of the L1 capsid proteins (29).
E1^E4 – The E1^E4 protein is the most divergent protein in sequence and length among the different papillomavirus types. The mRNA is initiated from the P3 promoter and is spliced from the E1 ORF to a site in the E4 ORF. Thus, the encoded protein is comprised of the amino terminal several amino acids of the E1 ORF with the rest of the protein encoded by the E4 ORF. Both the protein and the mRNA are the most abundant among all viral products. E4 ORF exactly overlaps the central hinge region of the E2 protein (reviewed by 13). In benign lesions, this protein is primarily detected in the upper strata in cells containing high copies of viral DNA and the L1 antigen (reviewed by 58). This expression profile reflects that E1^E4 is additionally present as the first ORF in a bicistronic late message, which also encodes the L1 or the L2 protein (reviewed by 13). Ectopic expression of the E1^E4 protein causes the collapse of the cytokeratin intermediate filaments in submerged monolayer cultures, although this was not observed in differentiated cells in the squamous epithelium (59, 60). Ectopic E1^E4 can sequester cyclin B/cdk1 to the cytokeratin, causing G2 arrest (reviewed by 61). However, in the raft culture model with a fully productive HPV-18 program (see Part VI), the elevated cytoplasmic cyclin B1 was located in the lower and mid-spinous cells where viral DNA amplification initiated, whereas the E1^E4 protein was detected in the upper spinous cells coincidental with high viral DNA, but not with cyclin B. These results suggest that E1^E4 alone is not responsible for the cytoplasmic accumulation of cyclin B1 to cause G2 arrest. The dramatic up-regulation of E4 protein in the upper strata could have been the consequence of elevated viral DNA templates for the transcription of monocistronic or bicistronic E1^E4 mRNAs. Indeed, the expression of HPV E7 alone in differentiated cells causes not only S-phase reentry but also a prolonged G2 phase (N.S. Banerjee, T.R. Broker, and L.T. Chow, unpublished results). The possible involvement of the E1^E4 protein of CRPV and several HPV types in DNA amplification has also been directly investigated by mutational analyses in rabbit skin and in organotypic culture of immortalized cell lines, respectively. The results were not consistent. The function of the highly abundant E1^E4 protein remains to be elucidated.
E5 – The E5 ORF is located immediately upstream of the early poly-A site and is present in all early region mRNAs. Two reports indicate, respectively, that E5 can be translated from an mRNA which also encodes the E2 or the E1^E4 protein (62, 63). E5 is a small, multi-functional membrane protein, predominantly localized to the endoplasmic reticulum (64 and references therein). It interacts with the 16-kDa vacuolar-ATPase and prevents the acidification of early endosomes, thereby altering the trafficking, turnover, and signal transduction of epidermal growth factor receptor (EGFR) and related receptor tyrosine kinases, hence modulating cell growth (65–67). Thus, E5 may have an important role in establishing and expanding the infected basal/parabasal cell population during the tissue repair phase after the wounding during which the virus first gains entry. In vitro, E5 increases the immortalization efficiency of HR HPV E6 and E7. Moreover, EGFR and its activities are elevated in laryngeal papillomas and in cultured papilloma cells (68). However, the role of E5 in the viral life cycle is not understood, as genetic dissection in organotypic cultures has not yielded clear answers (69, 70). Considering that efficient E1 protein nuclear import depends on phosphorylation by MAPKs, which are downstream effectors of receptor tyrosine kinase signal transduction, E5 is expected play a major role in the efficiency of viral DNA amplification, and it indeed does in the new model system in which organotypic cultures are developed from PHKs (J-H. Yu, T.R. Broker, and L.T. Chow, unpublished observation). Along with E6 and E7, E5 can down-regulate host immune responses to viral infection. Therefore, E5 would be a contributing factor in the early stages of viral oncogenesis, although E5 is not expressed in HPV cancers (21). A transgenic mouse system also indicated a role of E5 in cervical carcinogenesis (71).
The late capsid genes L2 and L1 – Papillomaviruses switch to late transcription by (i) dramatically increasing the number of template copies of DNA as a result of vegetative amplification, (ii) diminishing or eliminating the activity of the P1 and P2 promoters, leading to up-regulation of the P3 promoter, (iii) suppressing the utilization of the early polyadenylation site such that transcripts from the P3 promoter are elongated through to the late poly-A site; and (iv) gaining stability of late RNAs, possibly because specific RNA instability factors are lost in terminally differentiated keratinocytes. The L1 protein is primarily encoded by the E1^E4-L1 bicistronic mRNA, although minor mRNA species also exist, which have other 5′ ends, implicating additional promoters (reviewed by 13). A long transcript spanning E1^E4, E5, L2, and L1 could be a splicing intermediate for the E1^E4-L1 mRNA or could be the mRNA for the L2 protein.
L1 forms pentameric capsomeres that comprise the major portion of the icosahedral virion, while a single copy of the L2 protein is axially embedded into each of the 72 pentamers and helps establish shape and stability (72). Some post-translational modifications to L1 such as limited glycosylation apparently occur, but their function remains unknown. Papillomavirus virions do not have a membrane envelope. Because the L1 capsid proteins are cross-linked by disulfide bonds, they are notoriously stable to environmental extremes (29, 73–75). Virus-like particles (VLPs) comprised of the L1 protein alone form the basis for current anti-HPV vaccines, as described below.
The amino-terminal domain of the L2 protein has a furin cleavage site that is highly conserved among PV types. During de novo infection, virion binding to extracellular matrices induces a conformational change, exposing this site, and proteolysis is essential to binding to a co-receptor on the cell surface and to particle uptake into the cell (76, for a review, see 77). Presumably, the reducing atmosphere of the living cell breaks the disulfide cross-links of the L1 protein, and the acidic environment of the endosome dissociates the capsid, releasing the chromatinized viral genome into the cytoplasm where it traffics to the nucleus.
Part IV. Natural history of papillomavirus infections
HPV infections and interactions with the host cells need to be considered in four distinct phases: (i) latent, persistent infections dependent on long-term maintenance of the viral genome as autonomous plasmids. Such infections can remain subclinical for years, or they can become activated, particularly as a result of wounding or immunosuppression. (ii) Activation of viral gene expression leads to a warty lesion that can but does not necessarily result in extensive replicative amplification of the viral DNA and its packaging into infectious particles. Most benign HPV lesions of the mucosal epithelia, especially laryngeal papillomas, produce very few virions as inferred from the sparse cells positive for the capsid antigen (Fig. 4). (iii) Persistent infection by the HR HPV types can at a low frequency undergo neoplastic progression to high-grade dysplasias and (iv) to carcinomas, where the viral DNA is often integrated into host chromosomes. This review will focus primarily on the HPVs of the mucosa comprising the upper respiratory tract and anogenital tract as they have been most intensively studied.
Latent infections
Latent infections have been difficult to examine because the viral DNA and RNA are scarce, and cells in culture exhibit a proliferative ‘wounding’ phenotype that activates latent infection. PCR detection of the DNA and RT-PCR analysis of the transcripts have, nonetheless, determined that HPV can be harbored in cells/tissues completely subclinically at and beyond the apparently normal margins of many active, visible lesions and in patients in remission (12, 78). There is also good evidence that a significant fraction of the population carries latent HPV DNA in their upper airway with no history of papillomatous disease (79, 80). Latency is the most likely outcome of HPV infection when considered on a per-cell basis, because individuals with HPV-induced diseases such as RRP have a relatively small number of lesions but extensive latent infection throughout the airway. When the basal cells divide, the HPV must replicate to keep up the low copy number in these cells. To replicate viral DNA, the E1 and E2 replication proteins are required, and the E6 and E7 proteins are almost certainly needed as well. Notably, E6 and E7 mutant genomes cannot be stably maintained in transfected human keratinocytes (22–25). Once the basal cells have withdrawn from the cell cycle and returned to quiescence, there is no need for the early viral gene expression. These extremely low levels of viral gene expression may well be one of the viral strategies to minimize evidence of its presence, to ‘fly below the radar’ of the host immune system.
Active infections
The basal and parabasal cells maintain low viral DNA copy number, requiring minimal expression of viral early proteins when the cells periodically cycle. In an active infection, the parabasal cycling compartment expands to many cell layers before the daughter cells permanently exit the cell cycle to differentiate into spinous cells. However, when the daughter cells have withdrawn from the cell cycle to undergo squamous differentiation, it becomes imperative for the virus to restore the host replication machinery to enable viral DNA amplification and progeny virus production. In these lesions, not only does the viral DNA amplify in a subset of differentiated cells, but also the host DNA replicates to become tetraploid (9, 29, 81), accounting for the enlarged nuclei in a subset of spinous cells typical of papillomas, condylomata and low-grade squamous intraepithelial lesions (SIL). Although the induction of host DNA replication proteins such as the PCNA by the E7 protein in the differentiated strata of a squamous epithelium is an infallible indicator of HPV infections (8, 9, 29), only some of the PCNA-positive cells replicate host and viral DNA (see 3, 4) (8–10, 29, 35) This is attributable to the sequestration of cyclin E/cdk2 by its inhibitors p27kip1 and p21cip1 that are constitutively transcribed in the differentiated cells, but with varied protein stabilities (81–85). Moreover, benign lesions do not necessarily harbor many cells with amplified viral DNA or productive of the L1 capsid protein (see 3, 4). Such a modulated virus–host interaction contributes to the long-term persistence in the hosts without arousing the immune system to eliminate infected cells and hence virus from the host. This interpretation is consistent with highly elevated viral activities in immune-suppressed patients and in infected human xenografts implanted in immune-compromised mice (86, for reviews, see 87, 88).
Neoplastic progression
Given the strategy of the viral productive program, which depends on the viral oncoproteins to disrupt host cell cycle regulatory proteins, one can appreciate that persistent over-expression of the HR HPV oncogenes in the basal compartment can lead to excessive cell cycling and accumulation of deleterious host gene mutations selected for survival and growth. Thus, persistent infections with any of the HR HPV genotypes pose a small but very real risk of progression that can result in moderate and high-grade dysplasias in which the zone of cycling cells further expands to comprise the majority or even the entire thickness of the squamous epithelium, with little or no differentiation in the upper strata. The potential to support viral DNA amplification and virion production is correspondingly reduced. There is evidence that integration of viral DNA into the host chromosomes often occurs in preneoplastic lesions and these lesions progress faster than those containing entirely extrachromosomal HPV DNA (89).
HPV-associated anogenital cancers
Further neoplastic progression can lead to invasive and metastatic carcinomas. In such lesions, the viral genome is often integrated into host chromosomes. HPV-18 has been reported to integrate preferentially near c-myc (90). The most prominent example is the co-amplification and translocation of HPV-18 DNA adjacent to the c-myc gene in the HeLa cells derived from a cervical adenocarcinoma (91). The viral integration site is invariably located in and disrupts the E1 or E2 gene and is often accompanied by deletion of some of the downstream viral sequences, encompassing the E5 gene and early poly-A addition site (3). The consistent deletion of the DNA-binding domain of the E2 gene in HPV cancers results in the loss of negative feedback regulation of the P1 promoter. Accordingly, the E6 and E7 RNA transcripts are over-expressed. Because of the downstream deletion of the viral early poly-A site, the viral mRNA is chimeric, and transcription extends into the flanking host DNA, where a particularly stable cellular poly-A sequence is hijacked (92). In the most rapidly growing tumor cells, the levels of E6 and E7 transcription remain unchecked and invariably highly elevated. Analyses of a cervical cancer cell line, an oropharyngeal cancer, and epidermal keratinocytes immortalized in vitro by HPV-16 or HPV-18 (93) unexpectedly revealed that only a single copy of the papillomaviral genome or isogenic HPV copies arising from the loss of heterozygosity were transcriptionally active, no matter how many copies of the viral genome were integrated or at how many different host chromosomal loci (94). In cases of tandemly integrated viral genomes, only the downstream 3′ border copy, which was interrupted in the E2 gene, was transcriptionally active, while the upstream intact copies were silenced by DNA methylation, as were all other non-transcribed copies at other loci. Such selection invariably resulted in cells lacking expression of a functional E2 or E2-related protein.
What triggers the neoplastic progression of benign lesions harboring HR HPVs? The early region gene expression is up-regulated as a result of wounding and healing or following local or systemic immunosuppression. Such over-expression can begin to exert real and permanent damage through the combination of excessive cell cycling and interference with the DNA damage control functions of p53, resulting in the accumulation of mutations or chromosomal aberrations and pushing the cells toward immortalization and transformation. Indeed, continuous expression of the viral oncoproteins E6 and E7 is necessary to maintain the transformed phenotype, as ectopic E2 expression in cervical carcinoma cell lines that express the HR HPV oncogenes shuts off the P1 promoter, resulting in senescence or apoptosis (95–97).
Part V. HPV infections in the H&N region and lungs
Recurrent respiratory papillomatosis
Infections – Recurrent respiratory papillomatosis and associated lesions in the upper airway are extremely difficult to treat, not only because of their location, but also of their tendency to recurrent regrowth (98) (see also the articles by Syrjänen, by Smith, and by Larson and Derkay). The primary treatment modality is surgical removal under general anesthesia, most notably with the microdebrider and with lasers. Typically the seemingly normal tissues surrounding overt papillomas as well as more distant tissues in the airway are latently infected with HPV (11, 12), and papillomas can regrow because of activation of viral gene expression in the surgical margins or nearby squamous tissues during the wound healing phase, quickly reestablishing the obstructive papillomas. In addition, papillomas of the upper esophagus can be seen in RRP patients. Whether this is due to migration of HPV-infected cells from a primary lesion in or near the larynx or is a direct infection remains unknown.
Adult-onset RRP is probably caused by a new infection associated with oral sex. In contrast, juvenile-onset RRP is attributed to vertical transmission from mothers with genital condylomata, possibly during passage through the birth canal. Intriguingly, trophoblasts, which help establish the placenta early in pregnancy, can be infected with and support HPV replication in vitro (99). Indeed, HPV DNA has been found in placental tissues and in umbilical cord blood (100, 101), possibly leading to trans-placental infections. HPV has also been found in near-term amniotic fluid (102), and some babies do have laryngeal lesions at birth. Moreover, some children delivered by cesarean section go on to develop RRP. Thus, some oral and airway infections are established in utero.
Modification of normal squamous epithelial biology in HPV-induced papillomas – While HPV expression and replication are dependent on cellular factors that are characteristic of squamous epithelium, some of these factors are altered in active HPV infections. Studies of recurrent respiratory papilloma tissues and primary cells derived from those papillomas have helped elucidate alterations that occur in benign HPV-induced lesions. Globally, the transcriptional profile of the tissues when analyzed on gene expression arrays is significantly altered, with a pattern of induction or suppression of many genes that is quite characteristic of carcinomas, although the lesions are benign (103). The papillomas are characterized by abnormalities in differentiation that lead to an expansion of basal-like cells and accumulation of spinous cells, with reduced cell loss at the tissue surface (104). Although involucrin is expressed in the suprabasal cells, the switch from basal cell-like to differentiation-specific keratins occurs in only a small subset of cells. Most cells maintain basal-cell markers, and the terminal differentiation marker (pro)filaggrin is essentially absent. This perturbation of squamous differentiation in RRP might be one reason why respiratory papillomas produce very little virus relative to genital condylomas.
HPV6/11-infected papilloma cells have significant alterations in signal transduction pathways. They over-express surface EGFR because of recycling of the receptor following ligand binding and internalization, and have enhanced sensitivity to EGF so that the threshold for Erk activation is at least 10-fold lower than in normal cells (68). Other signal transduction alterations, many linked to the constitutive activation of the EGFR in vivo, include activation of phosphoinositol-3-kinase, p38 MAP kinase, and Nuclear Factor kappa B (NFκB), and elevated levels of the signaling intermediate Rac1, a small GTPase that regulates many signaling pathways (105–108). These alterations contribute to the abnormal phenotype of papilloma cells in vitro (109), and Woodworth et al. (110) have reported that EGFR signaling is required for the formation of HPV-induced papillomas in a transgenic animal model.
EGFR signaling through Rac1 also mediates expression of the enzyme cyclooxygenase 2 (COX-2) and its downstream product prostaglandin E2 (PGE2) in papilloma cells (107, 108). COX-2 is not only normally induced during inflammation and wound healing, but is also expressed in benign tumors and many carcinomas. COX-2 expression can inhibit epithelial differentiation and apoptosis, and inhibition of COX-2 in laryngeal papilloma cells with the selective inhibitor celecoxib suppressed cell growth and enhanced spontaneous apoptosis in vitro (107). COX-2 can be induced in normal laryngeal cells by the expression of the HPV-11 E7 protein, and treatment of papilloma cells with celecoxib reduces HPV-11 transcription (R. Wu, A. Abramson and B. Steinberg, unpublished data). Thus, virally induced alterations in signal transduction culminating in COX-2 expression might be an important contributor to papillomavirus formation. However, we have recent evidence (A. Lucs, R. Wu, N. Jamal, A. Abramson and B. Steinberg, unpublished data) showing that COX-2 is constitutively expressed in clinically normal airway tissues of respiratory papilloma patients. Thus, constitutive expression of COX-2 might also be a risk factor in the development of disease following HPV infection.
Pulmonary involvement – The HPV-driven laryngeal papillomas or oropharyngeal cancers can be found extending down the airway into the trachea, bronchi, and most rarely in the lungs in a subset of patients. The risk of extension into the trachea and lower airway is significantly increased if a tracheotomy is performed to create an alternative airway when the narrow laryngeal region is largely or fully obstructed with papillomas (111). The spread might be caused by cell migration. Papilloma cells can block the natural clearing action of ciliated airway lining in removing secreted mucus and microbial infections. On the other hand, it might be due to the activation of latent HPV infection in response to wound healing or due to the squamous metaplasia induced by tracheotomy, as similar amounts of viral DNA can be detected in the trachea and in the larynx. Approximately 5% of juvenile-onset laryngeal papillomatosis cases eventually progress to lung involvement (RRP Foundation Patient Data Analysis: http://www.RRPF.org). These tend to be patients who had early and severe laryngeal papillomas. Such expansion of the lesions usually occurs over a span of 10–20 years. In the lung, even LR HPV types can also cause neoplasias and cancers. Pulmonary involvement is determined by CT scan and biopsy. HPV lesions in the lungs are especially dangerous because they cannot be accessed readily by surgery and they have so far proved recalcitrant to adjuvant pharmacotherapies. Without the opportunity for periodic debulking, pulmonary papillomas can become obstructive for exchange of air, leading ultimately to necrosis and cavitation of the lung tissue, and to death.
Oral and ocular infections
HPV-13 and HPV-32 have been found in oral focal epithelial hyperplasia (i.e. Heck’s disease) (112). HPV-6 and HPV-11 among other genotypes can infect the ocular conjunctiva and induce papillomas that are cosmetically disfiguring and can grow sufficiently to obstruct vision (113) (see the article by Syrjänen).
Head and neck cancers and lung cancers
From a substantial fraction of oral and other H&N lesions, papillomavirus DNA can be isolated, PCR-amplified, and genotyped. Overall, about 20% of H&N cancers are believed to be a result of HPV-mediated oncogenesis (see the article by Smith). Most of these cases are oropharyngeal and tonsillar cancers. Interestingly, HPV-associated cancers have a generally better long-term prognosis than HPV-negative oral cancers. Thus, screening for HPV in oral lesions has become a new standard of diagnosis when confronted with H&N cancers, as this information can guide the choice of therapies.
Lung cancers that are very rare sequelae to upper airway papillomas and oropharyngeal cancers have been reported, almost always caused by HPV-16 or HPV-11 (cf. 114). In addition, HPV-16 has also been detected in lung cancers in patients without RRP or oropharyngeal cancers. The viral DNA was detected by PCR and the copy number was very low (below 1 genome equivalent/cell). To provide unambiguous determination that HPV is causative and not a passive passenger in the lung tumors, tests that are more specific are essential. One is the recovery and characterization of spliced HPV messenger RNA from tissue extracts using reverse transcription-PCR (RT-PCR). A more definitive approach is to perform in situ hybridization to demonstrate that HPV E6 and E7 mRNAs are expressed in the cancer tissues. Through the 1980s and 1990s, this type of assay was instrumental in ascertaining that HPVs are the causative agents for the wide spectrum of anogenital lesions (e.g. 1–3, 115).
In summary, HPV infections of the H&N region are becoming recognized as a very important cause of morbidity and mortality. Considerable further investigation is urgently needed. Upon generating definitive linkage of HPV infections to diseases other than in the anogenital mucosa, a far greater appreciation of the capabilities and consequences of HPV infections will alter current medical standards for diagnosis and clinical care. Perhaps most notably, such information is likely to change public perceptions that HPV is primarily a sexually transmitted disease and should motivate the necessary funding for new research and for development of effective therapeutic approaches.
Part VI. Organotypic epithelial cultures as experimental models to study HPV pathobiology
Several key capabilities necessary to investigate HPV infections in a meaningful and realistic experimental setting have been: (i) a steady-state model with which to generate stratified, differentiating epithelium; (ii) efficient ways to introduce the viral sequences to study their regulation and interaction with the host in the squamous epithelium; (iii) efficient ways to introduce the complete viral genome and recapitulate the productive program and to perform genetic analyses; and (d) infectious virions to infect keratinocytes and verify the productive program. All these studies of course require probe technologies to evaluate viral and host cell gene expression in the context of the differentiating squamous epithelium (29, 116). Several probe technologies are illustrated in 3, 4.
Organotypic epithelial (raft) cultures
The initial conundrum for tissue culture models is to generate adequate amounts of non-cycling, differentiating cells, virtually a contradiction in capabilities. The possibilities offered by organotypic epithelial tissue cultures were first suggested by Broker and Botchan (117). The key capability was the raft culture system initially developed to produce tissues for skin grafts (118) in which healthy primary human keratinocytes from a patient in need of surgical repair are grown on a dermal equivalent consisting of collagen matrix and human fibroblasts. Initially, the keratinocytes are grown submerged in an appropriate culture medium where the cells divide and spread laterally across the collagen surface in a manner akin to wound healing until they are nearly confluent. The assembly is then ‘lifted’ to and maintained at the liquid medium–air interface such that the upper surface of the epithelial cells is exposed to the atmosphere in the cell incubator, while the dermal equivalent is kept moist and supplied with nutrients and various growth factors secreted by the embedded fibroblasts. Continuous capillary movement of media through the collagen is driven by the evaporation of water from the upper surface of the developing epithelium. The keratinocytes begin to divide asymmetrically, establishing a cycling basal stratum and suprabasal, post-mitotic differentiated spinous strata. Within 10 days, a full thickness squamous epithelium is developed, from basal, lower and upper spinous strata to superficial granular strata covered by the stratum corneum (reviewed by 119). Because of the short duration, the TA stratum is not reestablished as in native squamous epithelia. These cultures can be sustained for about two weeks, after which the spinous strata become progressively thinner, as the cell division from the basal stratum diminishes or ceases, while the terminal differentiation and programmed cell death continue to occur normally at the superficial stratum. Very similar cultures are achieved by placing pieces of epithelial tissue biopsy directly on the collagen–fibroblast matrix. Cycling epithelial cells migrate across the surface and then stratify and differentiate into a squamous epithelium. Notably, the type of epithelium that emerges is entirely comparable with the morphology of the source of the biopsy (120). For example, Fig. 5 is an explanted laryngeal papilloma grown as an organotypic raft. Thus, the first experimental challenge was met: the production of squamous epithelial tissue cultures.
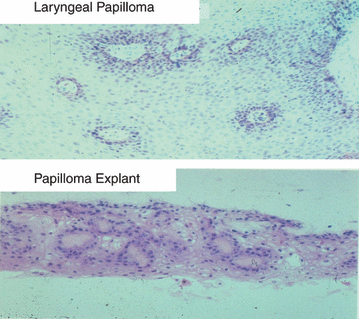
Explanted laryngeal papilloma tissues cultured as organotypic rafts. Top panel: histology of a laryngeal papilloma revealed by hematoxylin and eosin staining. Bottom panel: histology of explanted papilloma tissues from the same lesion grown as an organotypic raft culture. Explants and photographs were produced by Dr. Delf Schmidt-Grimminger from the Chow/Broker laboratory.
The Chow–Broker laboratory and that of Laimonis Laimins independently developed organotypic raft culture systems to recapitulate the HPV productive phases (121, 122). Virions were produced in the outgrowth of an explanted HPV-11 condyloma or in an HPV-31-containing cell line derived from a dysplastic patient specimen. The ‘raft’ tissue model has become a standard for experimental studies of HPVs.
Introduction of subgenomic HPV sequences into PHKs to investigate virus–host interactions
The next challenge was to introduce sub-genomic HPV DNA into the keratinocytes destined for organotypic culturing. It is difficult to perform DNA transfections of PHKs because the efficiency is low and many cells do not survive the common protocols such as calcium-mediated transfection or electroporation. As an alternative, retrovirus-mediated gene transfer (e.g. with MuLV or lentivirus vectors) has proven very efficient and has been used extensively for genetic analysis of HPV transcription regulatory sequences and E7 genes. For example, the HPV-18 and HPV-11 URR-P1 promoters linked to a β-galactosidase reporter have been transduced into the PHKs and the reporter expression examined in submerged PHKs and in raft cultures. The URR-P1 was active in submerged cultures, which resembles the wound healing state. However, in the squamous epithelium, the reporter activity was only detected in the differentiating epithelium unless histone deacetylases were inhibited, demonstrating the changing activity of the URR in different milieu. Mutagenic analyses reveal that the binding sites for transcription factors AP1, Oct, and Sp1 act synergistically and that mutation of any of the three significantly reduces the promoter activity (123–126). HPV-18 URR-P1 promoter-driven HPV-18 E7 or the LTR-driven HPV-11 E7 or HPV-1 E7 can each promote S-phase reentry by a subset of the spinous cells without sacrificing squamous differentiation while inducing a mildly dysplastic histology. Mutagenic analyses have also been performed to identify motifs critical to E7 activity. Indeed, these experiments have revealed p130 to be the E7-targeted pocket protein in differentiated strata (9, 10, 35, 126). On the other hand, when the HR HPV E6 and E7 oncogenes were under the control of the constitutive LTR promoter and then introduced into PHKs by acute retrovirus infection, the resulting raft cultures resembled high-grade dysplasias (33). Similarly, raft cultures display severely dysplastic histology comparable with high-grade SIL or carcinoma when HR HPV oncogene-immortalized keratinocytes or HPV-transformed cervical carcinoma cell lines are used. There was limited or no differentiation (10, 93, 127–129). Collectively, these experiments demonstrate that elevated HPV oncogene expression drives neoplastic progression.
Introduction of the HPV genome into keratinocytes to recapitulate the productive program in raft cultures
Traditionally, HPV genomes have been excised from recombinant plasmids and transfected into PHKs (reviewed by 130, 131). However, the transfection efficiency of non-supercoiled DNA is low compared with that of supercoiled DNA. Furthermore, without the proper negative supercoiling, such DNAs are poor templates for RNA transcription and DNA replication. Thus, it is necessary to select for and expand the transfected cells using a drug resistance marker gene expressed from a cotransfected plasmid. Such selection can take weeks if not months and thus the ability of the HR HPV oncoproteins to extend the life span and to immortalize the PHKs comes into play. LR HPV or HR HPV mutants that cannot persist cannot be studied. As an alternative approach, selected immortalized epithelial cell lines were used as the recipient (reviewed by 132; see also 133). However, raft cultures developed from extensively passaged or immortalized epithelial cells have not supported a highly productive program of the introduced viral DNA, compromising the ability to perform genetic analyses, probably because of altered growth and differentiation properties.
Recently, a new method has been developed in which HPV genomic plasmid is generated in PHKs from transfected supercoiled recombinant plasmids (29). The entire HPV genome linearized in a non-critical position in the URR and then flanked by bacteriophage P1 LoxP recombination sites was cloned into a bacterial plasmid containing a neomycin drug-selectable gene. When cotransfected into PHKs with a second plasmid that expresses the phage P1 Cre recombinase targeted to the nucleus (nlsCre), the Cre-mediated excision recombination generates two plasmids, the HPV genome and the cloning vectors, each harboring a single residual LoxP site of 34 base pairs. After a 4-day G418 drug selection, about 30% of the PHKs survive the selection and uniformly harbor HPV genomes. When these cells are placed on the dermal equivalent and grown as raft cultures, the HPV-18 genomes replicate as autonomous plasmids in the nuclei of the keratinocytes and amplify to high copy numbers in a large proportion of the mid- and upper spinous cells. Viral capsid genes are expressed in the superficial cells, and the newly replicated viral DNA is packaged into virions, which complete their maturation in the stratum corneum. These patterns are identical to those in benign lesions, and the unchecked high productivity most closely approximates what is seen in benign lesions from HIV/AIDS patients and in HPV-infected human xenografts in immune-deficient mice (86). Importantly, using detailed time course studies coupled with metabolic labeling, the entire productive program was elucidated, as already discussed in Part III.
In this new experimental model system, the progeny HPV particles are highly infectious. For the first time, the HPVs can be passaged in raft cultures of naïve PHKs and the entire productive program repeated with exceptional efficiency. Moreover, only a high multiplicity of infection (MOI) of naïve PHKs can elicit a productive infection. Below a certain threshold of MOI, the virus expresses its early genes, persists in the infected cells, and promotes cellular DNA replication in the differentiated cells, but very few cells are able to support viral DNA amplification and virion production. The reason remains to be elucidated. Such relatively non-productive infections are often observed in benign lesions in immune-competent patients.
As this method of introducing HPV genome into PHKs bypasses the need for immortalization functions of the HR HPV, one should in principle be able to analyze mutant genomes incapable of immortalization and also LR HPV genotypes, which do not extend the lifespan of PHKs. Indeed, an HPV-18 E6 mutant genome was successfully studied in raft cultures and the results show that the E6 protein is critical for efficient viral DNA amplification. Site-directed mutations of additional HPV genes can also be similarly studied.
Part VII. Translational applicability of basic HPV research
Clinical diagnosis
Detection of cervical infections can be achieved in multiple ways: (i) when the tissue is exposed to dilute acetic acid/vinegar, regions of active HPV infection of the mucosa will briefly turn a cloudy white (‘aceto-whitening’); this test is utilized in low-resource settings. (ii) The Papanicolaou smear of exocervical and endocervical cells is the current ‘gold standard’ for the diagnosis of active HPV infections, based on a scale of cytological abnormalities. The optimal approach uses liquid-based cytology, as exfoliated cells are less clumped and easier to visualize and characterize by light microscopy. The Pap smear has been one of the most successful diagnostic tests in all of medicine. It has reduced deaths resulting from cervical cancer to just 20% of the pre-Pap mortality in populations of sexually active women who are screened at least every few years, starting several years after sexual debut through to older ages (e.g. 60 or so if the cytology remains normal). (iii) If cytology is abnormal, the appropriate follow-up calls for colposcopically directed biopsies and pathological evaluation according to diagnostic criteria established by the ‘Bethesda’ consensus, ranging from low- to high-grade squamous intraepithelial lesions, to CIS and to invasive cancer. (iv) The biopsy can be examined for the induction of host S-phase biomarkers such as PCNA, MCM7, and KI67 (8, 134) across the spectrum of HPV diseases or for the accumulation of p16ink4 in cases of HR HPV-induced cancers (135 and references therein). p16ink4a inhibits the assembly of cdk 4 or 6/cyclin D, which normally phosphorylates and inactivates pRB as the initial step to promote the G1–S transition. Upon pRB destabilization by the HR HPV E7, the lack of feedback control then leads to increased p16ink4a protein. Indeed, p16ink4a has been immunohistochemically detected in a variety of HPV-associated high-grade lesions. (v) Screening for high expression of HR viral E6-E7 transcripts in cervico-vaginal lavages using RT-PCR. (vi) Testing for HR HPV DNA in Pap samples or cervico-vaginal lavages using one of several molecular probe technologies, some of which can identify specific HPV genotypes as well as coinfections with multiple types, a common occurrence (136). A combination of Pap smear and HR HPV DNA typing has been used effectively to identify preneoplastic lesions, while reducing the false-negative incidence of the Pap smear alone and increasing the intervals between Pap smear screenings (137).
Aceto-whitening is not used to detect H&N infections. Rather, initial diagnosis is based on the presence of exophytic papillomas or evidence of possible malignancy, confirmed by the histology of biopsies. As only a subset of H&N cancers is caused by HPV, and not all benign lesions are exophytic, determination of possible HPV-associated disease is dependent on detecting HPV DNA or (preferably) RNA in biopsies or exfoliated cells, supplemented with analyses of biomarkers, as just described.
These molecular tools for clinical diagnosis have evolved from decades of basic scientific assessment of HPV biology and viral interactions with the host cells in naturally infected tissue samples. They are excellent examples of clinical importance of basic research in support of translational research. The medical and epidemiologic fields concerned with papillomavirus diseases are in a degree of flux with regard to choices among the many new diagnostic options. Efforts to produce even better molecular diagnostic tests that are fast, sensitive and specific, predictive of disease outcomes, and affordable in developing countries remain high priorities.
Prophylactic vaccines
One of the greatest sources of excitement in recent HPV research has been the development and clinical validation of prophylactic vaccines that prevent primary HPV infection and persistence (for a review, see 138). These vaccines are based on VLPs composed of the L1 major capsid protein only. The neutralizing antibodies recognize conformational epitopes on the virus particles, accounting for the need to use VLPs instead of unassembled L1 monomers. For the same reason, the prophylactic vaccines are HPV type-specific, but they do have some limited cross-reactivity with very closely related genotypes (138). VLPs spontaneously self-assemble in cells expressing the L1 protein if present in sufficient concentrations, and this includes production in the bacterium Escherichia coli, yeast Saccharomyces cerevisiae, and eucaryotic insect cells Sf9 when infected with baculovirus expression vectors. VLPs can also self-assemble in vitro, and this property enables the dissociation–reassociation to remove traces of molecular contaminants during manufacture. Notably, the VLPs are devoid of any viral genomic material or any other DNA or RNA and, as such, they pose absolutely no risk of infection. Ironically, the absence of DNA is also one of their potential limitations. Unlike viral vaccines based on live, attenuated viruses such as the Sabin polio vaccine, the HPV vaccine does not establish a prolonged stimulation to the immune systems. Thus, the VLPs are mixed with an adjuvant to improve the initial response. Vaccination requires at least two injections, preferably three (with a primary shot at time 0 followed by a booster at 1 or 2 months and again at 6 months), to induce high titers of antibodies against the VLPs. Post-vaccination tracking indicates that the induced serological titers fall from peak values but stabilize at levels well above those seen in natural infections and that they remain protective for many years. What is not yet known is (i) the length of time before titers fall below a threshold necessary to prevent new infections; (ii) whether subsequent natural exposure can elicit a rapid enough memory response to prevent establishing new infections; and (iii) whether booster shots would be needed periodically, at a schedule to be determined through careful monitoring of vaccinated populations.
The requirement for maintaining native conformation adds considerably to the difficulty of manufacture, storage, and delivery of vaccines. As the L1-based VLPs are largely HPV type-restricted and expensive to produce, additional approaches are being proposed. One is to use capsomeres assembled from five L1 monomers. The antibodies elicited are also type specific but the antigen can be produced rather inexpensively in bacteria (139–141). Another approach is to develop a cross-reactive vaccine. A region of L2 is relatively conserved among diverse papillomaviruses, and a peptide corresponding to that region indeed elicits broadly cross-reactive antibodies in preclinical studies (for a review, see 142). Both are being explored as possible next-generation prophylactic HPV vaccines.
Therapeutic vaccines
The concept is to treat patients with serious HPV infections such as RRP and other refractory benign lesions and also HPV-associated cancers with vaccines composed of HPV proteins that could stimulate a cell-mediated immunity against the cells expressing the viral proteins. Success in optimizing therapeutic vaccines for papillomavirus infections depends on an understanding of the immunology of these diseases and the reason(s) why patients with persistent HPV infections do not mount an effective immune response (see the article on the immunology of recurrent respiratory papillomatosis by Bonagura). One current approach uses DNA-based vaccines to express a detoxified E7 protein, which no longer can bind the pRB family of pocket proteins. This has been efficacious in preclinical studies. A few clinical trials are ongoing (reviewed by 143).
Part VIII. Therapeutic approaches to existing HPV infections
The most common practice is to removal the lesions using a variety of surgical procedures including cold knife, microdebrider, various types of laser, cryosurgery, and electrocautery, according to the anatomic site. A number of small molecules have also been used either alone or as adjunctive therapies, as described below.
Small molecule drugs to treat genital warts
Topically delivered potions have been used to treat papillomavirus infections from time immemorial, just as traditional medicines derived from natural products have been used against many other common ailments. Some treatments have been partially efficacious and are still in widespread use and acceptance today to treat anogenital disease. Notably among these are podophyllotoxin (isolated from the May apple) and a formulated version called podophyllin or Condylox, which have been used to suppress genital warts (144). One of the major problems is that podophyllotoxin is anti-mitotic and does not readily allow for the re-epithelialization of healthy skin to replace the erosion caused by its application. Imiquimod (Aldara), an immune response modifier, has been used to treat genital lesions with good results (145–147). Neither can be used to treat RRP because of scarring or pain. Systemic injection of interferon has been used successfully in some of the patients but relapse can occur after discontinuation of treatment (148). A single study reported that treating cervical dysplasia patients with celecoxib, a selective COX-2 inhibitor, prevented disease progression and increased regression (149).
Small molecule drugs to treat RRP
In attempts to slow down or to curb post-surgical regrowth of airway papillomas, various adjunctive pharmacotherapies and immunotherapies have been attempted. The persistent clinical dilemmas are that (i) none of the agents used to treat HPV infections, including those described below, is reliably effective in all patients and (ii) collateral damage to normal host epithelium can be considerable. Building on the wealth, breadth, and depth of knowledge gained in the past decades of intensive worldwide research on papillomaviruses, selective inhibitors of different pathways essential to viral replication and long-term persistence are being sought (reviewed by 150). As almost all laryngeal papillomas are caused by HPV-6 or HPV-11, clinical application of future type-specific inhibitors would be a possibility. However, in almost all other anatomic sites, HPV type-specific agents are clinically impractical because of the large number of possible genotypes responsible for the lesions.
Vitamin A (all-trans-retinoic acid) treatment prevents keratinocyte differentiation, which of course then blocks much of the HPV reproductive program. However, this also prevents full healing, leaving incompletely differentiated squamous epithelia subject to painful sores and possible microbial infections.
Indole-3-carbinol and its derivatives such as diindoylylmethane (DIM) have received well-deserved attention because of their ability to suppress laryngeal papillomas in some patients, possibly by diminishing the production of pro-estrogenic metabolites in favor of anti-estrogenic derivatives (151, 152). I3C is naturally conjugated to several possible sugars as a gluconate in many cruciferous vegetables. In the acidic environment of the stomach, I3C is a very reactive compound and, among other possibilities, can dimerize to DIM. The active agent can also be chemically prepared as DIM, minimizing unpredictable reactions after ingestion. The proposed mode of activity is that I3C and DIM have structural similarities to estrogen (β-estradiol). The natural elimination pathway for any hydrophobic steroid hormone involves hydroxylation to increase solubility. β-estradiol is subject to conversion either to 16-α-hydroxy estrone (a strong pro-estrogenic derivative) or 2-hydroxy estrone (an anti-estrogenic derivative). Speculation is that the cytochrome P450 hydroxylase induced by I3C and DIM to make these aromatic compounds more aqueous soluble happens collaterally to convert β-estradiol to the 2-hydroxyestone and ultimately to suppress mitogenic stimulation to papillomas. Many RRP patients benefit from I3C or DIM intake on a daily regimen and, while consistent use can prolong intervals between surgeries, only rarely is this sufficient for complete remission.
Interferon-α generally blocks viral reproduction and is an integral part of natural anti-viral defenses in mammals. High-dose systemic injections of IFN-α can suppress RRP regrowth post-operatively, but at the price of inducing fevers and other significant side effects. Long-term delivery is not well tolerated. Some patients do experience successful remission of their papillomas, but most have a rapid rebound after termination of IFN treatments.
HPMPC (Cidofovir/Vistide) and other acyclic nucleoside analogs have been used off-label and compassionately in RRP patients. The pro-drugs are delivered to the tissue by intralesional injection, and they require phosphorylation once inside the cells to become a tri-phosphate equivalent, the immediate substrate for DNA or RNA polymerases. It is not known which kinase is responsible for their conversion to the tri-phosphate equivalent. On a precautionary note, cidofovir is not a chain terminator after incorporation (in contrast to most other nucleotide analogs used to inhibit DNA or RNA polymerases). Rather, because it has a 3′ hydroxyl group, it can be incorporated within growing polynucleotides. Its ultimate mechanism of inhibition is that polymerases slow down when encountering acyclic nucleoside phosphonates, as the normal steric orientation of the sugar ring is absent. Incorporated HPMPC apparently causes RNA and DNA polymerases to pause or stop. Of particular concern is that the incorporated nucleotide analog can be mutagenic because of poor templating. The long-term consequences of cidofovir in people treated for RRP remain unknown, and considerable caution in its use should be exercised. Some patients respond well to cidofovir and their lesions are minimized over time, but many others have little or no benefit (153) and may even experience vocal cord ‘stiffness’, perhaps because healing is delayed or prevented.
Recently, several new agents have entered clinical trials (Cox II inhibitors), have been advanced by RRP patients (artemisinin), or are in use by some ENT surgeons (e.g. intralesional injections with the mumps vaccine or Avastin).
Cox II inhibitor (e.g. celecoxib/Celebrex) and other new agents
As discussed above, the enzyme COX-2 and its major product PGE2 are expressed in HPV-induced respiratory papillomas and promote the growth and survival of the cells. A pilot clinical trial of celecoxib therapy for RRP showed striking responses, with 3/3 patients free of disease (A. Abramson, G. Mullooly, B. Steinberg, unpublished data). A large multi-centered double-blinded placebo-controlled study of celecoxib therapy has been funded by the National Institutes of Health and is currently underway. Included in the design of the trial are a number of studies to address the mechanism of celecoxib efficacy in vivo.
Artemisinin, artesunate, and related compounds are antimalarial agents derived from wormwood. They are cytotoxic against HPV-immortalized cells or cervical carcinoma cells, but not against normal cervical cells (154). Delivered orally, they have shown anecdotal benefits against laryngeal papillomatosis. The mechanism of action may involve selective generation of free radicals and oxidative stress in active papillomas. Additional research and validation are essential.
Mumps vaccine (or the MMR vaccine) injection directly into papillomas can result in remarkable regression of recalcitrant papillomas in some patients (155). A presumed mechanism of action is local boosted immune response (because essentially all RRP patients received mumps or MMR vaccination as infants) with collateral suppression of HPV activity.
Bevacizumab (Avastin) is an anti-angiogenic agent that, when injected sublesionally following angiolytic KTP laser treatment, can prevent rapid regrowth of papillomas of the larynx and nearby upper aerodigestive tract sites (156).
In summary, adjuvant treatments of RRP have involved a great deal of trial and error. The described approaches benefit some but definitely not all patients. They remain rather non-specific and appear to be limited by idiosyncracies associated with individual patients for reasons not understood.
Proscriptions and contraindications – Pharmaceutical compounds such as acyclovir and gancyclovir have shown efficacy in treating infections caused by the herpesviruses because these large viruses encode a unique thymidine kinase which is capable of turning these analogs into substrates for nucleic acid synthesis. However, HPVs strictly rely on host enzymes to supply the substrates and hence these agents would not have any specificity against HPV lesions. Other treatments (5-fluorouracil) that have been tried clinically should be avoided as they are likely to block cellular DNA synthesis or introduce unwanted mutations in cellular genes. In no case should ionizing radiation be used to treat any benign papilloma lesion, as the resulting DNA damage and strand breaks could promote chromosomal DNA instability and viral DNA integration and accelerate neoplastic progression. Such unintended consequences did result from attempts to treat laryngeal papillomas with radiation in the early days of clinical radiology.
Conclusions
Successful implementation of control measures – prevention, diagnosis, and treatment – ultimately comes down to (i) effective communication through various media to the general public, to policy makers, and to funding sources, (ii) education of health-care providers, (iii) development of inexpensive but reliable products for prevention and clinical care, and (iv) a sustainable ‘business plan’ for long-term delivery. These objectives are especially challenging in underdeveloped nations and regions because of competing health care and economic priorities and also because socially acceptable approaches and procedures can differ widely among cultures. Future efforts to bring HPV-associated diseases under control must continue to remain highly creative and adaptable, with the appreciation that the papillomaviral diseases are among the most promising targets for research, development, and delivery of clinical and public health benefits. With unwavering motivation and commitment of resources, success can and will come in global control and management of the HPV-associated diseases.
Acknowledgments
We thank Hsu-Kun Wang with assistance in preparing the micrographs. Over the years, much of the research in the laboratory of Louise Chow and Tom Broker was supported by grants from the US Public Health Service/National Institutes of Health/National Cancer Institute: CA36200, CA83679, and CA107338. Bettie Steinberg has been supported by a number of grants from the National Institute for Deafness and Other Communication Disorders/National Institutes of Health: DC00203, DC007946, and DC008579; and grant AI38029 from the National Institute for Allergy and Infectious Diseases.




