Drinking water supplementation with ascorbate is not protective against UVR-B-induced cataract in the guinea pig
Abstract.
Purpose: To study if ascorbate supplementation decreases ultraviolet radiation (UVR)-induced cataract development in the guinea pig.
Methods: Sixty 6–9-week-old pigmented guinea pigs received drinking water supplemented with or without 5.5 mm l-ascorbate for 4 weeks. After supplementation, 40 animals were exposed unilaterally in vivo under anaesthesia to 80 kJ/m2 UVR-B. One day later, the animals were killed and lenses were extracted. Degree of cataract was quantified by measurement of intensity of forward lens light scattering. Lens ascorbate concentration was determined with high-performance liquid chromatography (HPLC) with UVR detection at 254 nm. Twenty animals were used as non-exposed control.
Results: Supplementation increased lens ascorbate concentration significantly. In UVR-exposed animals, mean 95% confidence intervals (CIs) for animal-averaged lens ascorbate concentration (μmol/g wet weight lens) were 0.54 ± 0.07 (no ascorbate) and 0.83 ± 0.05 (5.5 mm ascorbate). In non-exposed control animals, mean 95% CIs for animal-averaged lens ascorbate concentration (μmol/g wet weight lens) were 0.72 ± 0.12 (0 mm ascorbate) and 0.90 ± 0.15 (5.5 mm ascorbate). All non-exposed lenses were devoid of cataract. Superficial anterior cataract developed in all UVR-exposed lenses. The lens light scattering was 39.2 ± 14.1 milli transformed equivalent diazepam concentration (m(tEDC)) without and 35.9 ± 14.0 m(tEDC) with ascorbate supplementation.
Conclusion: Superficial anterior cataract develops in lenses exposed to UVR-B. Ascorbate supplementation is non-toxic to both UVR-B-exposed lenses and non-exposed control lenses. Ascorbate supplementation does not reduce in vivo lens forward light scattering secondary to UVR-B exposure in the guinea pig.
Introduction
Epidemiological information supports an association between exposure to ultraviolet radiation (UVR-B) from sunlight and the development of cortical cataract in humans (McCarty & Taylor 2002). In addition, sunlight has been correlated with the development of the retinal disease age-related maculopathy, primarily as a consequence of blue light exposure (Algvere et al. 2006). Sunlight is therefore a significant cause of eye diseases in humans.
Experimental studies on animals, including rats, mice and rabbits, link UVR-B exposure to the development of cortical cataract (Pitts 1977; Jose & Pitts 1985; Söderberg 1988, 1990; Hightower & McCready 1993; Wegener 1994; Michael et al. 1996). In addition, experimental studies on young rats link UVR-B exposure to the development of nuclear cataract (Dong et al. 2003; Lofgren et al. 2003).
For continuous dose–response functions – in this particular case an increase in lens light scattering after UVR exposure – the concept of maximum tolerable dose (MTD) was recently developed as an index of toxicity (Söderberg et al. 2002). We have previously reported the MTD for 300 nm UVR for the 6-week-old albino rat to be 3.65 kJ/m2 (Söderberg et al. 2002), for the 6-week-old pigmented rat to be 4.20 kJ/m2 (Kakar MK, et al. IOVS 2003;44: Arvo E-Abstract 296) and for the 6-week-old pigmented mouse to be 3.24 kJ/m2 (Meyer et al. 2005). Pitts (1977) determined the threshold for the development of permanent lenticular opacity in the pigmented rabbit to be 5.0 kJ/m2. We have found the pigmented guinea pig to be highly resistant to developing UVR-B cataract. UVR-B doses ranging from 5.0 to 20.0 kJ/m2 were not sufficient to induce cataract formation in the pigmented guinea pig (VC Mody, unpublished data). We have found that a dose of 42 kJ/m2 induces faint cataract (Mody Jr VC, et al. IOVS 2004;45: ARVO E-Abstract 385) and a dose of 80 kJ/m2 results in mild superficial anterior cataract (Mody Jr VC, et al. Acta Ophthalmol Scand 2006;84;S239: EVER Poster Abstract 227). We determined MTD for young adult pigmented guinea pigs to be 69.0 kJ/m2 (Mody Jr VC, et al. IOVS 2004;45: ARVO E-Abstract 385).
Ascorbate
Ascorbic acid, or ascorbate, has two ionizable –OH groups with pKa1 = 4.25 and pKa2 = 11.8. Ascorbate is the favoured form at physiological pH (Halliwell & Gutteridge 1999). Therefore, we use the name ascorbate throughout this article.
Ascorbate is an essential nutrient in both the human and the guinea pig, with the lens ascorbate concentration being much higher in diurnal than in nocturnal animals (Long 1961a; Varma 1991).
Ascorbate is of particular interest because of its role as an antioxidant in a number of tissues, including the lens (Halliwell & Gutteridge 1999; Wu et al. 2004) and lens DNA damage (Reddy et al. 1998). A drawback with studies on scorbutic animals is that the animals lose weight and become ill (Malik et al. 1995; Wu et al. 2004). Two other studies show that short-term or prolonged ascorbic acid deficiency does not affect the antioxidant status of the lens in the guinea pig (Ohta et al. 2001, 2004). Although ascorbic acid deficiency does not affect lens antioxidant status, we use ascorbate supplementation in this study. We have shown that drinking water supplementation increases lens ascorbate concentration in a previous study (Mody et al. 2005a). The rationale for using guinea pigs in this study is that lens ascorbate concentration may be easily modulated by supplementation in this unique animal model.
The guinea pig is an appropriate model for studying ascorbate effects in cataractogenesis, because the ascorbate level in the aqueous humor and lens may be easily modulated by either dietary restriction or supplementation. The lens ascorbate concentration in the guinea pig is high compared to nocturnal animals (Long 1961b), and ascorbate is required in the diet of the guinea pig (Committee on Animal Nutrition NRC 1987). Both findings are consistent with that reported in the human. We have found that it is possible to increase lens ascorbate concentration levels in the guinea pig by 40% through drinking water supplementation (Mody et al. 2005a).
The significance of the guinea pig being a diurnal animal is that the human is also diurnal. The guinea pig is much more resistant to the development of UVR-induced cataract compared to the rat and mouse, which are nocturnal animals. Like the human, the guinea pig is adapted to daily solar UVR exposure outdoors, and therefore more closely correlates to the human model. We are studying the guinea pig model secondary to an acute dose of UVR-B, which correlates directly to humans for instance welders. From the data of the acute animal model, a chronic animal model may be studied later in order to correspond with long-term human cataract development.
In vitro (Reddy & Bhat 1999; Hegde & Varma 2004; Sasaki H, et al. IOVS 2000;41: ARVO Abstract 1076) studies in the rat and mouse show that ascorbate is protective against cataract formation and damage to lens constituents (including essential proteins and DNA) from various oxidative stresses, including UVR.
Evidence suggests that oral intake of ascorbate protects against cataract formation in the human lens (Leske et al. 1991; Robertson et al. 1991; Jacques et al. 1997; Taylor et al. 2002a). Furthermore, lenses with increasing degrees of cataract and browning secondary to protein oxidation were associated with lower ascorbate content (Tessier et al. 1998).
In diurnal animals, including the guinea pig, the concentration of ascorbate in the aqueous humor is higher than in plasma and further higher in the ocular lens than in the aqueous humour because of a concentration gradient maintained by active transport (Garland 1991). Our data supports the mechanism of active transport, because the increase is saturable (Mody et al. 2005a). Recently, the sodium-dependent ascorbate transporter (SVCT 2) has been identified in the human lens epithelium cell line (HLE-B3) (Kannan et al. 2001).
The purpose of the present investigation was to determine if drinking water supplementation with ascorbic acid reduces in vivo UVR-B-induced lens light scattering in the guinea pig.
Materials and Methods
Animals
Sixty 6–9-week-old pigmented guinea pigs were used in this study. Throughout the experiment, every animal was fed standard chow containing 0.125 mol l-ascorbate/kg chow in order to maintain the health of the animals. This was also a requirement for the ethical approval obtained from the Northern Stockholm Animal Experiments Ethics Committee. The experiments adhered to the Association for Research in Vision and Ophthalmology (ARVO) Statement on the Use of Animals in Ophthalmic and Vision Research.
Ascorbate supplementation
For a period of 4 weeks prior to experimental exposure to UVR, the animals received either regular drinking water without ascorbate supplementation or drinking water supplemented with 5.5 mm l-ascorbate.
All drinking water flasks, both supplemented and not supplemented, were wrapped in black plastic to minimize oxidation of the supplement ascorbate in the flask. Further, the content of the flasks was changed every 12 hr. Fresh ascorbate powder was added to the flask at each change. The interval for regular change of the contents of the drinking water flasks was based on the observation from a previous study that a significant percentage (70%) of ascorbate remains in the reduced state for up to 12 hr after preparation (Mody et al. 2005a).
Exposure to ultraviolet radiation
The radiation from a high-pressure mercury lamp was collimated, passed through a water filter and a double monochromator, and finally projected on the cornea of the exposed eye (Michael et al. 1996). The spectrum of radiation is shown in Fig. 1.
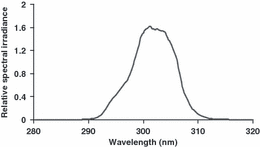
Spectrum of UVR lamp source.
Ten minutes preceding the exposure, the animal was anaesthetized with a mixture of 40 mg/kg ketamine and 5 mg/kg xylazine injected intraperitoneally. Five min after the injection, 1% tropicamide was instilled in both eyes. Each animal was exposed unilaterally to 80 kJ/m2 UVR-B for 1 hr. Ascorbate supplementation in the drinking water continued for 1 day after exposure to UVR-B.
Macroscopic imaging and measurement of intensity of forward light scattering
At 24 hr after exposure to UVR-B, the animal was killed with pentobarbital overdose. The 1 day post-ictal interval was chosen because a maximum intensity of forward light scattering develops at 1 day after UVR-B exposure in the guinea pig and remains constant until 8 days after exposure (Mody Jr VC, et al. Acta Ophthalmol Scand 2006;84;S239: EVER Poster Abstract 227). Thereafter, both eyes were enucleated; the lenses were extracted and placed in Ringer’s acetate and remnants of ciliary body were removed. Finally, the lens was transferred to a custom-built cuvette for light-scattering measurements.
The intensity of forward light scattering was measured with a Light Dissemination Meter (Söderberg et al. 1990). The original meter apparatus was adapted to the larger size of guinea pig lenses. We used a cuvette with a larger (15 mm) perforating hole and deeper (10 mm) well, and a photodetector equipped with a larger (3.9 × 3.9 mm) photodiode (S1226-44BK; Hamamatsu Photonics KK, Japan).
Then, each lens was photographed in darkfield illumination. Finally, the lens was frozen to −80°C and saved for later ascorbate determination.
Ascorbate measurements
Preparation of drinking-water samples
Forty-five water samples from the 5.5 mm ascorbate group were randomly tested throughout the experiment, both immediately after preparation and 12 hr after preparation. The sample size was based on our previous experience on variability in water solution of ascorbate (Mody et al. 2005a). The water samples were diluted 1:1 in 2.5% metaphosphoric acid and stored at −80°C. After thawing, the samples were diluted further 1:100 in 2.5% metaphosphoric acid. One aliquot per sample was measured using high-performance liquid chromatography (HPLC) with UVR detection.
Preparation of lens samples
The procedure for measuring lens ascorbate is described previously (Mody et al. 2005b). In short, each lens was homogenized, centrifuged and the ultrafiltered supernatant was subjected to HPLC with UVR detection.
At the end of the supplementation period, the guinea pigs were killed with pentobarbital overdose. Each lens was extracted, photographed, wet weight measured, homogenized and centrifuged in 1.0 ml of 0.25% metaphosphoric acid (Hallström et al. 1989).
When processing the lens samples for ascorbate measurement, it is important to add an agent to the sample to prevent metal-catalysed oxidation of ascorbate. The lens samples in the study were prepared in metaphosphoric acid. Besides protein denaturation, metaphosphoric acid prevents oxidation of ascorbate to dehydroascorbate because of high acidity and metal ion chelation (Washko et al. 1992; Koshiishi et al. 1998).
The supernatant was ultrafiltered and the ultrafiltrate injected into an HPLC column.
Measurement of lens ascorbate concentration
The selection of our method for ascorbate measurement from the other methods published (Omaye et al. 1979) was based on the fact that HPLC allows efficient separation of small molecules with higher sensitivity and better specificity than other methods (Hallström et al. 1989). The current HPLC method has the advantage over previously described methods of detecting even small concentrations of ascorbate in biological tissues (less than 0.06 μm) in the injected sample.
The column was an ion-exchange, reversed-phase column. This column was chosen based on previous experience in other studies (Hallström et al. 1989). The mobile phase used in the HPLC system was 2 mm sulphuric acid, pH 2.4. This mobile phase was also selected based on experience from previous studies (Hallström et al. 1989) to avoid oxidation of ascorbate during the passage through the HPLC column.
Ascorbate was detected as UVR absorption at 254 nm using an UVR absorbance detector at the expected elution time, checking for the pattern of surrounding peaks in the chromatogram. UVR provides specific detection with high sensitivity for ascorbate in biological tissues (Johnsen et al. 1985; Hallström et al. 1989).
We chose 254 nm to maximally accept ascorbate signal and reject dehydroascorbate signal, respectively (Fig. 2).
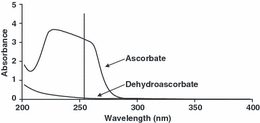
Ultraviolet radiation spectra for 1 mm ascorbate and 1 mm dehydroascorbate in 2.5% metaphosphoric acid. The vertical line denotes absorbance for the two spectra at 254 nm.
The absorbance ratio for the oxidized form of ascorbate, dehydroascorbate and ascorbate was found to be 4.1% at 254 nm (Fig. 2). This implies that 96% of the signal at 254 nm is ascorbate.
Figure 3 shows a typical chromatogram for ultrafiltered whole lens homogenate. Retention time for ascorbate was 5.7 min under the HPLC conditions used in the experiment.
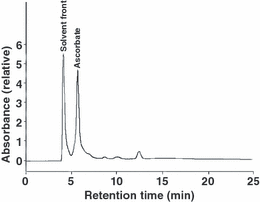
Chromatogram for ultrafiltered whole lens homogenate. Ultraviolet radiation detection at 254 nm.
The peak shape and symmetry allowed for resolution of the ascorbate peak. In order to analyse sequential samples, we used chromatography software that was programmed to identify the ascorbate peak based on retention time and shape.
The ascorbate concentration was calculated based on calibration against an external 10 μm l-ascorbate standard prepared from a commercially available ascorbate standard solution (Merck, Darmstadt, Germany). The low concentration standard was prepared by weighing a standard amount of commercially available ascorbate standard solution and adding water. The added amount of water was also weighed. The final concentration of ascorbate was calculated from the added masses of ascorbate standard solution and water, respectively.
There is always a risk that external calibration causes an error in both sensitivity and level of calibration. We estimated this error by comparing external and internal calibration on a pooled ultrafiltrate of rat lenses.
External standard with ascorbate solutions ranging from 0 to 20 μm were prepared and measured on HPLC. Absorbance increased linearly as a function of concentration (r2 > 0.99; data not shown). Internal standard addition samples were created by adding known amounts of ascorbate ranging from 0 to 20 μM to samples of a portion of pooled, processed, whole rat lens ultrafiltrate solution. The samples were measured with HPLC. As for the external standard, the increase of absorbance as a function of concentration of standard added was linear (r2 > 0.98; data not shown). The ascorbate concentration in the rat lens ultrafiltrate was estimated at 1.87 μm with the internal standard addition technique and 1.95 μm with the external calibration technique. Considering the insignificant difference between the two methods, it was decided to only use the external calibration for future experiments.
The quantitative recovery of ascorbate in measurements was also determined. The supernatant was obtained after ultracentrifugation of grinded lens in metaphosphoric acid. The pellet was re-extracted after ultracentrifugation. The ascorbate concentration in the supernatant after re-extracting the pellet was 46 % of that in the supernatant of the lens homogenate (data not shown). This finding indicates that ascorbate in the pellet is released into solution upon re-extraction.
Experimental design
Sixty animals were randomly divided into one group of 40 animals (used for the principal experiment) and another group of 20 animals (used for verification of ascorbate content).
Principal experiment
Twenty animals received drinking water with 5.5 mm ascorbate supplementation and another 20 animals received drinking water without ascorbate supplementation for a period of 4 weeks. Each animal was exposed to UVR-B on one side while the contralateral eye was left unexposed. Each lens was measured for intensity of forward light scattering three times. The wet weight of each lens was determined three times. Furthermore, ascorbate was measured once in each lens.
The sample size was based on our previous experience on variability of light scattering in pigmented guinea pig lenses (Mody Jr VC, et al. Acta Ophthalmol Scand 2006;84;S239: EVER Poster Abstract 227).
Verification of lens ascorbate
Ten animals received drinking water with 5.5 mm ascorbate supplementation and another group of 10 animals received drinking water without ascorbate supplementation for a period of 4 weeks. The sample size was determined by our previous experience on measurements of lenticular ascorbate (Mody et al. 2005a).
Statistical parameters
The significance limit and the confidence coefficient were set to 0.05 and 0.95, respectively. A two-way analysis of variance (anova) was used to detect the impact of main and interaction factors with lens light scattering as a dependent variable. Lens ascorbate data was analysed with two-way anova. In order to compare parameters, the equality of variances for the different groups was analysed with an F-test. If heterogeneity of variances was indicated, approximate t-tests were planned, in contrast to t-tests for independent groups in case of homogeneity of variance.
Results
Some animals were lost in the experiment because of the presence of congenital cataract. Final sample size was 35 in the principal UVR-B experiment and 16 in the verification experiment.
Drinking water ascorbate content
There was a 42% decrease in ascorbate content in the drinking water flasks during the 12 hr interval between refilling. The 95% confidence interval (CI) for the initial ascorbate concentration value before 12 hr was 8.29 ± 0.73 mm (n = 45); the final value after 12 hr was 5.96 ± 0.77 mm (n = 45). The 95% CI for the mean difference of ascorbate content between newly prepared flask and flask before renewal of content was 2.33 ± 0.24 mm (n = 45).
Lens ascorbate content
In both UVR-exposed animals and non-exposed animals, the animal-averaged lens ascorbate concentration was significantly higher in the 5.5 mm supplemented group than in the non-supplemented group (anova; factor ascorbate P < 1 × 10−6; interaction factor ascorbate versus UVR animal P = 0.24; Fig. 4).
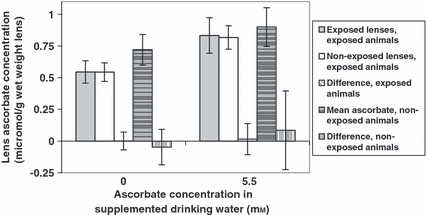
Lens ascorbate in ascorbate-supplemented guinea pigs after UVR-B exposure. Error bars represent 95% confidence intervals. There are five bars for the two supplementation groups, from left to right: mean lens ascorbate concentration from UVR-B-exposed lenses from UVR-B-exposed animals; mean lens ascorbate concentration from non-UVR-B-exposed lenses from UVR-B-exposed animals; mean paired difference in lens ascorbate concentration between UVR-B-exposed and non-UVR-B-exposed lenses from UVR-B-exposed animals; animal-averaged lens ascorbate concentration in non-UVR-B-exposed animals; mean-paired difference in lens ascorbate concentration in non-UVR-B-exposed animals.
Animal-averaged lens ascorbate was significantly lower in UVR-exposed animals than in non-exposed animals, in either of the 0 mm or the 5.5 mm supplementation groups (anova; factor UVR animal P = 0.006; interaction factor ascorbate versus UVR animal P = 0.24; Fig. 4).
Lens ascorbate in UVR-exposed lenses did not differ significantly from that in non-exposed lenses, in either of the supplementation groups (anova; factor UVR lens P = 0.73, interaction factor ascorbate versus UVR lens P = 0.38; Fig. 4).
Cataract development
Superficial anterior cataract developed in UVR-B-exposed lenses from both the 0 mm and 5.5 mm drinking water supplementation groups (Fig. 5B,D). No cataract developed in non-exposed animals from either group (Fig. 5A,C). In the non-exposed animal group that was supplemented with ascorbate in the drinking water, no cataract developed in either the 0 mm or 5.5 mm group (Fig. 5E,F).
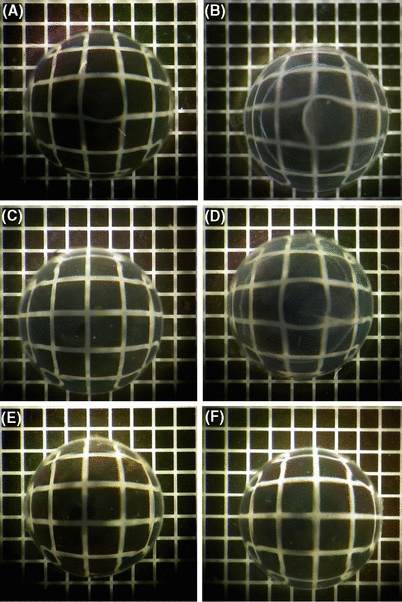
Lenses from guinea pigs exposed to UV-B radiation after drinking-water supplementation with 0 mm and 5.5 mm ascorbate. (A) Non-exposed lenses, 0 mm. (B) Exposed lenses, 0 mm. (C) Non-exposed lenses, 5.5 mm. (D) Exposed lenses, 5.5 mm. Lenses from non-exposed guinea pigs supplemented with ascorbate in the drinking water: (E) 0 mm; (F) 5.5 mm.
Lens light scattering
In both supplemented and non-supplemented animals, the lens light scattering was significantly higher in UVR-exposed lenses than in contralateral non-exposed lenses (anova; factor UVR P < 10−6; interaction factor ascorbate versus UVR P = 0.74; Fig. 6).
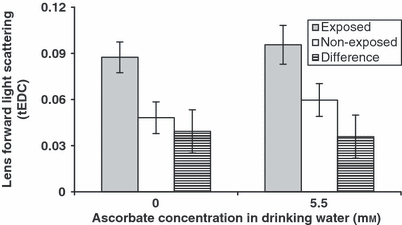
Lens light scattering after UVR-B exposure in ascorbate-supplemented guinea pigs. Error bars represent 95% confidence intervals. There are three bars for the two supplementation groups, from left to right: mean lens forward light scattering for UVR-B-exposed lenses from UVR-B-exposed animals; mean lens forward light scattering for non-UVR-B-exposed lenses from UVR-B-exposed animals; mean paired difference in lens forward light scattering for UVR-B-exposed lenses minus non-UVR-B-exposed lenses from UVR-B-exposed animals.
There was no significant effect of ascorbate supplementation on lens light scattering in either of the UVR-exposed or non-exposed groups (anova; factor UVR P = 0.06; interaction factor ascorbate versus UVR P = 0.74; Fig. 6).
Lens wet weights
In both UVR-exposed and non-exposed animals, there was no difference in animal-averaged lens wet weight between the two supplementation groups (t-test; P = 0.61 for UVR-B-exposed animals and 0.94 for non-exposed animals; Fig. 7).
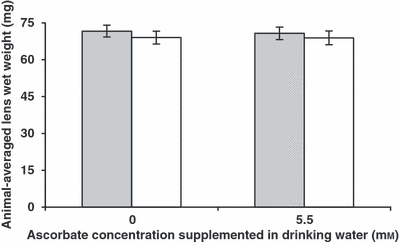
Animal-averaged lens wet weight in ascorbate-supplemented guinea pigs after UVR-B exposure. Grey, UVR-B-exposed animals; white, non-exposed animals. Error bars represent 95% confidence intervals.
In both supplementation groups, there was no difference in wet weight between the UVR-B-exposed lens and the contralateral non-exposed lens in each animal. The 95% CI for the paired-sample mean difference in the 0 mm group was –0.11 ± 1.06 mg, and 0.07 ± 0.72 in the 5.5 mm supplementation group.
Discussion
This investigation was undertaken to determine if ascorbate added as a supplement in drinking water can protect against in vivo UVR-B-induced light scattering. Although there was 9% less lens light scattering in the UVR-B-exposed 5.5 mm supplementation group than in the 0 mm supplementation group, the difference was not statistically significant (Fig. 6).
Glutathione is the major antioxidant in the lens (Bernat & Bombicki 1968; Giblin 2000). Combinative interactions of the water-soluble ascorbate and the lipid-soluble vitamin E play a role in the antioxidant defence of the lens cell and help reduce glutathione (Shang et al. 2003; Kutlu et al. 2005). Ayala & Söderberg (2005) have shown that α-tocopherol (vitamin E) fed in the diet significantly reduces lens light scattering in the UVR-B-exposed albino rat. Instilled vitamin E liposomes have been shown by Ohta et al. (2000) to retard cataract induced by galactose feeding in rats (Ohta et al. 2000). Oxidative damage to DNA, protein and lipid are factors involved in the development of cataracts. Being lipid-soluble, vitamin E exerts its effect on the lipids of cell membranes, including the nucleus and organelles. Ascorbate directly affects DNA – including charged DNA – and protein, and through interaction with vitamin E and coenzyme Q it plays a role in the protection of biomembranes (Beyer 1994). Vitamin E requires ascorbate in order to function, and the effect between the two vitamins may be greater than additive (Beyer 1994).
The specific interaction of ascorbate with vitamin E is that ascorbate reduces vitamin E utilizing glutathione. The interaction of ascorbate with vitamin E may be required for a protective effect.
Lens transparency depends on the regular structure of its cells and proteins. Oxidation in the lens results in enzyme inhibition and protein aggregation, which is manifest macroscopically as opacity or cataract formation. The most significant UVR filters in the lens are tryptophan-based proteins, for which ascorbate is not able to protect (Taylor et al. 2002b). Oxidative stress may also induce differential gene expression in lens epithelial cells, changing the protein composition and therefore the organization of the lens cell, resulting in cataract (Carper et al. 1999; Spector et al. 2002; Goswami et al. 2003). The additive effect of ascorbate and vitamin E may explain why there was no significant reduction in in vivo forward light scattering induced by UVR-B in the guinea pig in this study.
Biochemically, there are at least three possible mechanisms for the loss of lens ascorbate secondary to UVR exposure. The loss of ascorbate secondary to UVR may explain its ineffectiveness. Firstly, the consumable ascorbate may be directly oxidized by UVR-B (Reddy 1996). Alternatively, ascorbate may be consumed while serving its function as an antioxidant in the lens. Finally, consumable ascorbate may be lost by leakage from lens cells damaged by UVR.
Ascorbate was determined in the lens, but not in the aqueous humor or plasma, in this study. It was difficult to obtain an adequate amount of aqueous humor from guinea pigs for ascorbate measurement, and plasma samples are too unclean for ascorbate measurement using our technique of ultrafiltration with UVR detection.
UVR-B-exposed animals contained 19% lower animal-averaged lens ascorbate concentration than non-exposed animals (Fig. 4). This finding is consistent with a previous rat study where UVR-B exposure consumed lens ascorbate (Mody et al. 2006). The finding that UVR-B exposure consumed lens ascorbate is consistent with that reported in a study of UVB irradiation on the metabolic profile of aqueous humor in rabbits analysed by 1H NMR spectroscopy (Midelfart 2005). In contrast, there was no difference in ascorbate concentration in UVR-exposed lenses compared to non-exposed lenses from the same UVR-exposed animals (Fig. 4). This is of interest because it indicates a signalling effect to both lenses, despite the strictly unilateral UVR exposure. The reason for the bilateral effect is unknown, although the bilaterality may be the result of ascorbate requirement and high ocular concentration in the guinea pig in contrast to the rat and most other species.
Superficial anterior cataract developed in all lenses exposed to a supra-threshold UVR-B dose of 80 kJ/m2 1 day after UVR-B exposure. The type of cortical cataract that develops after 80 kJ/m2 UVR-B dose is consistent with that found in a study by Wu et al. (2004) in which guinea pigs were exposed to 82 kJ/m2 (Wu et al. 2004). The finding is also consistent with a previous guinea pig UVR-B study from our group (Mody Jr VC, et al. Acta Ophthalmol Scand 2006;84;S239: EVER Poster Abstract 227). The previous studies show that the induction of cortical cataract in the pigmented guinea pig requires a much higher UVR dose and is of less severity than that of the pigmented rat (Kakar MK, et al. IOVS 2003;44: Arvo E-Abstract 296). We chose a dose of 80 kJ/m2 because, although it is a high dose in other species, the dose is not high in the guinea pig and a lower dose does not induce reliable cataract development in the guinea pig. The mechanism for this extraordinary tolerance to UVR in the guinea pig is unknown. A concentration as high as 10% of the unique ζ-crystallin has been reported in the guinea pig in contrast to the rat (Rao et al. 1997). ζ-crystallin, through its function as a NADPH:quinone oxidoreductase, may significantly increase the concentrations of pyridine nucleotides in the lens, therefore protecting the lens from oxidative stress (Rao & Zigler 1992; Rao et al. 1997).
Non-exposed lenses do not develop cataract in either exposed or non-exposed animals. This finding is important because it demonstrates that cataract does not develop from anaesthesia and that ascorbate supplementation in drinking water is non-toxic to the guinea pig lens. In addition, in our data the ascorbate-supplemented group developed a little less cataract, consistent with the hypothesis that there is no photosensitizing effect of ascorbate supplementation in the lens. To the best of our knowledge, there are no other comparable prior studies in the guinea pig.
The cataract that develops in pigmented rodents exposed to close-to-threshold UVR is usually superficial anterior, with little or no equatorial or nuclear involvement. The similar lens wet weights in this study show that any osmotic changes in the anterior region are too small to be detected by whole-lens measurements.
Significant lens light scattering developed in guinea pigs exposed to the supra-threshold UVR-B dose of 80 kJ/m2; this occurred in guinea pigs supplemented with both 0 mm and 5.5 mm ascorbate in the drinking water. Although ascorbate serves as an antioxidant in the lens, simple modulation of one factor in the lens does not result in significant protection. We know that asorbate supplementation changes the antioxidant status in the lens by increasing lens ascorbate. Therefore, this finding does not contribute to the negative result in this study.
Conclusions
Drinking-water supplementation with ascorbate for a period of 4 weeks increases lens ascorbate concentration significantly in both non-UVR-B-exposed guinea pigs and UVR-B-exposed guinea pigs. Lens ascorbate concentration is greater in non-UVR-B-exposed animals than in UVR-B-exposed animals, while there is no significant difference in lens ascorbate concentration between non-UVR-B-exposed and UVR-B-exposed lenses from the same exposed animal. Superficial anterior cataract develops in lenses exposed to UVR-B both in animals given drinking water that is supplemented with ascorbate and those whose drinking water is non-supplemented. Ascorbate supplementation does not protect against UVR cataract development in the pigmented guinea pig.
Acknowledgements
The authors are grateful to Ms Monica Aronsson for her dedicated and hard work in maintaining the animals. Parts of the study have been presented at the Association for Research in Vision and Ophthalmology Conference in Fort Lauderdale, Florida, USA in 2005 and 2006. The study was supported by the Swedish Society for Medical Research, the Swedish Radiation Protection Institute, the Karolinska Institutet Research Foundation, the Crown Princess Margareta Foundation, the Gun and Bertil Stohnes Foundation, the Karin Sandqvist Foundation, the St Erik’s Eye Research Foundation, Swedish Research Council Project no. K2004-74KX-15035-01A and Swedish Council for Working Life and Social Research Project no. 2002-0598.