SUPPORT FOR THE IMMUNOCOMPETENCE HANDICAP HYPOTHESIS IN THE WILD: HORMONAL MANIPULATION DECREASES SURVIVAL IN SICK DAMSELFLIES
Abstract
The immunocompetence handicap hypothesis (ICHH) states that hormones enhance sexual trait expression but impair immunity. Previous tests of the ICHH have been hampered by experimental design problems. Here, we report on an experimental test of the ICHH that includes manipulations of both hormones and infections in males of the territorial damselfly, Hetaerina americana, with accurate survival measurements. We conducted a fully factorial experiment subjecting each individual to one of three topical treatments: methoprene (a juvenile hormone analog), acetone, or control, and one of three injection treatments: bacteria, PBS, or control. We measured survival of manipulated males in both the wild and in captivity. As predicted, survival was most heavily impaired in methoprene-bacteria males than in the other groups in the wild, and no survival differences emerged in captive animals. This result confirms that survival is one cost an animal pays for increased hormonal levels. This corroborates theoretical predictions of the ICHH.
Fitness depends, to a large extent, on how an individual optimizes resource allocation to reproduction and survival (Stearns 1992). To this end, animals have evolved physiological means to shift resource allocation to these functions as environmental conditions change (Stearns 1992; Flatt et al. 2005). Hormones are key mediators of communication between environmental cues and the organism's internal state and therefore natural subjects for investigations of plastic responses to exogenous cues (Flatt et al. 2005; Hau 2007). The immunocompetence handicap hypothesis (ICHH) indicates that hormones are responsible for resource allocation between sexual and immune functions (Folstad and Karter 1992). According to the ICHH, sexual expression and immune function will be traded-off, that is, animals whose hormonal levels promote sexual trait expression will become immune-depressed.
A number of studies have tested the ICHH in both vertebrates and invertebrates (reviewed by Jacobs and Zuk 2010). The usual approach is to experimentally manipulate hormonal levels (via castration, direct hormone implantation, or using endocrine antagonists that suppress hormonal action), and then assess changes in immune function via measurements of immune component levels (e.g., Belliure et al. 2004; Ros et al. 2006; Ashley et al. 2009) and/or intensity or prevalence of pathogens (e.g., Uller and Olsson 2003; Deviche and Parris 2006; González-Tokman et al. in press). Support for the ICHH has been mixed: increased hormonal levels have not always led to a weakening of the immune system (Roberts et al. 2004; Hasselquist 2007). One major drawback of many studies is that although hormonal level is experimentally manipulated, infection usually is not manipulated. There may be additional noncontrolled factors (e.g., risk of exposure to infection) that may arise as confounding factors after hormonal levels have been manipulated. One example of a confounding factor is that encounters with pathogens depend on a number of factors that are not necessarily driven by testosterone levels. If the assumption that hormonally increased and control animals are encountering pathogens with the same probability is violated, then differences between experimental groups may arise for reasons other than the factor of interest (e.g., due to contact among cospecifics or aerial transmission; reviewed by Rudolf and Antonovics 2005). Therefore, it is important to experimentally manipulate both hormonal levels and immune challenges. In the only two studies that have achieved this, both have provided support for the ICHH (Lindstrom et al. 2001; Mougeot et al. 2006). A second shortcoming of many prior studies that investigate the ICHH is that survival has only been rarely studied; instead proxies for survival have been used (reviewed by Kotiaho 2001). A key prediction of the ICHH is that increased hormonal levels lead to reduced survival (Kotiaho 2001). Related to this, survival in the wild has to be assessed using modern techniques that can distinguish between survival and recapture rates. Such techniques are essential especially for groups that are likely to have biases in longevity, which may be the case for experimental individuals whose longevity is usually underestimated (Lebreton et al. 1992; Williams et al. 2002). In this article, we have solved the above issues using a territorial calopterygid damselfly as a study subject.
Previous findings in males of territorial calopterygid damselflies have provided evidence that increased levels of juvenile hormone (JH) lead to an increase in territorial aggression and wing pigmentation, two key sexual traits used during mate acquisition, with a reduction in immunocompetence (Contreras-Garduño et al. 2009, 2011). However, such hormonal manipulation did not show convincingly that survival was impaired (Contreras-Garduño et al. 2009). Here, we manipulate both hormonal levels (with methoprene acid, an analog of JH, JHa) and infection in the American rubyspot (Hetaerina americana Fabricius) and assess survival, predicting it to decrease in methoprene acid treated and infected animals. Because our second prediction is that decreased survival is explained by an increase in territorial behavior, we assess this indirectly by manipulating hormonal level and infection using animals whose territorial behavior was prevented. Our working hypothesis is that elevated hormonal levels will negatively affect survival.
Materials and methods
The study was carried out in Tetlama River, Morelos, Mexico (18o45′ 55″N, 99o14′ 45″W) in November–December 2010. Our study site (500-m long) was a sunny area delimited by large, shaded parts, (damselfly density and activity are extremely low in shady areas; all González-Tokman et al., pers. obs.). Animals were captured with a butterfly net. Captures and surveys were carried out in the sunny portion of the river. To avoid potentially confounding effects of the age on the measured variables, we only used young mature males that can be distinguished from younger or older males because of the texture and appearance of their wings and thorax (age class 3 according to Plaistow and Siva-Jothy 1996). At this stage, males show the highest immunity and territorial activity (Contreras-Garduño et al. 2008). Using an indelible marker, we marked each male by writing a three-digit number on the clear part (i.e., not the wing spot) of his left anterior wing. Also, we measured the left anterior wing with a digital caliper (± 0.01 mm) as an approximation of body size. After experimental manipulation (see below), animals were released where they were originally collected.
TREATMENTS
Each male received a topical application and an injection. Topical applications consisted of one of three treatments: hormonal increase (experimental), sham, or control. The hormonal-increase treatment consisted of methoprene acid, a JHa, which is known to modify the behavior and physiology of odonates and their parasites at the same dose used in the present study (Contreras-Garduño et al. 2009, 2011; González-Tokman et al. in press). From a dilution of 5 mg of methoprene acid mL−1 distilled water, we took 1 μl and diluted it 1:1000 in acetone. Using a micropipette, we took a 3 μl drop (15 ng methoprene acid) of the methoprene + acetone treatment (Met) and applied it topically on the dorsal part of the head. We used 3 μl of acetone (Ac) as sham treatment. Met and Ac treatments penetrate rapidly the cuticle near the corpora allata (Contreras-Garduño et al. 2009), the organ where JH is naturally synthesized (Flatt et al. 2005). Finally, individuals were handled but not given a topical application for the control group. All individuals received an injection immediately following the topical application. Injections consisted of one of three treatments: infection, sham, or control. Individuals in the infection group were infected with the gram-negative bacterium Serratia marcescens. This bacterium is common and highly lethal in wild American rubyspot populations in Central Mexico (González-Tokman et al. 2011). We resuspended bacteria from a laboratory culture (Instituto Nacional de Salud Pública, Cuernavaca, México) in phosphate buffer saline (PBS 1×, pH = 7) in a concentration of 700 colony formation units (CFU) μl−1. We injected 1 μl of the mixture of bacteria + PBS (Bac) in the dorsal thorax at the location where wings are inserted and the exoskeleton is not rigid. We injected 1 μl of PBS as sham treatment. Finally, individuals were handled but not injected for the control group. Topical treatments never made direct contact with the injury caused by the injection.
SURVIVAL IN THE FIELD AFTER METHOPRENE AND BACTERIAL TREATMENTS
We determined whether Met had different effects on the survival of infected (Bac) and healthy (PBS, Control) animals under natural conditions. To estimate survival in the field, we used MARK 6.1 software (White and Burnham 1999). We used a capture–recapture approach (for similar approaches see Munguía-Steyer et al. 2010; Buzatto et al. 2011) that allows dissociation of survival (ϕ) and recapture (p) probabilities by calculating maximum likelihood estimates from encounter histories of regular surveys (Lebreton et al. 1992; Williams et al. 2002). These methods have rarely been applied to experimental data despite the fact that they allow comparing survival of individuals of different treatments. In our study case, where treatments could modify not only survival but also recapture probabilities (i.e., due to dispersal), capture–recapture approach is a correct method for estimating survival accurately. We tested 61 different models (Table S1) that included the different combinations of predictors of survival and recapture: Top treatment, Inj treatment and time, plus additive and interactive models (Table S1).
From November 18–25, we collected 476 males and assigned them to any of the nine different combinations of one topical (Top) treatment (Met, Ac, Control) and one injected (Inj) treatment (Bac, PBS, Control). Sample sizes were as follows: Met–Bac = 54, Met–PBS = 52, Met–Control = 53, Ac–Bac = 52, Ac–PBS = 53, Ac–Control = 53, Control–Bac = 52, Control–PBS = 53, and Control–Control = 54. We marked each individual and released it to the river after no longer than 2 min of manipulation. After manipulation and marking, we recorded the presence of adult marked males during 21 consecutive days, from November 24 to December 15. We used this 21-day period given that residual longevity of males we used was about 15 days (González-Tokman et al., unpubl. data). Detection of individuals was based on surveys done by three observers, from 1100 to 1400 h, the time at which animals are more active (Contreras-Garduño et al. 2008).
SURVIVAL IN CAPTIVITY AFTER METHOPRENE AND BACTERIAL TREATMENTS
In November 18, additional 156 males were captured and manipulated with the same treatments as above (Top + Inj). After manipulation, males were kept in captivity in 5-mL assay tubes with a perch and a cap of humid cotton for keeping a temperature of about 26°C. During the experiment males were not fed. The experiment ended when the last male died. Males were monitored every 4 h to record the time to death. Sample sizes were as follows: Met–Bac = 16, Met–PBS = 15, Met–Control = 17, Ac–Bac = 17, Ac–PBS = 18, Ac–Control = 17, Control–Bac = 15, Control–PBS = 14, and Control–Control = 27. Although starvation can have different effects in infected and noninfected insects (González-Tokman et al. 2011), and in animals that differ in parasite resistance (Valtonen et al. 2010), it helps to homogenize individual resource availability and to avoid confounding effects of adopting different feeding strategies when infected (e.g., Adamo et al. 2010; González-Tokman et al. 2011).
STATISTICS
For analyzing survival under natural conditions, we employed Comarck–Jolly–Seber (CJS) capture–recapture models that estimate survival and recapture parameters based on encounter histories (Lebreton et al. 1992). The global model included time, Top treatment, Inj treatment, and the interaction between both treatments: ϕ(Top × Inj + t) P(Top × Inj + t). We tested the goodness of fit of the global model assessing if there is overdispersion estimating the c-hat using the median c-hat approach (White and Burnham 1999; Buzatto et al. 2011). Overdispersion factors greater than 3 indicate structural deficiencies in the model. Our global model had slight overdispersion (c-hat = 1.093). For this reason, we employed the Akaike Information Criteria for overdispersed data (QAIC; Burnham and Anderson 2002) to select the best of the competing models (i.e., the model with the lowest QAIC value). Given that two models had similar QAIC values (ΔQAIC = 0.85, see results), we used likelihood ratio tests (LRTs) to determine significant differences among models. Specifically, we tested the significance of the interaction Top × Inj present in the global model compared with a reduced model that was an additive model (Top + Inj). Because there was variation in survival along time, we estimated the mean survival parameters for the combinations of Top and Inj treatments using a variance component approach (Williams et al. 2002).
Survival in captivity was analyzed with a proportional hazard Cox regression model that included Top + Inj + Body size + Top:Inj. The best model was selected based on AIC.
Prior to parametric tests, homogeneity of variances was tested with the Fligner–Killeen test (Crawley 2007). The presence of outliers was explored with Cook's distance, but no outlier was detected (Cook's distance < 1). Analyses were done in R (R Core Development Team 2009, version 2.10.0) and MARK 6.1 software (White and Burnham 1999).
Results
SURVIVAL IN THE FIELD AFTER METHOPRENE AND BACTERIAL TREATMENTS
Survival parameters differed along time, Top, Inj treatments, and the interaction of Top × Inj treatments (Table 1). Male damselflies treated with the combination of methoprene and bacteria (Met–Bac) had a significantly lower probability of surviving in the field than males with any other combination of treatments (Table 1, Fig. 1). The bacteria groups had a decreased survival when compared with control treatment groups. However there was no difference between bacteria and PBS treatments with the exception of when Met was also applied (Table 1, Fig. 1).
| Parameter | Treatment | Mean Estimate | Error | 95% CI | ||
|---|---|---|---|---|---|---|
| Topical | Injected | Lower | Upper | |||
| ϕ | Met | Bac | 0.5439 | 0.0611 | 0.4242 | 0.6636 |
| Met | PBS | 0.8372 | 0.0330 | 0.7726 | 0.9018 | |
| ϕ | Met | Control | 0.9203 | 0.0191 | 0.8828 | 0.9578 |
| Ac | Bac | 0.7900 | 0.0411 | 0.7094 | 0.8706 | |
| ϕ | Ac | PBS | 0.8068 | 0.0378 | 0.7327 | 0.8810 |
| Ac | Control | 0.9277 | 0.0176 | 0.8931 | 0.9623 | |
| ϕ | Control | Bac | 0.7662 | 0.0445 | 0.6789 | 0.8534 |
| Control | PBS | 0.8645 | 0.0290 | 0.8077 | 0.9213 | |
| ϕ | Control | Control | 0.9076 | 0.0211 | 0.8662 | 0.9489 |
| P | Met | Bac | 0.5858 | 0.0481 | 0.4914 | 0.6802 |
| P | Met | PBS | 0.6020 | 0.0345 | 0.5342 | 0.6697 |
| P | Met | Control | 0.6944 | 0.0257 | 0.6440 | 0.7449 |
| P | Ac | Bac | 0.4462 | 0.0440 | 0.3600 | 0.5324 |
| P | Ac | PBS | 0.4628 | 0.0363 | 0.3916 | 0.5340 |
| P | Ac | Control | 0.5642 | 0.0273 | 0.5107 | 0.6177 |
| P | Control | Bac | 0.4421 | 0.0445 | 0.3549 | 0.5293 |
| P | Control | PBS | 0.4587 | 0.0337 | 0.3927 | 0.5247 |
| P | Control | Control | 0.5601 | 0.0283 | 0.5047 | 0.6156 |

Estimates of mean daily survival probabilities of wild American rubyspot males exposed to one Top and one Inj treatment. Estimates were calculated from a variance components approach of the best fitted model ϕ(Top × Inj + t) P(Top + Inj).
The LRT comparing the model with an additive survival term: ϕ(Top + Inj + t) and the model with an interactive survival term ϕ(Top × Inj + t), both with P(Top + Inj), was significant (χ2= 10.747, df = 4, P= 0.030), which means that adding the interaction term to the model resulted in a better fit. In the most supported model, time was a good predictor of survival (Table 2), which means that probability of survival is not constant across time. Recapture probabilities were best explained by the additive effects of Top and Inj treatments (Tables 1 and 2, Fig. 2). In general, the Met treatment is associated with higher recapture probabilities (Table 1, Fig. 2)
| Model description | QAICc | Δ QAICc | QAICc weight | Model likelihood | No. of parameters | Q deviance | |
|---|---|---|---|---|---|---|---|
| Survival components | Recapture components | ||||||
| ϕ(Top×Inj+t) | P(Top+Inj) | 3280.851 | 0 | 0.45260 | 1 | 33 | 3213.246 |
| ϕ(Inj+t) | P(Top+Inj) | 3281.706 | 0.85 | 0.29518 | 0.6522 | 27 | 3226.629 |
| ϕ(Top+Inj+t) | P(Top+Inj) | 3283.234 | 2.38 | 0.13748 | 0.3038 | 29 | 3223.993 |
| ϕ(Top×Inj+t) | P(Top×Inj+t) | 3284.920 | 4.07 | 0.05916 | 0.1307 | 56 | 3168.277 |
| ϕ(Top×Inj+t) | P(Top) | 3287.154 | 6.30 | 0.01936 | 0.0428 | 30 | 3225.826 |
| ϕ(Inj+t) | P(Top) | 3288.264 | 7.41 | 0.01112 | 0.0246 | 24 | 3239.411 |
| ϕ(Top+Inj+t) | P(Top+Inj+t) | 3289.749 | 8.90 | 0.00529 | 0.0117 | 47 | 3192.489 |
| ϕ(Inj+t) | P(Top+Inj+t) | 3289.873 | 9.02 | 0.00497 | 0.0110 | 46 | 3194.751 |
| ϕ(Top×Inj+t) | P(Top+Inj+t) | 3289.977 | 9.13 | 0.00472 | 0.0104 | 52 | 3181.980 |
| ϕ(Inj+t) | P(Inj) | 3291.528 | 10.68 | 0.00217 | 0.0048 | 24 | 3242.675 |
| ϕ(Top+Inj+t) | P(Top) | 3291.729 | 10.88 | 0.00197 | 0.0044 | 27 | 3236.652 |
| ϕ(Top×Inj+t) | P(Inj) | 3293.689 | 12.84 | 0.00074 | 0.0016 | 30 | 3232.361 |
| ϕ(Top+Inj+t) | P(Inj) | 3294.717 | 13.87 | 0.00044 | 0.0010 | 26 | 3241.718 |
| ϕ(Top+Inj+t) | P(Top+t) | 3299.390 | 18.54 | 0.00004 | 0.0001 | 46 | 3204.268 |
- The best supported model is in bold. Top, topical treatment (Met, Ac, Control); Inj, injected treatment (Bac, PBS, Control); t, time.
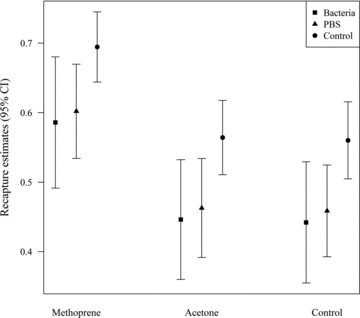
Estimates of mean daily recapture probabilities of wild American rubyspot males exposed to one Top and one Inj treatment. Estimates were calculated from a variance components approach of the best fitted model ϕ(Top × Inj + t) P(Top + Inj).
SURVIVAL IN CAPTIVITY AFTER METHOPRENE AND BACTERIAL TREATMENTS
Survival of males in captivity was not explained by any of the treatments or covariates included in the model. The model selection procedure excluded Top (χ2= 0.856, P= 0.652) and Inj (χ2= 1.376, P= 0.503) treatments and body size (χ2= 1.279, P= 0.258) as well.
Discussion
Our results strongly support the ICHH, as males that were experimentally infected with bacteria (Bac) died faster than noninfected animals when supplemented with a JH analog (Met), but not under natural hormonal levels. Thus, our results indicate a fitness cost of hormones when animals are sick. Our results have important implications for future evaluations of the ICHH. Manipulation of both infection and hormonal levels is needed but had not been evaluated yet. If experimentally infected animals are not used, hormonal supplementation costs may arise but it may not be clear whether a hormone treatment in combination with an infection affects survival too. It seems likely that mortality was the highest in Met–Bac-treated animals because bacteria grew faster in Met males that were presumably immunosuppressed. Despite we did not measure any immune parameter, immunosuppression is a common consequence of high levels of JH (Rantala et al. 2003; Contreras-Garduño et al. 2009). Given that immunosuppressed animals can become more susceptible to predation (Rantala et al. 2011), we cannot discard predation as a consequent source of mortality associated to immunosuppression caused by Met in infected animals. This source remains to be tested.
Immunosuppression is not the only effect of JH in adult insects, so one has to keep in mind other sources of mortality associated to the combination of methoprene and bacteria. For example, resistance to environmental and physiological stress has been shown to decrease with JHa (e.g., Salmon et al. 2001; Tatar et al. 2001). Related to this, Drosophila melanogaster flies treated with high doses of methoprene are less resistant to starvation so that they die faster than control flies (Salmon et al. 2001). Such situation could be intensified in the presence of additional stressors such as pathogens (e.g., Bac treatment), leading infected animals to be more susceptible when JH levels are high (e.g., Met treatment). However, male survival was not affected by methoprene and/or bacteria in our experiment in captivity, where starvation was a main source of stress. Hence, we can argue that the main causes of male mortality in Met–Bac animals in the field experiment were more probably related to changes in behavior or immunosuppression, which are common effects of JH in damselflies (Contreras-Garduño et al. 2009, 2011).
Physical activity seems responsible of differences in survival, given that treatment did not affect survival in captivity (where movement was prevented) whereas it did in the wild. In our study species, territorial defense can be an extremely costly activity (Contreras-Garduño et al. 2008). During territorial contests, male lipid resources are heavily used (Plaistow and Siva-Jothy 1996; Contreras-Garduño et al. 2008) and are not renewable during adulthood (Raihani et al. 2008). Moreover, nonterritorial animals that have had their lipidic reserves exhausted survived for a shorter period during an experimental infection compared to territorial animals when both animals were kept inactive (Contreras-Garduño et al. 2007). This implies a close relationship between lipid resources and immunocompetence (Hernández-Hernández et al. 2003). Costs of territoriality could have driven to fast mortality in highly territorial males (for a similar rationale see Munguía-Steyer et al. 2010), especially if territoriality was enhanced with Met and the immune system was simultaneously activated with Bac.
The recapture rates (detectability) we found give support to the idea that Met increased territorial activity in a long term: Met-treated males were easier to detect during the daily surveys, presumably because they became more territorial and therefore more faithful to their defended sites (Munguía-Steyer et al. 2010). The reduced effect of bacterial treatment on recapture probabilities seems unexpected according to previous findings in other calopterygids, in which immune response activation has been associated with enhanced dispersal (Suhonen et al. 2010) and reduced territorial behavior (Rantala et al. 2010), which would lead to lower recapture rates in infected animals. Unfortunately, our capture–recapture approach does not allow determining precise causes for the observed recapture rates. However, our results are consistent with long-term effects on site fidelity observed in other species of territorial odonates (e.g., Munguía-Steyer et al. 2010).
Tests of the ICHH have been primarily conducted in vertebrates. Support for the ICHH has been mixed in vertebrate studies. Apart from the methodological challenges we outlined in the introduction, one physiological reason is that the action of testosterone on sexual selection and immunocompetence is far from simple (Marsh 1996). Contrary to expectations from the ICHH, testosterone in some vertebrates has shown to affect immunocompence positively and sexual traits negatively (Roberts et al. 2004). Research in invertebrates has provided a clearer support for the ICHH despite most studies failing to manipulate hormonal levels (Marsh 1996). In those cases where hormonal levels have been experimentally augmented, sexual traits have been found to increase in expression while immune functions become negatively affected (e.g., Rantala et al. 2003; Fedorka and Mousseau 2007; Contreras-Garduño et al. 2009, 2011). One reason of why ICHH has found stronger support in invertebrates than in vertebrates is that the action of JH is more ubiquitous (controlling more functions), direct (i.e., acting directly on gene expression), and thus less complex than testosterone (e.g., Flatt et al. 2005; Riddiford 2008). Here, we have shown under natural conditions that JH is an important mediator of life-history trade-offs in insects (Flatt et al. 2005).
Associate Editor: R. Bonduriansky
ACKNOWLEDGMENTS
A PAPIIT-UNAM grant (IN-204610) covered expenses of this research. To the Posgrado en Ciencias Biológicas and a CONACYT grant to DMG-T. D. Pera provided logistic support. D. Ruiz-Silva, H. Hernández-Córdoba, I. Pagán, A. Rodríguez, G. Jiménez-Cortés, and S. Martínez-Zamilpa helped during fieldwork. C. Anderson provided key comments and grammar revision. Two anonymous reviewers provided important opinions. All authors declare not to have any conflict of interest.




