GENOME SIZE IS NOT CORRELATED WITH EFFECTIVE POPULATION SIZE IN THE ORYZA SPECIES
Abstract
Genome sizes vary widely across the tree of life and the evolutionary mechanism underlined remains largely unknown. Lynch and Conery (2003) proposed that evolution of genome complexity was driven mainly by nonadaptive stochastic forces and presented the observation that genome size was negatively correlated with effective population size (Ne) as a strong support for their hypothesis. Here, we analyzed the relation between Ne and genome size for 10 diploid Oryza species that showed about fourfold genome size variation. Using sequences of more than 20 nuclear genes, we estimated Ne for each species after correction for the effects of demography and heterogeneity of mutation rates among loci and species. Pairwise comparisons and correlation analyses did not detect a negative relationship between Ne and genome size despite about 6.5-fold interspecies Ne variation. By calculating phylogenetically independent contrasts (PICs) for Ne, we repeated correlation analysis and did not find any correlation between Ne and genome size. These observations suggest that the genome size variation in the Oryza species cannot be explained simply by the effect of effective population size.
With a vast spectrum spanning several orders of magnitude across the tree of life, genome size remains an aspect of concerns in population genetics and comparative genomics because it serves as the most fundamental property of a genome (Petrov 2001; Lynch 2007). According to Plant DNA C-value database (Bennett and Leitch 2005a), genome sizes range nearly 2000-fold across angiosperms and about 100-fold in Poaceae. It is widely accepted that varying amounts of noncoding DNA contribute to the large-magnitude variation of genome sizes (Lynch 2007; Flowers and Purugganan 2008) and the mechanisms for both increase and decrease in DNA content have been well addressed (Lynch 2007; Hawkins et al. 2008; Whitney et al. 2010). To date, different hypotheses have been proposed to account for the tremendous diversity of the genome sizes across major lineages of organisms and invoked extensive controversy (Petrov 2002; Lynch and Conery 2003; Charlesworth and Barton 2004; Bennett and Leitch 2005b; Yi 2006; Charlesworth 2008; Hawkins et al. 2008; Whitney et al. 2010, 2011; Boussau et al. 2011; Lynch 2011).
Lynch and Conery (2003) proposed a nonadaptive theory arguing that evolution of genome complexity was driven mainly by nonadaptive stochastic forces rather than by adaptive evolution. They predicted that more harmful noncoding DNA in lineages with small Ne would accumulate due to the preponderance of random genetic drift over natural selection and thus the genome sizes in these lineages became larger. They presented the observation that genome size was negatively correlated with effective population size (Ne) across prokaryotes and eukaryotes as a strong support for their hypothesis. In a comparison between freshwater and marine fish species, Yi and Streelman (2005) detected an obvious negative relationship between genome size and Ne independent of phylogeny, body size, and generation time, consistent with Lynch and Conery's (2003) hypothesis. By examining the ratio of nonsynonymous to synonymous substitutions (Dn/Ds) among recently diverged taxa that differ in genome size, Boussau et al. (2011) found that genomic duplications (causing larger genome size) occurred concomitantly with smaller Ne (higher Dn/Ds) in the mitochondrial genomes of tetrapods, again supporting the contribution of nonadaptive processes to the mitochondrial genome size.
Nevertheless, objections to the idea that genome size is determined by population size have been frequently raised based on theoretical and empirical investigations (e.g., Charlesworth and Barton 2004; Gregory and Witt 2008; Whitney and Garland 2010; Whitney et al. 2010). First, the analyzing strategies in Lynch and Conery (2003) were problematic in that (1) using heterozygosity as a proxy for Ne failed to consider substantially varying mutation rates in different lineages (Charlesworth and Barton 2004; Daubin and Moran 2004); (2) bacterial species were difficult to distinguish from each other so that polymorphism levels might be falsely elevated (Daubin and Moran 2004); (3) estimate of Ne based on polymorphism using molecular markers was sensitive to recent demographical histories (Daubin and Moran 2004; Yi 2006; Gregory and Witt 2008); (4) the negative correlation between genome size and Ne disappeared when using phylogenetically independent contrasts (PICs) (Whitney and Garland 2010). Second, empirical investigations on different lineages of organisms were not in accordance with Lynch and Conery's (2003) hypothesis, including bacteria (Daubin and Moran 2004; Kuo et al. 2009), mammals (Vinogradov 2004), Arabidopsis thaliana versus A. lyrata (Wright et al. 2002; Charlesworth and Barton 2004), maize versus fly (Charlesworth 2008), and seed plants (Whitney et al. 2010). For example, Daubin and Moran (2004) indicated that small Ne in symbiotic bacteria might result in reduced genomes through gene loss and thus the relation between Ne and genome size in bacteria was the opposite of that proposed by Lynch and Conery (2003). In a recent study based on a dataset including 205 seed plant species, Whitney et al. (2010) found no relationship between genome size and Ne using PIC analyses.
Despite these debates, relatively few studies have been conducted on related species that share a common evolutionary history and differ in much fewer properties (Charlesworth and Barton 2004; Charlesworth 2008; Flowers and Purugganan 2008). The rice genus (Oryza L.) has increasingly become an important model for a variety of studies on biological questions thanks to the completion of rice genome sequencing of two rice cultivars and the development of the Oryza Map Alignment Project (OMAP) that aims to build a genome-level experimental system for Oryza studies (Shimamoto and Kyozuka 2002; Wing et al. 2005). The genus Oryza consists of two cultivated and approximately 22 wild species distributed across the world (Vaughan 2004). These species can be classified into 10 genome groups (A-, B-, C-, E-, F-, G-, BC-, CD-, HJ-, HK-genomes) (Ge et al. 1999; Wing et al. 2005) and serve as a proper study system to test Lynch's hypothesis with the following advantages: (1) About fourfold genome size variation was found for the diploid species (Ammiraju et al. 2006; Miyabayashi et al. 2007); (2) Phylogeny of Oryza is fully resolved, especially for the diploid species (Ge et al. 1999; Zou et al. 2008); (3) Shared evolutionary history helps eliminate the effects of other factors like ancient demography, when comparing different species for genome size and effective population size.
In this study, we obtained sequences of more than 20 nuclear gene fragments for 10 Oryza species representing all diploid genome types in the genus, which avoids the confounding effect of polyploidy. We corrected for the effects of demography and heterogeneity of mutation rates among loci and species before estimating Ne. By pairwise comparisons and correlation analyses, we found no significant correlation between genome size and Ne in the genus Oryza, providing the first fine-scale comparison among closely related species.
Materials and Methods
SPECIES SAMPLING AND LOCI STUDIED
We sampled 10 diploid Oryza species, representing all six diploid genome types (A-, B-, C-, E-, F-, and G-genomes) in the genus (Table 1), of which four (B-, E-, F-, and G-) genomes each have a single species (Vaughan 2004; Zou et al. 2008). For each species, we sampled at least eight accessions that covered the distribution range of the species except for O. rhizomatis from which four accessions were used (Table 1) because this species is endemic to Sri Lanka. Information on the sampled accessions is listed in Table S1.
| Taxon | Genome type | C value (pg)1 | N2 | Number of loci | πs3 | Ne (×106)3 |
|---|---|---|---|---|---|---|
| Oryza nivara | A | 0.93 | 11 | 22 | 0.0054 (0.0053) | 0.28 (0.28) |
| Oryza rufipogon | A | 0.88 | 15 | 22 | 0.0054 (0.0054) | 0.23 (0.23) |
| Oryza barthii | A | 0.94 | 13 | 26 | 0.0021 (0.0019) | 0.13 (0.12) |
| Oryza punctata | B | 0.86 | 10 | 20 | 0.0011 (0.0010) | 0.08 (0.07) |
| Oryza officinalis | C | 1.28 | 12 | 23 | 0.0050 (0.0050) | 0.35 (0.35) |
| Oryza rhizomatis | C | 1.92 | 4 | 23 | 0.0063 (0.0066) | 0.47 (0.49) |
| Oryza eichingeri | C | 1.39 | 8 | 23 | 0.0073 (0.0069) | 0.52 (0.51) |
| Oryza australiensis | E | 1.92 | 10 | 20 | 0.0044 (0.0046) | 0.32 (0.33) |
| Oryza brachyantha | F | 0.61 | 10 | 20 | 0.0033 (0.0035) | 0.24 (0.23) |
| Oryza granulata | G | 2.38 | 14 | 20 | 0.0032 (0.0030) | 0.19 (0.19) |
- 1The genome size estimates (C values, picograms, pg) of the 10 species were obtained from Miyabayashi et al. (2007) except for that of O. nivara that was from Ammiraju et al. (2006). 2The number of accessions sampled for each species in this study. The number of accessions sampled for Oryza nivara and O. rufipogon (Zhu et al. 2007), and O. barthii (Li et al. 2011) was 12, 18, and 20, respectively. 3Average πs and Ne across loci. The figures in parentheses were calculated based on the filtered datasets in which all sequences from nonneutral loci were removed.
In our previous studies on population genetics of the Oryza species, we have obtained sequences of 10 loci for five species (O. rufipogon, O. nivara, O. officinalis, O. rhizomatis, and O. eichingeri) (Zhang and Ge 2007; Zhu et al. 2007), and 14 loci for O. barthii (Li et al. 2011). For these species, we further sequenced additional 12 or 13 loci in this study. For the remaining four species (O. punctata, O. australiensis, O. brachyantha, and O. granulata), we sequenced 20 loci (Table 1). Therefore, we obtained sequences from 20 to 26 unlinked nuclear loci for each of the 10 species (Table 1). Detailed information on the sampled loci is provided in Tables S2 and S3.
DNA EXTRACTION, AMPLIFICATION, AND SEQUENCING
Total DNA was extracted from fresh or silica gel-dried leaves, using the CTAB (hexadecyltrimethylammonium bromide) method as described in Ge et al. (1999). PCR amplification and purification of the products were performed generally following those in previous studies (Zhang and Ge 2007; Zhu et al. 2007). Sequencing was done on an ABI3730XL automatic sequencer (Applied Biosystems, Foster City, CA). Purified products were sequenced either directly or after cloning into pGEM T-easy vectors (Promega, Madison, WI) if direct sequencing failed or dual peaks were found. At least three cloned DNA fragments were sequenced for each individual. The number of clones per individual was added by three until the haplotype was shared among at least two clones, so as to exclude artificial singleton (Zhang and Ge 2007). All sequences have been deposited in GenBank, with the accession numbers JQ414289–JQ415911.
SEQUENCE ANALYSIS
Sequences were assembled with the ContigExpress program (Informax Inc., North Bethesda, MD) and aligned with ClustalX 1.83 (Thompson et al. 1997) before additional manual refinements. As well established in population genetics (Charlesworth 2009), the expected level of nucleotide diversity in a sample of a population (π) is 4Neμ under the standard model, where Ne is the effective population size and μ is the mutation rate per nucleotide. Because of the heterogeneity of evolutionary rates across genes, we first used the method of Zhang and Ge (2007) to estimate μ at silent sites for each locus by μ=μadh1×Ksil/Ksadh1, where Ksil and Ksadh1 are silent distances between the target species and its corresponding outgroup at that locus and at Adh1 locus, respectively (Table S3). μadh1 is estimated to be 7.0 × 10−9 substitutions per synonymous site per year, a fossil-calibrated synonymous rate of Adh1 divergence in grasses (Gaut et al. 1996). Then, we calculated average pairwise difference per basepair between sequences at silent sites (πs) for each locus using DnaSP version 5.10.00 (Librado and Rozas 2009). Ne can be estimated from silent nucleotide site diversity by Ne=πs/(4μ) (Charlesworth 2009).
Because nonneutral data may bias the Ne estimate, we performed several neutrality tests to confirm whether the loci were neutral. We calculated Tajima's D (1989) and D* and F* of Fu and Li (1993) for each locus to test for the neutral equilibrium model of evolution across species. The associated one-tailed P-values were obtained by computing 1000 coalescent simulations, with recombination taken into account. The minimum number of recombination events (Rm) was estimated with the four-gamete test (Hudson and Kaplan 1985). These tests were performed using the program DnaSP. To discriminate between selection forces and population demography, the multilocus HKA test (Hudson et al. 1987) was performed with the HKA package (http://genfaculty.rutgers.edu/hey/software#HKA). Individual runs of the HKA test were performed for the contrast between each of 10 species and its corresponding outgroup. Detailed information about the loci and the outgroups in HKA runs is provided in Table S4.
TEST THE RELATIONSHIP BETWEEN NE AND GENOME SIZE
Pairwise comparison of πs and Ne among the 10 species was conducted and the significance was evaluated by paired t-test. We performed correlation analysis between Ne and genome size. Correlation coefficient and its significance were calculated for geometric mean Ne estimates across loci, and all values were log10 transformed prior to analysis.
Because shared phylogenetic history may violate the assumption of statistical independence, we further used PICs (Felsenstein 1985) to repeat the correlation analysis. The Oryza phylogeny of the 10 species (Fig. 1) was basically obtained from Zou et al. (2008) with slight modification in which O. sativa was replaced by O. nivara because these two species are most closely related (Zhu and Ge 2005) and O. sativa is a cultivated species. The Oryza phylogeny with branch lengths was imported to Mesquite version 2.7.4 (Maddison and Maddison 2010). We did ancestral reconstruction for genome size in Oryza with the parsimony ancestral state method of Mesquite (Maddison and Maddison 2010). To test whether the relationship between the traits in Oryza exhibited phylogenetic signals, we first used BayesTraits (Pagel and Meade 2009) to calculate λ that varies from 0 (entirely phylogenetical independence) to 1 (entirely phylogenetical dependence). Then, we obtained PICs for Ne and genome size using the PDAP:PDTREE module in Mesquite (Midford et al. 2002). We obtained standardized contrasts for further correlation analyses by dividing the raw contrasts by the standard deviations (Garland et al. 1992).
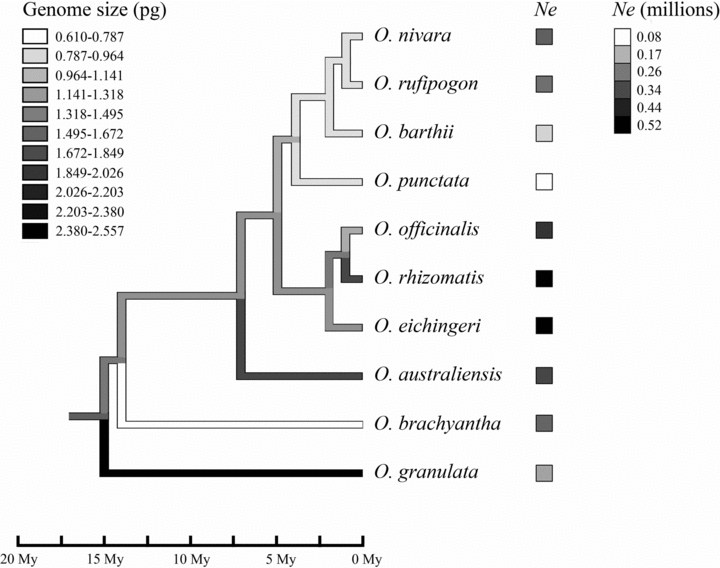
Phylogeny for 10 Oryza species with a reconstruction of ancestral genome sizes. Shades of the branches on the tree represent the genome sizes, and shades of squares after the species names indicate the mean Ne estimates for the species. Time scale is provided below the tree.
Results
A total of 2463 sequences from 29 loci were obtained from 10 diploid Oryza species. The length of aligned sequences for each locus ranged from 418 to 1467 bp, with a total of 23,915 bp (including 6429 bp of coding region) in length (Table S2). Of the 29 loci, 26 contained both coding and noncoding sites and the remaining three contained either intron (Adh1, TFIIAγ-1) or 5′-flanking (CatA) regions. The schematic diagrams of the 29 genes are shown in Figure S1. The number of loci used for each of 10 species varied from 22 to 26 (Table 1). Standard statistics of sequence variation for each locus for each species are summarized in Table S3. At the species level, average estimates of silent nucleotide diversity (πs) across loci varied substantially among 10 species, ranging from 0.0011 (O. punctata) to 0.0073 (O. eichingeri) (Table 1; Fig. S2). Given the heterogeneity of evolutionary rates across genes, we performed the paired t-test between all pairs of 10 species and found substantial variation of diversity levels among species. As shown in Figure 3a and Table S5, 23 species contrasts showed significant t-test values (P < 0.05) and the remaining 22 contrasts showed no significance. Of the significant contrasts, 15 and eight have positive or negative values, respectively (Fig. S3a). These observations indicated that the species with larger genome size might not have smaller πs values.
Summary of the tests of Tajima's D (1989) and D* and F* of Fu and Li (1993) for each locus for each species are shown in Table S3. No significance was observed for a majority of values, except for Adh1 in O. nivara and O. rufipogon, Cbp1 in O. rufipogon, and NP70 in O. granulata, in which all three tests were significant (Table S3). A significant departure from neutrality at a specific locus may not necessarily indicate the signature of selection because these statistics are sensitive to population demography. We further conducted a multilocus HKA test that is robust to population structure and demography. Signature of departure from the neutral model was detected in four contrasts (O. rhizomatis/O. punctata, O. eichingeri/O. punctata, O. brachyantha/O. granulata, and O. granulata/O. brachyantha) (Table S4). The HKA test was repeated with exclusion of the loci with the largest contribution to the overall statistics until the statistic dropped below the critical value (Table S4). Significant departure can be explained by a larger variance in the polymorphism/divergence ratio than that expected under a neutral equilibrium model, which might be attributed to selection on some loci for some species.
Ne estimates for each locus for each species are listed in Table S3. Consistent with the πs values, the average Ne estimates across loci varied substantially among the 10 species, with the maximum difference between species being about 6.5-fold (Table 1). When pairwise comparison of Ne was performed with paired t-test, similar pattern to the πs values was detected as expected. Twenty-three of 45 pairs of species comparisons were significant (P < 0.05), with 14 being positive and nine negative (Fig. S3b, Table S5). To assess the relationship between Ne and genome size, we first performed correlation analysis between Ne and genome size using the log-transformed trait values. Using geometric mean Ne across loci, we did not detect significant relationship between Ne and genome size (Fig. 2a). Then, we repeated correlation analysis to account for the phylogenetic nonindependence. We obtained λ= 0.95, indicating strong phylogenetic signal for the relationship between the two traits. We thus calculated PICs for the Ne and C after standardization (Table S6). The correlation patterns using PICs (Fig. 2b) were similar to those using the phylogenetically uncorrected data (Fig. 2a).
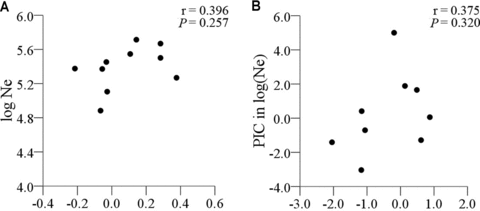
Plot of the correlation between mean Ne and genome size (C) without (a) and with (b) phylogenetical corrections based on all sequences.
As indicated above, a few of loci did not evolve neutrally in some species, including Adh1 in O. nivara and O. rufipogon, Adh1-C in O. eichingeri, Cbp1 in O. rufipogon, Lhs1 in O. brachyantha and O. granulata, NP70 in O. granulata, and Waxy in O. rhizomatis, O. brachyantha, and O. granulata (Tables S3 and S4). To account for the potential impact of the nonneutral loci on evaluation of the correlation between genome size and effective population size, we filtered the dataset by removing the sequences from nonneutral loci and obtained the πs/Ne values using the filtered dataset (Table 1). Although the πs/Ne estimates were slightly different, paired t-test generated similar results, in which about half of total 45 species comparisons was not significant for both πs and Ne (Figs. S3c and S3d, Table S5). Correlation analysis with the filtered data did not find significant relationship between Ne and genome size either (Fig. S5).
Lynch (2011) indicated that Neμ (as estimated by πs), rather than Ne was the relevant predictor variable to correlate with genome size. Thus, we performed correlation analyses between genome size and πs based on the filtered datasets. The results indicated that no significant correlation was detected between genome size and πs, both with (r= 0.277, P= 0.470) and without (r= 0.317, P= 0.372) phylogenetical corrections. Together, all analyses above provide no evidence that genome size is correlated with effective population size in the genus Oryza.
Discussion
The negative correlation between genome size and effective population size hypothesized by Lynch and Conery (2003) was based on nearly neutral theory and population genetic principles and seemingly applied in many cases across the web of life (Yi 2006; Lynch 2007; Boussau et al. 2011). The explanation power of Ne for several evolutionary questions is commonly accepted and selection efficiency related to Ne may explain for variation of some other biological attributes (e.g., codon bias, evolutionary rate, proportion of adaptive substitution, etc.) among different lineages of organisms (Lynch 2007; Charlesworth 2009). However, as pointed out by many authors (Charlesworth and Barton 2004; Daubin and Moran 2004; Yi 2006; Gregory and Witt 2008), the potential biases for obtaining reliable estimates of Ne such as mutation rate heterogeneity, taxonomy difficulties, sensitivity of molecular markers to demography should be considered cautiously. Phylogenetical nonindependence should be also taken into consideration in multiple species analysis (Whitney et al. 2010; Whitney and Garland 2010). In the present study, we tested Lynch and Conery's (2003) hypothesis using 10 diploid Oryza species in which fourfold genome size variation is present across species. We did not detect a negative relationship between Ne and genome size as predicted by Lynch and Conery (2003), despite the fact that alternate analyses were performed to account for the potential biases mentioned above.
It should be noted that genome size evolution is a complex process influenced by numerous evolutionary forces (Charlesworth and Barton 2004; Lynch 2007; Hawkins et al. 2008; Whitney et al. 2010). For instance, using multiple regression analysis with PICs for the relation among Ne, genome size, and outcrossing rate in seed plants, Whitney et al. (2010) found no relationship between Ne and genome size but a weak relationship between outcrossing and genome size. In our case, 10 Oryza species diverged within the last 15 million years (Fig. 1 and Tang et al. 2010) with a majority of features in common. However, different mating systems have been recorded for the Oryza species (Vaughan 2004) despite few extensive investigations. Based on previous studies, we chose seven Oryza species with available records and divided them into two groups according to their mating systems: (1) predominantly inbreeding species, including O. nivara (Barbier 1989), O. barthii (Vaughan 2004), O. eichingeri (Jayasuriya and Vaughan 2003), and O. granulata (Qian et al. 2001); and (2) outcrossing species, including O. rufipogon (Barbier 1989), O. officinalis (Gao et al. 2001; Jayasuriya and Vaughan 2003), and O. rhizomatis (Jayasuriya and Vaughan 2003). Then, we compared the genome sizes between the two species groups with contrasting mating systems and found no significant relationship (P= 0.917), suggesting that the genome size variation in Oryza cannot be simply explained by mating system either.
A number of studies have detected recent bursts of several LTR-retrotransposon families in some Oryza species, which was considered as the main cause of the fourfold genome size variation in the diploid Oryza species (e.g., Piegu et al. 2006; Zuccolo et al. 2007). Fine-scale comparative analyses based on orthologous Oryza BAC sequences also supported the TE activity (Ammiraju et al. 2008; Lu et al. 2009; Sanyal et al. 2010). These reports indicated the important contribution of the noncoding elements like TE, which is one of the prerequisites for Lynch and Conery's (2003) hypothesis. However, the lineage-specific genome expansion could not be exclusively explained by the effect of Ne, as demonstrated by this study. As reviewed in Whitney et al. (2010), the principle theories for genome expansion could be classified as adaptive (Gregory and Hebert 1999; Bennett and Leitch 2005b), neutral (Petrov 2002; Oliver et al. 2007), and maladaptive (“junk DNA” theories) (Doolittle and Sapienza 1980; Lynch and Conery 2003). Although adaptive effects of larger genome size and numerous functions of noncoding DNA were proposed (Gregory and Hebert 1999; Bennett and Leitch 2005b), no direct evidence has been found in Oryza.
Petrov (2002) argued that the process of genome size evolution might fit a mutational equilibrium model, in which all insertions and deletions were neutral and organisms got their optimum genome sizes until DNA loss through small deletions was equal to DNA gain through long insertions. Although indel bias might be efficient for long-term evolution (Petrov 2001), it might not explain the vast change in genome sizes within such a short time scale in diversification of Oryza. Oliver et al. (2007) showed that the rate of genome size change was proportional to genome size, with a faster rate occurring in the larger genome. It was predicted that smaller genomes were more difficult to become large whereas larger genomes were easier to become small, and thus a skewed distribution toward smaller values would be found. Because this hypothesis might be proper for generalization over long time scales, it is expected that the extant C-values and reconstructed values at ancestral nodes in Oryza did not show such a skewed pattern (Fig. 1).
It is worth noting that a number of limitations might arise in our detection of correlation between genome size and effective population size. First, we used the method described in Zhang and Ge (2007) to correct for the heterogeneity of mutation rates across genes before estimating Ne. However, this correction was based on the divergence data between the target species and its corresponding outgroup under the molecular clock assumption that would be violated to some extents. Second, no significant correlation in our estimates may result partly from small sample size (10 data points). This cannot be avoided in our case that sampled all major lineages in Oryza, but should be taken into consideration in further investigations using closely related species. Finally, it is likely that Ne reflects the coalescence history while changes in genome size (e.g., due to TE proliferation, Piegu et al. 2006; Zuccolo et al. 2007) might be rapid and recent. Therefore, evolutionary time scale might have different impacts on the estimates of effective population size and genome size, and thus should be considered with caution. To sum up, genome size evolution is a complex process influenced by several evolutionary forces, and therefore the genome size variation in the diploid Oryza species could not be simply explained by one of the above theories. Investigation of the fitness significance of important LTR-retrotransposon families with large contribution to genome size should be considered in future study in Oryza. Particularly, multivariate analysis based on reliable estimates of Ne, in conjunction with phylogenetic correction, is required at different taxonomic levels to distinguish the relative contribution of correlated variables to genome size variation.
Associate Editor: A. Cutter
ACKNOWLEDGMENTS
We thank X. h. Zou, F. M. Zhang, L. L. Zang, L. Huang, X. M. Zheng, and other members of Ge's group for technical assistances, and A. Cutter, the associate editor, and two anonymous reviewers for valuable comments and suggestions on the manuscript. We are grateful to D. A. Vaughan for providing some leaf samples, and to the International Rice Research Institute (Los Banos, Philippines) for providing seed samples. This work was supported by the National Natural Science Foundation of China (30990240) and the National Basic Research Program of China (2007CB815704).




