SEXUAL SELECTION AFFECTS THE EVOLUTION OF LIFESPAN AND AGEING IN THE DECORATED CRICKET GRYLLODES SIGILLATUS
Abstract
Recent work suggests that sexual selection can influence the evolution of ageing and lifespan by shaping the optimal timing and relative costliness of reproductive effort in the sexes. We used inbred lines of the decorated cricket, Gryllodes sigillatus, to estimate the genetic (co)variance between age-dependent reproductive effort, lifespan, and ageing within and between the sexes. Sexual selection theory predicts that males should die sooner and age more rapidly than females. However, a reversal of this pattern may be favored if reproductive effort increases with age in males but not in females. We found that male calling effort increased with age, whereas female fecundity decreased, and that males lived longer and aged more slowly than females. These divergent life-history strategies were underpinned by a positive genetic correlation between early-life reproductive effort and ageing rate in both sexes, although this relationship was stronger in females. Despite these sex differences in life-history schedules, age-dependent reproductive effort, lifespan, and ageing exhibited strong positive intersexual genetic correlations. This should, in theory, constrain the independent evolution of these traits in the sexes and may promote intralocus sexual conflict. Our study highlights the importance of sexual selection to the evolution of sex differences in ageing and lifespan in G. sigillatus.
Senescence (or ageing) is the general decline in organismal fitness and performance with age and is an almost universal feature of multicellular organisms (reviewed in Hughes and Reynolds 2005). Evolutionary theory suggests that senescence evolves because extrinsic mortality means that few individuals reach old age in a population, which reduces the intensity of natural selection with age (Medawar 1946, 1952; Williams 1957). This weakening of natural selection with age may result in the accumulation of alleles with deleterious effects that are only expressed late in life (mutation accumulation,Medawar 1952) or that enhance fitness early in life at the expense of fitness late in life (antagonistic pleiotropy,Williams 1957). Under both scenarios, higher extrinsic mortality should result in the evolution of accelerated senescence (Medawar 1952; Williams 1957; Hamilton 1966; Rose 1991). Although this prediction has received support from experimental studies (e.g., Service et al. 1988; Stearns et al. 2000) and comparative analyses (e.g., Keller and Genoud 1997; Bryant and Reznick 2004), recent theory suggests that this outcome may not be realized under all conditions (Williams et al. 2006). For example, if high extrinsic mortality selects for increased investment in somatic maintenance, it is theoretically possible for a decelerated rate of senescence to evolve (Abrams 1993; Williams and Day 2003). This appears to occur in natural populations of guppies (Poecilia reticulata) subject to different predation regimes (Reznick et al. 2004).
More recently, there has been a growing appreciation that sexual selection also plays an important role in the evolution of senescence (Promislow 2003; Graves 2007; Bonduriansky et al. 2008). In most sexually reproducing species, one sex (male) produces numerous, small gametes that compete for access to smaller numbers of large gametes (female). This dichotomy in reproductive investment between the sexes means that males typically allocate more resources to competing for additional matings than females (Trivers 1972). As a result, male fitness is generally more variable than female fitness and this increases both the opportunity for and intensity of sexual selection in males (Bateman 1948; Trivers 1972). In general, female fitness is limited by the time investment and resource acquisition demands of offspring production and viability selection acting on females is expected to promote low-risk, low-wear-and-tear strategies with moderate rates of return over extended time periods (Bonduriansky et al. 2008). In contrast, sexual selection means that males are expected to pursue high risk, “live fast, die young” life-history strategies that have the potential to yield high fitness returns over a short time frame (Vinogradov 1998; Hunt et al. 2004; Bonduriansky et al. 2008; Punzalan et al. 2008) and theoretical models show that this may be achieved by trading lifespan for enhanced mating success (Kokko 1997, 2001). In support of this prediction, male mortality is higher than female mortality across a range of taxa (Finch 1990) and has been shown to covary with the intensity of sexual selection in both comparative studies (e.g., Promislow 1992; Clutton-Brock and Isvaran 2007) and experimental evolution (e.g., Maklakov et al. 2007). However, empirical support for this general prediction is far from universal (Williams et al. 2006; Bonduriansky et al. 2008) and this prediction may, in fact, be incorrect in species that exhibit age-dependent changes in reproductive effort (Partridge and Barton 1996; Graves 2007; Bonduriansky et al. 2008). In species where male reproductive effort increases with age (e.g., Mysterud et al. 2004), sexual selection may favor the evolution of a slower rate of ageing in males than in females if the increase in reproductive effort with age enhances male mating success (Partridge and Barton 1996; Graves 2007; Bonduriansky et al. 2008). In fact, theory suggests that an increase in male reproductive effort with age represents an evolutionary stable strategy (ESS) over a wide range of conditions and may even drive the evolution of female mating preferences for older males in the population (Kokko 1997).
Despite the potential importance of sexual selection to the evolution of lifespan and ageing, there are relatively few direct empirical studies that quantify the role of sexual selection in the evolution of lifespan and ageing (reviewed in Bonduriansky et al. 2008). One method that has been used is experimental evolution in which replicate populations are permitted to evolve with differing opportunities for sexual selection (i.e., monogamy or polygamy) (e.g., Promislow et al. 1998; Maklakov et al. 2007, 2010; Maklakov and Fricke 2009). Although this approach has provided valuable support for a role of sexual selection in the evolution of lifespan and ageing in the sexes (e.g., Promislow et al. 1998; Maklakov et al. 2007 but see Maklakov and Fricke 2009), a recent study suggests that this pattern may actually represent differential selection on life-history regimes rather than the effects of sexual selection per se (Maklakov et al. 2010). Furthermore, none of these studies have examined whether differences in the opportunity for sexual selection also lead to an evolutionary divergence in age-dependent reproductive effort in the sexes (Bonduriansky et al. 2008). This is, in part, due to the difficulties with accurately quantifying male reproductive effort in most species. In this regard, field crickets have proven to be an important model for ageing research as both male and female reproductive effort can be easily quantified: male reproductive effort can be measured as the time spent calling to attract a mate (Hunt et al. 2004, 2006; Zajitschek et al. 2007; Judge et al. 2008) and female reproductive effort can be measured as the number of eggs produced (Head et al. 2005; Hunt et al. 2006; Zajitschek et al. 2007). In a number of cricket species, the number of females attracted to a calling male on a given night is positively correlated with his calling effort (Bentsen et al. 2006; Jacot et al. 2008; Rodríguez-Muňoz et al. 2010) and is therefore, an important determinant of male reproductive success. Moreover, as calling is metabolically costly (Kavanagh 1987), nightly calling effort also constitutes an important form of male reproductive effort. Despite this, the majority of studies examining the relationship between age-dependent reproductive effort and ageing in crickets have focussed exclusively on lifespan (Hunt et al. 2004, 2006; Zajitschek et al. 2007; Judge et al. 2008), which may not always provide an accurate measure of the rate of senescence (Pletcher 1999; Monaghan et al. 2008).
Here, we examine the role that sexual selection plays in the evolution of lifespan and ageing in the decorated cricket, Gryllodes sigillatus. Using eight inbred lines, we first quantify differences between the sexes in lifespan, measures of ageing (Gompertz baseline mortality and rate of ageing), and how reproductive effort changes with age and use this to estimate the genetic basis of these traits in males and females. If sexual selection is important to the evolution of lifespan or ageing, we predict that these traits will be heritable and differ across the sexes, the direction of which should reflect how males and females alter their reproductive effort with age. We then estimate the genetic correlations between these traits, as well as between early and late-life reproductive effort, to test the hypothesis that the relationship between these traits differs between the sexes. Finally, we estimate the genetic correlations between male and female lifespan, measures of ageing and age-dependent reproductive effort. A positive genetic correlation for these traits across the sexes would indicate that these traits are unable to evolve independently in the sexes and may provide a signature of intralocus conflict if coupled with sex-specific differences in selection operating on these traits (Bonduriansky and Chenoweth 2009). To our knowledge, this is the first study to quantify genetic (co)variance between lifespan, ageing parameters, and age-dependent reproductive effort in both males and females and therefore provides compelling evidence demonstrating an important role for sexual selection in the evolution of lifespan and ageing in this species.
Materials and Methods
INBREEDING PROTOCOL AND CRICKET MAINTENANCE
Gryllodes sigillatus used in this study were descended from 500 adult crickets collected in Las Cruces, New Mexico in 2001, and used to initiate a laboratory culture maintained at a population size of approximately 5000 crickets and allowed to breed panmictically (hereafter, referred to as the outbred population). Nine inbred lines were created by subjecting a random subset of crickets from this population to 23 generations of full-sib mating followed by 12 generations of panmixis within each line (Ivy and Sakaluk 2005). Of the nine original lines, eight were used in our experiment, referred to as lines A, B, D–I, respectively.
Crickets were housed in 15-L plastic containers in an environmental chamber (Percival I-66VL) maintained at 32 ± 1°C on a 14 hours:10 hours light/dark cycle. Crickets were provided with cat food pellets (Friskies Go-Cat Senior®, Flyte So Fancy Ltd, Devon, UK) and water in 60-mL glass test tubes plugged with cotton wool ad libitum, as well as an abundance of egg cartons to provide shelter. As soon as adults were detected, moistened cotton wool was provided in a petri dish (10 cm diameter) as an oviposition substrate. Each inbred line was maintained in two containers and our outbred population in eight containers.
Each generation, crickets were randomly mixed between containers and were maintained at a density of approximately 300 crickets per container.
EXPERIMENTAL DESIGN
On the day of hatching, 270 nymphs from each of the eight inbred populations were isolated in individual plastic containers (5 cm × 5 cm × 5 cm). Each nymph was provided a piece of cardboard egg box for shelter and water in a 2.5-mL test tube plugged with cotton wool. Crickets were fed cat food pellets (Friskies GoCat Senior) and their enclosure cleaned once a week. For the first three feedings, food was provided in powder form in the lid of a 2.5-mL eppendorf. For subsequent feedings, two cat food pellets were provided every week to each cricket. As soon as nymphs reached final instar, they were checked daily for eclosion to adulthood. Adult survival was checked daily from eclosion until death.
We measured the reproductive effort of male and female G. sigillatus on days 10, 20, and 30 posteclosion and each cricket was measured on only one of these days, being allocated to these sampling periods at random prior to adult eclosion. Table 1 presents the number of crickets alive in each of these three sampling periods, plus at 40 days posteclosion. Our main rationale for choosing these sampling periods was to ensure that sufficient crickets were alive at each sampling period to ensure that an accurate measure of reproductive effort could be attained. As can be seen in Table 1, insufficient males from one of the inbred lines (line A) were alive to assess reproductive effort at 40 days posteclosion. This sampling regime has been used in other ageing studies on field crickets (Hunt et al. 2004, 2006; Zajitschek et al. 2007), thereby making our work directly comparable. For simplicity, we refer to these sampling periods as reproductive effort measured “early,”“mid,” and “late” in life, although we appreciate that this classification is not perfect for all lines or for both sexes (Table 1).
| Line | Mean LS±SE | Median LS | Reproductive effort | n | |||
|---|---|---|---|---|---|---|---|
| Day 10 | Day 20 | Day 30 | Day 40 | ||||
| Males | |||||||
| A | 34.06±2.37 | 29.5 | 46 | 39 | 21 | 14 | 48 |
| B | 49.75±1.83 | 49.5 | 105 | 102 | 91 | 75 | 108 |
| D | 60.04±2.19 | 61.0 | 132 | 128 | 110 | 98 | 133 |
| E | 51.55±4.55 | 52.0 | 45 | 40 | 35 | 34 | 51 |
| F | 63.60±2.16 | 60.5 | 120 | 118 | 114 | 101 | 120 |
| G | 74.87±2.36 | 75.0 | 122 | 121 | 118 | 114 | 125 |
| H | 40.44±1.78 | 38.0 | 93 | 84 | 64 | 44 | 96 |
| I | 68.73±2.27 | 67.0 | 127 | 124 | 120 | 114 | 127 |
| Outbred | 73.62±2.61 | 71.0 | 105 | 104 | 102 | 93 | 105 |
| Females | |||||||
| A | 35.88±1.83 | 35.0 | 61 | 55 | 45 | 21 | 65 |
| B | 39.31±1.43 | 40.0 | 88 | 79 | 62 | 37 | 88 |
| D | 41.03±1.50 | 41.0 | 79 | 75 | 59 | 41 | 80 |
| E | 39.48±2.40 | 39.0 | 61 | 54 | 42 | 28 | 64 |
| F | 48.06±1.55 | 50.0 | 102 | 96 | 84 | 71 | 103 |
| G | 49.18±1.35 | 51.0 | 119 | 114 | 104 | 83 | 119 |
| H | 36.81±1.40 | 37.0 | 103 | 91 | 70 | 35 | 105 |
| I | 53.47±1.45 | 52.0 | 103 | 100 | 93 | 84 | 103 |
| Outbred | 50.42±1.50 | 52.0 | 151 | 141 | 120 | 98 | 151 |
Prior to day 8 posteclosion, all animals were reared in isolation and were therefore virgins. On each of days 8, 18, and 28 posteclosion, every experimental cricket was paired with a cricket of the opposite sex taken at random from the outbred population and allowed 48 hours to mate. All animals were paired, irrespective of whether their reproductive effort was assayed or not, to incorporate the effects of mating on reproductive effort and survival. Pairs were then separated and all experimental females were provided with a small petri dish (5 cm diameter) full of moist sand for oviposition. Although all experimental females were given access to this substrate to oviposit across their lifetime, we measured female reproductive effort in a subset of females (see below) as the number of eggs produced in the 48 hours immediately following their allotted sampling period. The content of each petri dish was emptied into a container of water, swirled, and the eggs removed with fine forceps and counted.
In male crickets, the amount of time spent calling is a good measure of mating success because females strongly prefer males that call more in both the laboratory (Hunt et al. 2006) and field (Bentsen et al. 2006; Jacot et al. 2008; Rodríguez-Muňoz et al. 2010). It is also a good measure of reproductive effort because calls are metabolically expensive to produce (e.g., Kavanagh 1987). Consequently, we measured the amount of time each male spent calling on a given night (hereafter referred to as calling effort) using a custom-built electronic monitoring device. Each male was placed in an individual recording chamber (5 × 5 × 5 cm), with a condenser microphone (c1163, Dick Smith®) embedded in the lid, on his night of sampling. Each recording chamber was, in turn, placed inside a larger foam container (15 × 15 × 15 cm) to ensure each male was acoustically isolated. Each microphone was connected via leads to a data acquisition unit (DaqBook 120, IO-Tect, Cleveland) and computer (Dell™ OptiPlex™ 580). The data acquisition unit activates a single microphone at a time, which then relays the sound level to the PC board in which it is compared to the background noise. If the received signal is ≥ 10 dB, this is recorded as a call. The microphone is then deactivated and the next one in the series is activated, with each recording chamber being sampled and recorded 10 times per second. For the current analyses, we used the number of seconds a male called each night between 18:00 and 09:00. We expect that male calling effort is under strong sexual selection, while female reproductive schedules are shaped by natural selection. When we refer to sexual selection promoting divergent strategies of reproductive effort across the sexes, this is because it is predicted to affect male, rather than female, schedules of reproductive investment over time.
The use of inbred organisms in evolutionary research can be problematic, particularly if inbreeding depression results in a large reduction in the fitness (or fitness-related traits) of individuals or if the inbred lines do not represent a true random sample of genotypes from the outbred population from which they were derived (Hoffmann and Parsons 1988). Moreover, there is the potential for inbreeding to bias quantitative genetic parameters, particularly the sign and strength of genetic correlations between traits (for discussion see Rose 1984b). Therefore, in addition to the inbred lines, we also established 270 nymphs from the outbred population and measured lifespan, rates of ageing, and age-dependent reproductive as outlined above. It is important to note, however, that our aim was not to provide an explicit test of how inbreeding has influenced these traits, as this would require a vastly different experimental design, but rather to simply provide a baseline for comparison. Consequently, these outbred crickets are not included in any of our statistical analyses but are provided in our figures only for visual comparison. Importantly, trait means for our inbred lines were within the natural range documented in our outbred population, suggesting that inbreeding per se has not influenced trait expression (see Results). Our design, however, does not allow us to determine if inbreeding has influenced quantitative genetic estimates. Therefore, as with all studies employing inbred lines, our quantitative genetic estimates should be interpreted with a degree of caution.
With the exception of males from lines A and E, we measured the reproductive effort of 20 crickets of each sex at each sampling period (i.e. days 10, 20, and 30 posteclosion) per line. Due to higher nymph mortality in lines A and E, and the fact that we assigned animals at random to sampling periods prior to adult eclosion, calling effort was only measured in 15 males per sampling period. Our estimates of survival and ageing parameters come from a total of 1791 crickets (878 females and 913 males) and the sample sizes for each sex and line are provided in Table 1. Using simulation models, Pletcher (1999) provided a number of general guidelines on sample sizes needed to accurately estimate mortality parameters: (i) more than 50 individuals are needed to detect changes in the rate of ageing and identification of the correct mortality function, (ii) 75–100 individuals are required to detect leveling off in ageing late in life, and (iii) 100 individuals are needed to detect a change in baseline mortality. It is important to note that these guidelines are based on the use of outbred individuals and that the statistical power of these estimates is dramatically increased when inbred individuals are used (Pletcher 1999). Based on the sample sizes presented in Table 1, we are therefore confident in our estimated mortality parameters.
STATISTICAL ANALYSIS
Analysis of lifespan and survival
We examined differences in lifespan across the lines and sexes using a generalized linear mixed model (GLMM), including line as a random effect and sex as a fixed effect in the model, implemented in the “nlme” package (Pinheiro et al. 2011) in R (version R. 2.11.1; R_Development_Core_Team 2010). Lifespan could not be transformed to meet a normal distribution in either sex nor did it adhere to any other well-defined statistical distribution. We therefore tested the significance of the terms in our model using a randomization test where we compared the F values from the above GLMM (Freal) to those produced when the same GLMM was executed but lifespan was shuffled at random (without replacement) across lines and the sexes (Frandom)(Manly 2007). This shuffling procedure was iterated 10,000 times using a Monte Carlo simulation and the proportion of times (p) that Frandom exceeded Freal was calculated for each term in the model. Two-tailed significance values were calculated for each term in the model as 2p if p < 0.5 or as 2 (1 −p) if p > 0.5 (Manly 2007). All randomization tests were executed in R using a modified version of the “shuffle” function.
We analyzed differences in survival between lines and sexes using a Cox proportional hazards regression, implemented using the “coxph” function of the “survival” package (Therneau and Lumley 2011) in R. Line, sex, and their interaction were included as explanatory variables in this model.
Estimating and comparing rates of ageing
We used a maximum likelihood approach implemented in the “bbmle” package of R (Bolker 2009) to compare five statistical different models that describe the demographic rate of change in mortality with age: Gompertz, Gompertz-Makeham, Logistic, Logistic-Makeham, and Weibull. We compared these models separately for each sex per line and the best fitting model was taken as the one with the lowest Akaike's Information Criterion (AIC). In each instance, the Gompertz model always provided the best fit to the data with a ΔAIC always exceeding 10.

We compared Gompertz models that had either sex-specific α, sex-specific β, or sex-specific α and β, with models that included line-specific effects on these two parameters, respectively. To test for an ageing signature in mortality rate increase (β > 0), we constrained β to be zero in the Gompertz models fitted to each line, separately for males and females, and compared them to their unconstrained versions. For fitted Gompertz parameters, we estimated variance by randomly resampling 95% of the occurred ages at death in a sample and repeating this step 1000 times. For each resampled subsample, we then estimated the Gompertz parameters and calculated the variance of these 1000 values. For model comparison, we used AIC values and weights (Burnham and Anderson 2002).
Age-dependent reproductive effort
We examined differences in age-dependent reproductive effort across the lines and sampling periods using a GLMM, including line as a random effect and sampling period as a fixed effect in the model, implemented in the “nlme” package in R (Pinheiro et al. 2011). Female fecundity and male calling effort were not normally distributed, so we determined the significance of terms in our models using the randomization procedure outlined above for the analysis of adult lifespan. As our fixed effect had multiple levels (day 10, 20, and 30 posteclosion), we also used this randomization procedure to conduct post-hoc tests.
Quantitative genetic analysis
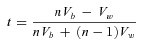
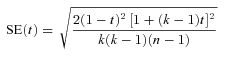
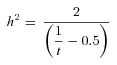

Gompertz parameters are population-based estimates and therefore the above analysis based on within and between line variance cannot be used to estimate the heritability of these parameter estimates. To estimate the heritabilities of Gompertz parameter (α and β) for males and females, we generated 1000 pseudo-Gompertz parameter estimates for each inbred line (separately for each sex) by sampling at random from a normal distribution where the mean was the actual Gompertz parameter estimate for the line and the standard deviation was calculated from the standard error of this actual estimate. We therefore assume that the standard error associated with each Gompertz parameter is not only a measure of the accuracy of the estimation routine, but can also be used to infer the variance of this parameter within each line. We then used these pseudoestimates for each line to extract the within and between line variance components for the Gompertz parameters using a linear mixed effects model implemented in R using the “lme4” package (Bates and Maechler 2009).

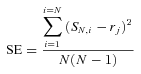
Simulation models have shown that this jacknife approach provides more accurate genetic estimates than those based on conventional inbred line means when fewer than 20 inbred lines are used in the analysis (Roff and Preziosi 1994). These estimates of genetic (co)variance from inbred lines contain variance due to dominance or epistasis and therefore should be considered broad-sense estimates (Falconer and Mackay 1996). As crickets were reared individually, we reduced the variance between lines due to common environment effects. Heritability estimates and genetic correlations were considered statistically significant if the estimates divided by their standard errors were greater than 1.96, rejecting the null hypothesis of no correlation based on a two-tailed t-distribution with infinite degrees of freedom.
Results
LIFESPAN AND RATES OF AGEING
There was significant variation in adult lifespan across lines (p= 1.00, P < 0.0001) and also across the sexes (p= 1.00, P < 0.0001), with males living an average of 15 days longer than females (male lifespan: 58.45 ± 0.94 days, female lifespan: 43.70 ± 0.60 days). However, the magnitude of this sexual asymmetry in lifespan differed significantly across lines (line × sex: p= 1.00, P < 0.0001) being small in some lines (e.g., lines A and H) and larger in others (e.g., lines G and I) (Fig. 1, Table 1). Qualitatively similar results were found when we analyzed the probability of survival using a Cox proportional hazards regression (line:χ2= 273.12, df = 7, P= 0.0001; sex:χ2= 214.94, df = 1, P= 0.0001; line × sex: χ2= 54.22, df = 7, P= 0.0001) (Fig. 1).
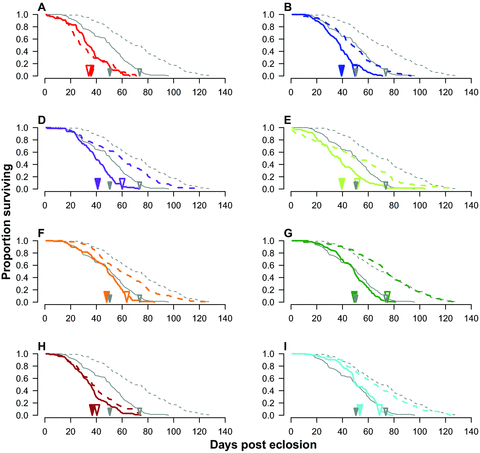
Adult survival curves for male (dashed lines) and female (solid lines) Gryllodes sigillatus from each of our eight inbred lines (thick, colored lines) and the outbred population (thin, grey lines). Figures A–I each represents a different inbred line (lines A, B, D–I, respectively). In each panel, the arrow heads represent mean lifespan for males (open symbols) and females (closed symbols) for each inbred line (colored symbols) and the outbred population (smaller, grey symbols).
We compared ageing parameters derived from the Gompertz model and found that the best-fit model included sex-specific baseline mortality (α) and ageing rate (β) demonstrating that the sexes exhibit different patterns of ageing (Table 2). Indeed, females always aged faster than males (Fig. 2). Consequently, we compared ageing parameters across lines separately for each sex (Table 3). For males, the best-fit model included line-specific baseline mortality and ageing rate (Table 3) indicating that both ageing parameters (α and β) differ across lines (Fig. 2B). In contrast, the best-fit model for females included a line-specific ageing rate only (Table 3), demonstrating that females from the same lines differed in their rate of ageing but not their baseline mortality (Fig. 2A).
| Model Name | Parameters | n | Δi | wi | |
|---|---|---|---|---|---|
| (A) Sex-specific baseline mortality and ageing rate | αmαf | βmβf | 4 | 0.00 | 0.839 |
| (B) Sex-specific ageing rate | βmβf | 3 | 3.35 | 0.161 | |
| (C) Line-specific baseline mortality and ageing rate | αaαbαdαeαfαgαhαi | βaβbβdβeβfβgβhβi | 16 | 97.90 | <0.001 |
| (D) Line-specific ageing rate | βaβbβdβeβfβgβhβi | 9 | 119.31 | <0.001 | |
| (E) Sex-specific baseline mortality | αmαf | β | 3 | 143.28 | <0.001 |
| (F) Line-specific baseline mortality | αaαbαdαeαfαgαhαi | 9 | 151.97 | <0.001 | |
| (G) Baseline mortality and ageing rate | α | β | 2 | 416.23 | <0.001 |
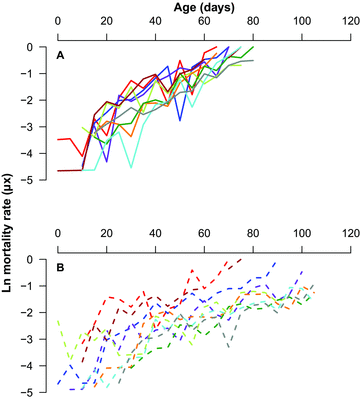
Age-dependent mortality rates in (A) female (solid lines) and (B) male (dashed) Gryllodes sigillatus from each of our eight inbred lines (colored lines) and the outbred population (grey lines). Line colors are as presented in Figure 1.
| Model name | Parameters | n | Δi | wi | |
|---|---|---|---|---|---|
| Males | |||||
| (A) Line-specific baseline mortality and ageing rate | αaαbαdαeαfαgαhαi | βaβbβdβeβfβgβhβi | 16 | 0.00 | 1 |
| (B) Line-specific baseline mortality | αaαbαdαeαfαgαhαi | β | 9 | 14.61 | <0.001 |
| (C) Line-specific ageing rate | βaβbβdβeβfβgβhβi | 9 | 22.10 | <0.001 | |
| (D) Baseline mortality and ageing rate | α | β | 2 | 203.51 | <0.001 |
| Females | |||||
| (A) Line-specific ageing rate | α | βaβbβdβeβfβgβhβi | 9 | 0.00 | 1 |
| (B) Line-specific baseline mortality | αaαbαdαeαfαgαhαi | 9 | 22.72 | <0.001 | |
| (C) Line-specific baseline mortality and ageing rate | αaαbαdαeαfαgαhαi | βaβbβdβeβfβgβhβi | 16 | 39.03 | <0.001 |
| (D) Baseline mortality and ageing rate | 2 | 74.52 | <0.001 | ||
AGE-DEPENDENT REPRODUCTIVE EFFORT
Females showed clear signs of reproductive ageing, with fecundity significantly decreasing with age (p= 1.00, P= 0.0001) (Fig. 3). Post-hoc tests revealed that female fecundity was significantly greater at 10 days of age than at 20 (p= 0.00, P= 0.0001) or 30 (p= 0.00, P= 0.0001) days of age but that fecundity did not change between 20 and 30 days of age (p= 0.44, P= 0.87) (Fig. 3). Although female fecundity varied significantly across lines (p= 1.00, P= 0.0001), females from the different lines showed a similar decline in reproductive effort with age (line × age: p= 0.90, P= 0.21)(Fig. 3).
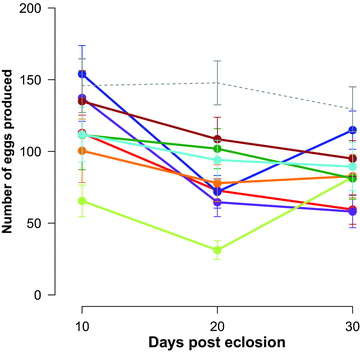
Age-dependent reproductive effort (±SE) of female Gryllodes sigillatus from each of our eight inbred lines (colored, solid lines) and the outbred population (grey, dashed lines). We measured female reproductive effort as the number of eggs produced (fecundity) in a 48-hour period commencing on days 10, 20, and 30 posteclosion to adulthood. Line colors are as presented in Figure 1.
In contrast to females, male reproductive effort significantly increased with age (p= 1.00, P= 0.0001) (Fig. 4). Post-hoc tests revealed that males spent more time calling per night at 30 days of age than at 10 (p= 0.00, P= 0.0001) and 20 (p= 0.002, P= 0.006) days of age, and more time calling at 20 than at 10 days of age, although this difference was not significant (p= 0.03, P= 0.057) (Fig. 4). As observed in females, male nightly calling effort differed significantly across lines (p= 0.00, P= 0.0001), but the increase in reproductive effort with age did not significantly differ across lines (line × age: p= 0.93, P= 0.133)(Fig. 4).

Age-dependent reproductive effort (±SE) of male Gryllodes sigillatus from each of our eight inbred lines (colored, solid lines) and the outbred population (grey, dashed lines). We measured male reproductive effort as time spent calling each night on either day 10, 20, and 30 posteclosion to adulthood. Line colors are as presented in Figure 1.
THE GENETICS OF LIFESPAN, AGEING, AND AGE-DEPENDENT REPRODUCTIVE EFFORT
Consistent with the large differences we observed between lines, we found significant heritability estimates for lifespan, ageing parameters, and age-dependent reproductive effort in both males and females (Table 4). In both sexes, there were consistent positive genetic correlations between reproductive effort measured at the different ages (Table 4). There were positive genetic correlations between lifespan and reproductive effort in males and in females but the strengths of these associations were typically much weaker in females (Table 4). Lifespan and baseline mortality were negatively genetically correlated in both sexes and between lifespan and the rate of ageing in males but not in females (Table 4).
| LS | α | β | ERE | MRE | LRE | |
|---|---|---|---|---|---|---|
| Males | ||||||
| LS | 0.97±0.02 | |||||
| α | −0.79±0.03 | 0.99±0.01 | ||||
| −0.42±0.10 | −0.26±0.40 | 0.99±0.01 | ||||
| ERE | 0.35±0.24 | −0.44±0.10 | 0.44±0.17 | 0.36±0.14 | ||
| MRE | 0.51±0.04 | −0.53±0.07 | 0.21±0.27 | 0.45±0.06 | 0.70±0.12 | |
| LRE | 0.49±0.05 | −0.40±0.05 | −0.06±0.14 | 0.15±0.07 | 0.95±0.00 | 0.52±0.14 |
| Females | ||||||
| LS | 0.94±0.03 | |||||
| −0.40±0.03 | 0.98±0.01 | |||||
| β | 0.09±0.15 | −1.16±0.23 | 0.99±0.00 | |||
| ERE | −0.11±0.11 | −0.98±0.45 | 0.97±0.06 | 0.48±0.14 | ||
| MRE | 0.32±0.09 | −0.99±0.46 | 1.02±0.88 | 0.77±0.35 | 0.76±0.10 | |
| LRE | 0.09±0.11 | −0.02±0.04 | −0.00±0.16 | 0.34±0.21 | 0.17±0.05 | 0.46±0.14 |
Importantly, in both males and females, there was a significant positive genetic correlation between early-life reproductive effort and the rate of ageing (Table 4). This genetic association was strongest in females and did not extend to mid or late-life reproductive effort in either sex (Table 4). Moreover, there were consistent negative genetic correlations between baseline mortality and reproductive effort measured at the different ages for both sexes (Table 4). This suggests that genes promoting elevated reproductive effort early in life were associated with those causing more rapid ageing but a reduced baseline mortality.
All measures of age-dependent reproductive effort, ageing, and lifespan exhibited positive genetic correlations between the sexes, although this association was not significant for early-life reproductive effort (Table 5). Likewise, the majority of intersexual genetic correlations between measures of age-dependent reproductive effort were positive, as were the intersexual genetic correlations between the rate of ageing and age-dependent reproductive effort (Table 5). In contrast, the intersexual genetic correlations between baseline mortality and measures of age-dependent reproductive effort were consistently negative (Table 5).
| ♀ ♂ | LS | α | β | ERE | MRE | LRE |
|---|---|---|---|---|---|---|
| LS | 0.88±0.00 | −0.33±0.09 | 0.08±0.04 | −0.10±0.05 | 0.19±0.11 | 0.08±0.17 |
| −0.71±0.01 | 0.76±0.02 | −0.66±0.24 | −0.57±0.14 | −0.62±0.12 | −0.37±0.10 | |
| β | −0.28±0.14 | −0.95±0.93 | 0.94±0.23 | 0.92±0.38 | 0.81±0.22 | 0.30±0.13 |
| ERE | 0.31±0.09 | −0.61±0.21 | 0.51±0.38 | 0.35±0.21 | 0.82±0.00 | 0.24±0.07 |
| MRE | 0.85±0.03 | −0.63±0.07 | 0.43±0.59 | 0.18±0.34 | 0.62±0.08 | 0.13±0.07 |
| LRE | 0.85±0.03 | −0.39±0.03 | 0.13±0.27 | −0.07±0.16 | 0.28±0.13 | 0.15±0.07 |
The intersexual genetic correlations involving lifespan were far less consistent in their pattern (Table 5). Although there were consistent negative genetic correlations between baseline mortality and lifespan across the sexes, the intersexual genetic correlations between ageing rate and lifespan differed in sign and magnitude (Table 5). Likewise, the intersexual genetic correlations between age-dependent reproductive effort and lifespan were largely positive, although the genetic correlation between early-life reproductive effort in females and lifespan in males was negative.
Discussion
Ageing is almost ubiquitous across multicellular organisms and understanding how this process evolves remains a central question in evolutionary biology (Hughes and Reynolds 2005; Williams et al. 2006; Bonduriansky et al. 2008; Monaghan et al. 2008). The role that natural selection plays in the evolution of lifespan and ageing is already well established (Medawar 1952; Williams 1957; Hamilton 1966; Rose 1991), but more recently there has been a growing appreciation that sexual selection may also play a significant role in shaping this process (Promislow 2003; Graves 2007; Bonduriansky et al. 2008). However, surprisingly little empirical evidence currently exists on how sexual selection contributes to the evolution of lifespan and ageing in the sexes (Bonduriansky et al. 2008). Studies that have attempted to quantify the influence of sexual selection on the evolution of lifespan and ageing have used experimental evolution (e.g., Promislow et al. 1998; Maklakov et al. 2007, 2010; Maklakov and Fricke 2009), but have not examined whether differences in the opportunity for sexual selection also lead to an evolutionary divergence in age-dependent reproductive effort in the sexes (Bonduriansky et al. 2008). This information is critical because differences in how the sexes alter their reproductive effort with age, and the effect that this has on sex-specific fitness, is predicted to determine the way that sexual selection will influence the evolution of lifespan and ageing in the sexes (Partridge and Barton 1996; Graves 2007; Bonduriansky et al. 2008).
Here, we show that sexual selection has facilitated divergence in the way that male and female decorated crickets (G. sigillatus) alter their reproductive effort across their lifetime; whereas males increase their reproductive effort with age, females show a decline in fecundity over time. These contrasting schedules of reproductive effort were associated with differences in lifespan and ageing between the sexes, with males living longer and ageing more slowly than females. As our measures of reproductive effort are important determinants of fitness in this species, these findings suggest that the selective value of lifespan and ageing differs considerably for the sexes. Mediating this sexual divergence, we found a positive genetic correlation between early-life reproductive effort and ageing rate in both sexes indicative of antagonistic pleiotropy, although this relationship was much stronger in females than in males. Despite clear sex differences in lifespan, ageing, and age-dependent reproductive effort, we found strong positive genetic correlations for these traits across the sexes suggesting that these traits are unlikely to evolve independently in males and females. Our results therefore highlight a clear and important role for sexual selection in the evolution of lifespan and ageing in the sexes of G. sigillatus.
Classic sexual selection theory predicts that males should maximize reproductive success by investing in sexual advertisement at the expense of lifespan (Kokko 1997, 1998; Getty 1998; Kokko et al. 2002), whereas females should adopt a low-risk strategy allowing time to accrue the resources needed to meet the relatively higher demands of reproduction (Promislow 2003; Graves 2007; Bonduriansky et al. 2008). This general prediction has received considerable empirical support, with males having higher mortality rates than females across a range of taxa (Comfort 1979; Finch 1990; Promislow and Harvey 1990), as well as a positive relationship between the intensity of sexual selection and male-biased mortality being documented across a variety of vertebrate species (Promislow 1992; Clutton-Brock and Isvaran 2007). However, this support is far from conclusive with many counter examples existing in which mortality rates are female biased (Promislow and Haselkorn 2002; Graves 2007; Reed et al. 2008) and in many species, males with more elaborate sexual traits have a longer lifespan (Jennions et al. 2001). Even within a single species, results can be conflicting. For example, in the seed beetle, Callosobruchus maculatus, males have a shorter lifespan and age faster than females (Fox et al. 2003, 2006; Maklakov et al. 2007), yet a number of natural evolution experiments have shown only a weak effect of sexual selection on the evolution of male and female lifespan and ageing (Maklakov et al. 2007, 2010; Maklakov and Fricke 2009).
One reason for these inconsistencies across empirical studies may be the lack of information on both male and female age-dependent reproductive effort for most study species. Theory suggests that how the sexes alter their reproductive effort with age, and the effect this has on fitness, is critical in determining the effect of sexual selection on the evolution of lifespan and ageing (Partridge and Barton 1996; Graves 2007; Bonduriansky et al. 2008). Indeed, sexual selection may promote the evolution of slower ageing in males than in females, if reproductive success increases with age in males to a greater extent than it does in females (Partridge and Barton 1996; Graves 2007; Bonduriansky et al. 2008). In agreement with this prediction, we found that male G. sigillatus live longer and age more slowly than females and that this pattern is associated with differences in how the sexes invest in reproductive effort across their lifetime. Calling effort is an important determinant of mating success in field crickets (Bentsen et al. 2006; Jacot et al. 2008; Rodríguez-Muňoz et al. 2010) and we found that males increased this form of reproductive effort with age. If this increase in late-life reproductive effort enhances male reproductive success in G. sigillatus, the strength of sexual selection on males will increase as they age and this will, in theory, select against genes that have negative pleiotropic effects late in life and the accumulation of deleterious mutations (Graves 2007; Bonduriansky et al. 2008) and promote enhanced somatic maintenance (Kirkwood 1977; Kokko 1997). In contrast, females exhibited clear reproductive senescence. This is expected to reduce the strength of sexual selection with age and enable genes with negative pleiotropic effects acting late in life and the accumulation of deleterious mutations to persist in the population (Graves 2007; Bonduriansky et al. 2008). This pattern of reproductive senescence is also expected to select against somatic maintenance in females, as there is little benefit to investing in somatic maintenance for a future that is unlikely to be reached (Kirkwood 1977). More work is needed, however, to determine how these evolutionary processes contribute to the evolution of sex-specific ageing in G. sigillatus, although it is unlikely that these alternatives (mutation accumulation and antagonistic pleiotropy) will be mutually exclusive (Williams et al. 2006).
Life-history theory, and particularly evolutionary theories of ageing, predicts that current reproductive effort will trade-off against future reproduction and lifespan (Roff 1992). We predicted that these trade-offs would be manifested as negative genetic correlations between early and late-life reproductive effort or between early-life reproductive effort and longevity. Contrary to this prediction, we found no apparent trade-offs between reproductive effort measured at different ages or between age-dependent reproductive effort and lifespan, with the genetic correlations between these traits being largely positive within the sexes. We did, however, find a positive genetic correlation between early-life reproductive effort and the rate of ageing in both sexes providing support for the antagonistic pleiotropy theory of ageing (Williams 1957; Rose 1991). This trade-off was particularly strong in females, which is consistent with their shorter relative lifespan and decline in reproductive effort with age. In general, trade-offs between age-dependent reproductive effort and lifespan (Rose and Charlesworth 1981; Rose 1984a) or rates of ageing (Tatar et al. 1993; Maklakov et al. 2010) appear well supported in females but fewer studies have successfully explored this relationship in males due to difficulties in quantifying reproductive effort in most species (Hunt et al. 2006; Zajitschek et al. 2007). For this reason, crickets have become a valuable model for ageing research because of the relative ease with which both male and female age-dependent reproductive effort can be measured. For example, in the Australian black field cricket (Teleogryllus commodus), a regime of divergent artificial selection on male lifespan resulted in a correlated response in age-dependent reproductive effort (Hunt et al. 2006). Males from lines selected for a shorter lifespan began calling earlier in adulthood and called more in total than males from lines selected for a longer lifespan (Hunt et al. 2006), suggesting that antagonistic pleiotropy has played an important role in the evolution of male lifespan in this species. A half-sib quantitative genetic study on this same species, however, failed to detect a genetic correlation between early-life reproductive effort and lifespan in either sex but did find a negative genetic correlation between average fecundity and lifespan in females (Zajitschek et al. 2007). Importantly, both of these studies were based on lifespan, which may not always provide an accurate measure of senescence (Pletcher 1999; Monaghan et al. 2008) because high intrinsic frailty may reduce longevity without accelerating the rate of ageing. Indeed, we found little evidence for a genetic association between early-life reproductive effort and lifespan in either sex of G. sigillatus. Our results therefore illustrate the importance of examining both lifespan and ageing parameters when attempting to understand the evolution of senescence (Pletcher 1999; Bonduriansky et al. 2008; Monaghan et al. 2008).
Intralocus sexual conflict occurs whenever selection on shared phenotypic traits in one sex displaces the other sex from its phenotypic optima, because many traits shared by the sexes have a common genetic basis but are subject to contrasting selection that prevents the sexes from evolving independently to their own phenotypic optima (Bonduriansky and Chenoweth 2009). Consequently, when intralocus sexual conflict is operating in a population, traits shared by the sexes will be characterized by strong and positive intersexual genetic correlations, as well as selection gradients that are different in sign and strength across the sexes (Bonduriansky and Chenoweth 2009). Recently, it has been proposed that sex differences in the optimal timing or relative costliness of reproductive effort may mediate intralocus sexual conflict over lifespan and rates of ageing (Zajitschek et al. 2007; Bonduriansky et al. 2008). Consistent with this view, we found that the trade-off between early-life reproductive effort and the rate of ageing was considerably stronger in females than in males. Moreover, we found a clear difference in the way that the sexes alter their reproductive effort with age that suggests males have more to gain by living longer and ageing more slowly than females. Despite this sexual divergence, we found positive genetic correlations between measures of age-dependent reproductive effort, lifespan, and ageing parameters across the sexes suggesting that these traits are not free to evolve independently in the sexes. Together these findings suggest that intralocus sexual conflict has the potential to play an important role in the evolution of sex differences in lifespan and ageing in G. sigillatus. However, confirmation that intralocus sexual conflict is indeed operating, as well as a direct estimate of its strength, requires formal estimation of the sex-specific fitness surfaces for age-dependent reproductive effort, lifespan, and ageing (Lewis et al. 2011). Although this is likely to prove difficult, Charmantier et al. (2006) showed in a natural population of mute swans (Cygnus olor) that sexual selection acting on the age at first and last reproduction is opposing in the sexes and that there was a positive genetic correlation between these two important life-history traits. Unfortunately, this study did not estimate the intersexual genetic correlations for these traits so that the importance of intralocus sexual conflict to the evolution of senescence could not be directly estimated (Charmantier et al. 2006). Considerably more work is needed, however, before the operation of intralocus sexual conflict can be confirmed in G. sigillatus and the role, if any, that it may play in the evolution of lifespan and ageing in this species.
In conclusion, our results suggest that sexual selection plays an important role in the evolution of sex differences in lifespan and ageing in G. sigillatus by promoting divergence in investment in reproductive effort with age by males and females and therefore the fitness consequences associated with lifespan and ageing. This sexual divergence was coupled with strong positive intersexual genetic correlations between lifespan and measures of ageing, raising the potential for intralocus sexual conflict over the evolution of sex differences in lifespan and ageing in this species. However, more research is needed to provide a definitive role for intralocus sexual conflict in this species. Our work highlights the need to consider these important, yet largely ignored, mechanisms when studying the evolution of lifespan and ageing (Graves 2007; Bonduriansky et al. 2008).
Associate Editor: R. Bonduriansky
ACKNOWLEDGMENTS
We thank M. Cupper, C. Mitchell, M. Neighbour, and C. Lowry for providing assistance in the laboratory. CRA was funded by a Natural Environment Research Council (NERC) studentship (awarded to JH and NR), NR and JH were funded by NERC, FZ by Wenner-Gren Foundations, and SKS by National Science Foundation. JH was funded by a Royal Society Fellowship and Equipment Grant.




