MULTIPLE GENETIC BENEFITS OF FEMALE PROMISCUITY IN A SOCIALLY MONOGAMOUS PASSERINE
Abstract
The adaptive function of female extrapair mating in socially monogamous passerines is currently debated. In the bluethroat (Luscinia s. svecica), a previous study showed that offspring sired by extrapair males had a higher cell-mediated immunity than their within-pair half siblings, suggesting an immunogenetic benefit of extrapair mating in this species. Here, we expanded that dataset with two more years and investigated the association between extrapair paternity and microsatellite multilocus heterozygosity, in addition to cell-mediated immunity. We found that extrapair offspring were more heterozygous than their within-pair half siblings, and corroborated the previous finding of enhanced cellular immunity in extrapair offspring in this four-year dataset. The increased heterozygosity among extrapair offspring appeared to be a result of extrapair mates being less genetically similar than pair mates, and also less genetically similar than expected by random choice. Together with previous findings in this species, showing that the majority of females participate in extrapair copulations, our results indicate a postcopulatory cryptic female choice of genetically dissimilar males. The enhanced cellular immunity and increased heterozygosity were not related to each other, and hence our results indicate two independent genetic benefits of extrapair mating in the bluethroat.
Among passerine birds, 86% of the species investigated have been found to produce extrapair offspring (Griffith et al. 2002). Whereas explaining the adaptive function of extrapair paternity for males is regarded as straightforward, the female function is more elusive (e.g., Trivers 1972; Griffith et al. 2002; Westneat and Stewart 2003). Recent studies have questioned whether extrapair fertilizations in passerines are adaptive for females at all, based on comparative evidence that the costs of such behavior in the form of reduced paternal care outweigh any benefits females might gain (Arnqvist and Kirkpatrick 2005; Albrecht et al. 2006). In contrast, there is a growing body of evidence indicating that females in several species of passerines increase their fitness through extrapair mating (e.g., Kempenaers et al. 1997; Sheldon et al. 1997; Johnsen et al. 2000; Foerster et al. 2003; Schmoll et al. 2005; Garvin et al. 2006; Stapleton et al. 2007). However, the type of benefit varies across species, and hence the search for one general explanation may prove futile.
In some species, females may gain nongenetic (direct) benefits by being allowed to forage inside the extrapair male's territory, by being helped by the extrapair male in predator defense (Gray 1997) or brood care (Blomqvist et al. 2005), or by insuring successful fertilization of her eggs (Sheldon 1994). Alternatively, females may gain genetic (indirect) benefits, by producing extrapair offspring of higher quality than within-pair offspring (e.g., Johnsen et al. 2000; Foerster et al. 2003; Garvin et al. 2006; Stapleton et al. 2007). In socially monogamous species, female mate choice can be constrained by the number of single males, and extrapair copulations may be a strategy to modify their initial choice (Møller 1992). For example, the occurrence of extrapair offspring is associated with high genetic similarity between social pairs in several passerine species (Freeman-Gallant et al. 2003; Eimes et al. 2005; Tarvin et al. 2005; Freeman-Gallant et al. 2006), and hence females seem to avoid the negative effects of inbreeding by engaging in extrapair copulations (Pusey and Wolf 1996). Alternatively, rather than only avoiding inbreeding, the females may be targeting specific extrapair males to maximize the genetic quality of their offspring, either by choosing high-quality extrapair males (the good genes hypothesis), or by choosing extrapair males whose genome best complements their own (the compatible genes hypothesis).
A powerful way to test for genetic benefits of extrapair fertilizations is to compare within-pair and extrapair offspring raised in the same nest, that is, maternal half siblings. These nestlings are exposed to the exact same environmental conditions, and originate from the same maternal genotype. Thus, any difference between them should be caused by the differential paternal genetic contribution (Sheldon et al. 1997). In addition, comparing paternal half siblings can reveal if a genetic benefit is due to good genes per se or compatible genes (e.g., Johnsen et al. 2000; Garvin et al. 2006): whereas a genetic effect through compatible genes also predicts a difference between paternal half siblings, an effect of good genes does not.
Individual heterozygosity is a prime example of a compatibility benefit of extrapair mating, because the level of heterozygosity to a large extent depends on the relative genetic similarity of an individual's parents (Brown 1997; Charlesworth and Charlesworth 1999). Heterozygosity is positively related to survival and reproductive success in many taxa (Coulson et al. 1998; Coltman et al. 1999; Amos et al. 2001; Hansson et al. 2001; Tregenza and Wedell 2002; Charpentier et al. 2005; Pujolar et al. 2005). In passerines, there are several examples that heterozygosity correlates positively with survival, territory size, song diversity, male plumage characters, clutch size, hatching success, fledging success, and fertilization success (Hansson et al. 2001; Foerster et al. 2003; Cordero et al. 2004; Seddon et al. 2004). Furthermore, extrapair offspring have been found to have a higher heterozygosity than their maternal half siblings in blue tits Cyanistes caeruleus (Foerster et al. 2003) and tree swallows Tachycineta bicolor (Stapleton et al. 2007).
In the socially monogamous bluethroat Luscinia s. svecica, a previous study revealed that extrapair offspring have an increased cell-mediated immunity, as expressed by the swelling response to phytohemagglutinin (PHA), compared to both their maternal and paternal within-pair half siblings, lending support to the compatible genes hypothesis (Johnsen et al. 2000). In the present study, we expanded that dataset with two more years, and investigated the effect of extrapair paternity on heterozygosity, in addition to cell-mediated immunity. We employed microsatellite markers to calculate multilocus heterozygosity and pairwise genetic similarity, and examined the association between these variables and extrapair paternity in the four-year dataset on bluethroat nestlings.
Material and Methods
STUDY SITE AND STUDY SPECIES
This study was carried out in Heimdalen, Øystre Slidre municipality, southern Norway (61°25′N, 8°52′E), during May–July in 1998, 1999, 2002, and 2003. The study area is located at an altitude of about 1100 m above sea level, in the subalpine vegetation zone (Vik 1978).
The bluethroat is a small (ca. 18 g), socially monogamous, migratory passerine (Cramp 1988). The female builds a nest on the ground, lays five to seven eggs that she incubates for 13–15 days (Johnsen and Lifjeld 1995). Both parents feed the nestlings, which leave the nest after 10–14 days (Anthonisen et al. 1997). The population of bluethroats in Øvre Heimdalen has been studied since 1991, and the breeding density has been estimated to 23–38 breeding pairs per km2 (Anthonisen et al. 1997; Johnsen et al. 2000).
GENERAL FIELD METHODS
Males were caught shortly after territory establishment and females shortly after clutch completion. The birds were aged as yearlings (one-year old) or older according to the presence or absence of whitish tips on the greater wing coverts (Svensson 1992). All adult birds were individually marked using one metal band and three additional colored leg bands. Blood samples (5–25 μl) were obtained by puncturing the brachial vein, and stored in a lysis buffer for genetic analyses.
All nests were frequently visited around the expected time of hatching. After hatching the nestlings were individually marked with a permanent marker and/or by nail clipping, and weighed (to the nearest 0.05 g using a Pesola 10 g spring balance). Blood samples (5–25 μl) were normally obtained on day 2 posthatch by puncturing the femoral vein, and stored in lysis buffer. The nestlings were also weighed on day 2 as part of the blood sampling, and on day 5 through 8 as part of the PHA assay.
PHA ASSAY
The protocol for the PHA assay is described in Johnsen et al. (2000). Briefly, 0.1 mg of PHA (product number L8754, Sigma-Aldrich, St. Louis, MO) was injected subcutaneously in the outer section (metacarpus) of the right wing on day 5 (sensitizing injection) and in the inner section (ulna) on day 7 (treatment injection). The PHA was dissolved in 40 μl phosphate buffered saline (PBS) in 1998 and 1999, and in 20 μl PBS in 2002 and 2003. An identical volume of PBS only was injected at the respective positions in the left wing as a control. The reduction of volume in 2002 and 2003 was done to ease the injection of the total solution, which should not affect the swelling response as the amount of PHA was the same. The thickness of each wing was measured with a micrometer (Mitutoyo Digimatic model 543–681, Mitutoyo, Aurora, IL) at the injection site immediately before injection and 24 ± 1 h after injection at the exact same position. All measurements of a particular brood were performed by one person only. The repeatability of these measurements is found to be high in this species (Johnsen et al. 2000). The difference in swelling between the right and left wing from day 7 to day 8 was used as an estimate of cell-mediated immunity (Stadecker et al. 1977; Fairbrother et al. 2004).
GENETIC ANALYSIS
DNA was extracted using QIAamp DNA Blood Kit (QIAGEN, Venlo, The Netherlands). Microsatellite markers were amplified by polymerase chain reaction (PCR) on an ABI Prism GeneAmp PCR System 9700 (Applied Biosystems, Foster City, CA), and ran on an ABI Prism 3100 Genetic Analyser using fluorescently labeled primers. Allele sizes were determined using ABI Prism Genemappper Software version 3.0. The markers Ase19, Cuμ4, FhU2, HrU7, Mcyμ4, PAT MP 2–43, Phtr2, and Ppi2 were run for all four years. In addition Lm6, FhU3, and Pocc5 were run for 1998 and 1999, whereas PmaC25 were run for 2002 and 2003 (mean: 8.78 loci, range: 4–11 loci, for details on genotyping and locus characteristics see Johnsen et al. (1998) and Fossøy et al. (2006)). Offspring were considered as extrapair when they showed two or more allelic mismatches with their putative father. Genetic fathers were assigned using CERVUS (Marshall et al. 1998). Because we increased the number of microsatellite markers from Johnsen et al. (2000), we could identify five more extrapair males from 1998 and 1999 than in the previous study. In 1998 and 1999, the Z-linked primer Pocc5 was used to determine the sex of most (N= 380) nestlings (see Johnsen et al. 2000), whereas the remaining nestlings in 1998 and 1999, and all nestlings in 2002 and 2003, were sexed using the primers P2 and P8 (Griffiths et al. 1998).
CALCULATIONS OF HETEROZYGOSITY AND GENETIC SIMILARITY
We calculated standardized individual heterozygosity by dividing the proportion of heterozygous loci for an individual by the mean observed heterozygosity for all loci typed for that individual (Coltman et al. 1999). By standardizing, we avoid any bias toward individuals that were successfully genotyped on all markers compared to those that did not amplify on one or more marker (Coltman et al. 1999). Moreover, we also make sure that each marker will have an equal contribution to the calculation of overall heterozygosity (Amos et al. 2001).
As a measurement of genetic similarity between individuals, we estimated pair-wise genetic relatedness (Queller and Goodnight 1989), using the software Relatedness version 5.0.8 (http://www.gsoftnet.us/GSoft.html). The pairwise genetic relatedness was calculated for 1998/1999 and 2002/2003 separately because of the somewhat different marker-sets used. The allele frequencies for each period were calculated using only adult, presumably unrelated, individuals, and assuring that each individual only was included once.
STATISTICAL ANALYSIS
Linear mixed model analyses (restricted maximum likelihood (REML)) were used to compare maternal and paternal half siblings. Compared to nested ANOVA, which is the traditional method for comparing half siblings, mixed models are less sensitive to unbalanced data (Shaw 1987). Brood identity was included as a random factor to control for nonindependence among maternal half siblings, whereas male identity was included as a random factor when comparing paternal half siblings. No renestings were included in the analyses. Year, and the interaction between year and paternity were included in all models but were removed by backward stepwise exclusion when P > 0.1. For the analyses of PHA response we included body mass at the time of the treatment injection (day 7), because this factor is known to affect the PHA response in bluethroats (Johnsen et al. 2000). The normal distribution of the models was checked by diagnostic plots of the residuals. All mixed model analyses were performed in SPSS 14.0.
We used randomization tests to compare the genetic similarity of social and extrapair partners against a random distribution of similarity values between each female and all other males in the population. For this purpose we included all males either breeding in the population or having paternity in at least one nest. Some males caught by us early in the season may still have been migrating and hence not have been present in the valley during the females' fertile period. For the randomization of genetic similarity between social mates we included all males for each female, whereas for the randomization of genetic similarity between extrapair mates, we excluded the social male for each female, because he could not be considered as a potential extrapair male for that female. Both randomizations were iterated 10,000 times. The randomization tests were performed in Resampling Stats version 5.0.
To test for heterozygosity-fitness correlations, we used generalized models (GLZ) in Statistica 7.1, and included all adults from all four years. Individuals breeding in the population for more than one year were only included in the first year to avoid pseudoreplication. In all models, we controlled for age and year, and the interaction between these and heterozygosity. For each fitness variable we also included other environmental and genetic factors known to affect this variable. We removed the factors by backward stepwise exclusion when P > 0.1, always removing the interaction terms first. We also reran the models without controlling for the other genetic and environmental factors, and in all cases the effect of heterozygosity was qualitatively similar. Hence, the effects of heterozygosity were not dependent on the other variables. For binomial response variables, we used a GLZ model with a binominal distribution and logit link. For clutch size we used a GLZ model with an ordinal multinomial distribution and logit link. Total fertilization success showed a left-skewed distribution and we therefore used a GLZ model with Poisson distribution. Normality was checked by diagnostic plots of the residuals of the model.
We tested for linkage between any of our microsatellite using GENEPOP version 3.4 (http://wbiomed.curtin.edu.au/genepop/genepop_op2.html), with the following Markov chain parameters: Dememorization = 10,000, Batches = 10,000, and Iteration per batch = 10,000. For this analysis we included all adults (n= 487) from all four years, but performed the analysis separately for 1998/1999 and 2002/2003 because of the different marker-sets used. However, we found no evidence of linkage between any pair of microsatellites in our dataset (data not shown).
Results
PATERNITY
Across all four years, 94 of 188 (50%) broods contained one or more extrapair offspring, and 267 of 1032 (26%) chicks were sired by an extrapair male (Table 1). We identified the genetic father of 197 (74%) extrapair offspring. Seventy-nine broods contained both within-pair and extrapair offspring, that is, mixed paternity broods, which could be used for comparisons of maternal half siblings. Among these we gathered data on PHA swelling response in 47 broods. We identified 45 males that sired both within-pair and extrapair offspring, which could be used for comparisons of paternal half siblings. Among these we gathered PHA response data for 28 comparisons.
| Year | Broods | Chicks | ||
|---|---|---|---|---|
| 1998 | 33/52 | 62% | 87/283 | 31% |
| 1999 | 24/49 | 49% | 74/270 | 27% |
| 2002 | 24/45 | 53% | 74/235 | 31% |
| 2003 | 13/42 | 31% | 32/244 | 13% |
| Total | 94/188 | 50% | 267/1032 | 26% |
COMPARISONS OF MATERNAL HALF-SIBLINGS AND EXTRAPAIR MALES
Extrapair offspring had both a larger swelling response to PHA and a higher heterozygosity compared to their maternal (within-pair) half siblings (Table 2A, Fig. 1A). There was a significant effect of year on PHA response, but for both PHA response and heterozygosity the direction of the effect was similar in all four years (Fig. 1A). When only including the two most recent years, paternity was no longer significantly associated with PHA response (REML: F= 1.29, P= 0.26, N= 15 broods, 77 offspring). A correlation between the difference in PHA response and the difference in heterozygosity revealed no significant association between the two indirect benefits (R2= 0.02, P= 0.35, N= 47, Fig. 2A). Extrapair offspring were not significantly heavier (REML: F= 0.23, P= 0.64, estimate ± SE: −0.09 ± 0.18, N= 49 broods, 245 offspring), and did not have significantly longer tarsi when they were eight days older than their maternal half siblings (REML: F= 0.64, P= 0.42, estimate ± SE: 0.16 ± 0.19, N= 46 broods, 234 offspring).
| N | Estimate±SE | df | F | P | |
|---|---|---|---|---|---|
| (A) Maternal | |||||
| PHA Response | |||||
| Paternity | 47 | 0.155±0.048 | 207.29 | 10.45 | 0.001 |
| Body mass | 0.035±0.019 | 214.94 | 3.42 | 0.066 | |
| Year | 43.56 | 3.18 | 0.033 | ||
| Heterozygosity | |||||
| Paternity | 79 | 0.037±0.016 | 422.55 | 4.89 | 0.028 |
| (B) Paternal | |||||
| PHA Response | |||||
| Paternity | 28 | 0.175 ± 0.056 | 182.57 | 9.91 | 0.002 |
| Body mass | 0.070 ± 0.018 | 186.63 | 15.43 | <0.001 | |
| Year | 21.67 | 4.41 | 0.014 | ||
| Heterozygosity | |||||
| Paternity | 45 | 0.000 ± 0.017 | 303.18 | 0.00 | 0.989 |
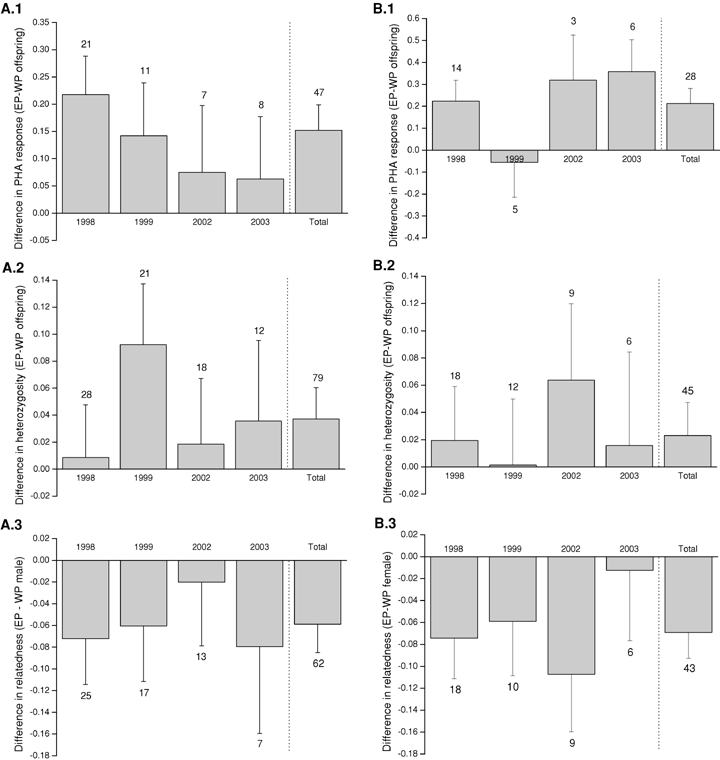
Annual variations of the differences in PHA response, heterozygosity, and parental genetic similarity for (A) maternal and (B) paternal half siblings. Bars indicate mean ± SE, and numbers above bars denote number of broods. WP, within-pair; EP, extrapair.
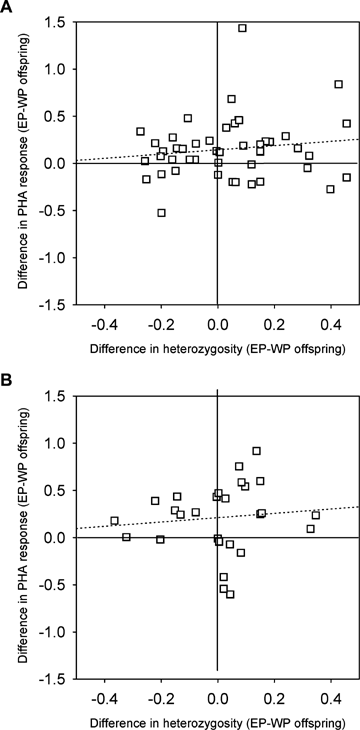
Comparison of the difference in PHA response and heterozygosity for (A) maternal and (B) paternal half siblings. The hatched lines are regressions of the difference in PHA response and the difference in heterozygosity. WP, within-pair; EP, extrapair.
To test whether the positive effects of extrapair paternity on offspring PHA response and heterozygosity was caused by local (known) or nonlocal (unknown) extrapair males, we analyzed the data separately for both male categories. Extrapair offspring sired by known males, that is, males caught by us during the field season, had a significantly larger PHA response than their within-pair half siblings (REML: F= 9.03, P= 0.003, estimate ± SE: 0.16 ± 0.05, N= 39 broods, Fig. 3A), whereas extrapair offspring sired by unknown males, that is, males not caught by us during the field season, did not (REML: F= 0.85, P= 0.36, estimate ± SE: 0.11 ± 0.12, N= 10 broods, Fig. 3A). Similarly, offspring sired by known males had a significantly larger heterozygosity than their within-pair half siblings (REML: F= 5.52, P= 0.019, estimate ± SE: 0.041 ± 0.017, N= 62 broods, Fig. 3B), whereas extrapair offspring sired by unknown males did not (REML: F= 0.022, P= 0.64, estimate ± SE: 0.017 ± 0.038, N= 23 broods, Fig. 3B).
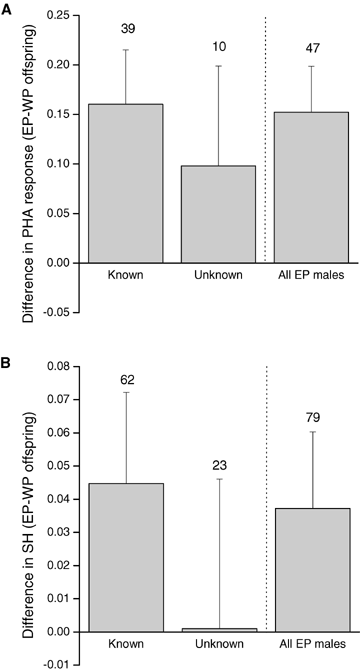
Comparison of the difference in (A) PHA response and (B) heterozygosity for maternal half siblings between known (identified sire) and unknown (unidentified sire) extrapair males. Bars indicate mean ± SE, and numbers above bars denote number of broods. WP, within-pair; EP, extrapair.
The probability that a brood would contain one or more extrapair offspring tended to increase with the genetic similarity between pair mates (GLZ: W= 3.51, P= 0.061, estimate ± SE: 1.92 ± 1.02, year effect: W= 10.04, P= 0.018, N= 182 broods). In 14 of these comparisons, the within-pair male sired none of the chicks in his brood. Because total loss of paternity might stem from a variety of causes, such as male infertility (Lifjeld et al. 2007), we reran the tests using only broods where the pair male sired at least one offspring, that is, mixed paternity broods. In this subset, the effect of genetic similarity was significant (GLZ: W= 3.90, P= 0.048, estimate ± SE: 2.14 ± 1.08, year effect: W= 9.24, P= 0.026, N= 168 broods, Fig. 4C). Furthermore, within-pair offspring from broods that contained no extrapair offspring were significantly more heterozygous than within-pair offspring from mixed paternity broods (Fig. 4B, REML: 175 broods, F= 11.77, P= 0.001, estimate ± SE: 0.07 ± 0.02).
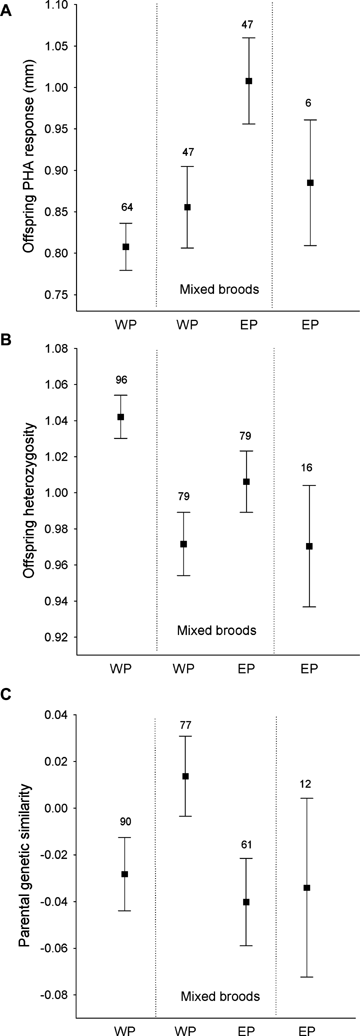
Offspring PHA response, heterozygosity and parental genetic similarity in relation to brood and paternity identity. Bars indicate mean ± SE, and numbers above bars denote number of broods. WP = within-pair, EP = extrapair.
Extrapair males tended to be less genetically similar to the female than the female's within-pair male (paired t-test: t74= 1.74, P= 0.086, within-pair males: mean =−0.003, extrapair males: mean =−0.045). When testing among males that sired at least one offspring, the difference was significant (paired t-test: t61= 2.21, P= 0.031, within-pair males: mean = 0.010, extrapair males: mean =−0.048, Fig. 1A.3). We also calculated genetic similarity based on each single microsatellite marker alone, and reran the statistical test for each marker separately. None of the microsatellite loci predicted a significant difference in genetic similarity alone (all P > 0.09), and hence the lower genetic similarity between the females and their extrapair males compared to their pair males is most likely caused by a genome-wide difference in genetic similarity rather than a difference at specific loci.
We also ran a randomization test to analyze the genetic similarity of within-pair and extrapair mates in relation to all males available in the study area. Whereas the genetic similarity of within-pair mates did not differ from a random choice of males, extrapair mates were significantly less genetically similar than expected by random choice (Combined P < 0.001, Table 3) .
| Observed | N | Randomized | P | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Social mates | ||||||
| 1998 | −0.0148 | 0.1491 | 53 | −0.0156 | 0.0182 | 0.521 |
| 1999 | 0.0133 | 0.1286 | 49 | −0.0131 | 0.0186 | 0.076 |
| 2002 | −0.0327 | 0.1612 | 50 | 0.0040 | 0.0151 | 0.006 |
| 2003 | 0.0053 | 0.1703 | 55 | −0.0009 | 0.0153 | 0.655 |
| Total | −0.0072 | 0.1491 | 207 | |||
| Extrapair mates | ||||||
| 1998 | −0.0447 | 0.1548 | 28 | −0.0152 | 0.0179 | 0.051 |
| 1999 | −0.0384 | 0.0989 | 20 | −0.0132 | 0.0189 | 0.094 |
| 2002 | −0.0423 | 0.1553 | 18 | 0.0000 | 0.0151 | 0.002 |
| 2003 | −0.0112 | 0.1926 | 7 | 0.0006 | 0.0139 | 0.197 |
| Total | −0.0392 | 0.1430 | 73 | |||
Extrapair males were not more heterozygous than the within-pair males they cuckolded (paired t-test: t76=−0.48, P= 0.64, within-pair males: mean = 0.99, extrapair males: mean = 1.00). There was also no difference in heterozygosity when testing among males that sired at least one offspring (data not shown).
COMPARISONS OF PATERNAL HALF SIBLINGS AND EXTRAPAIR FEMALES
Extrapair offspring also had a higher swelling response to PHA than their paternal, that is, the genetic offspring in the extrapair male's own nest, half siblings (Table 2B, Fig. 4A). When only including the two most recent years, the difference remained significant (REML: F= 8.38, P= 0.005, N= 9 comparisons, 74 offspring). However, extrapair offspring did not have a significantly higher heterozygosity than their paternal half siblings (Table 3, Fig. 4B). A correlation between the difference in PHA response and the difference in heterozygosity revealed no significant association (R2= 0.01, P= 0.60, N= 28 comparisons, Fig. 2B). Extrapair offspring were not significantly heavier (REML: F= 1.71, P= 0.19, estimate ± SE: −0.31 ± 0.24, N= 29 comparisons, 205 offspring), and did not have significantly longer tarsi when they were eight days older than their paternal half siblings (REML: F= 0.35, P= 0.56, estimate ± SE: 0.18 ± 0.30, N= 27 comparisons, 184 offspring).
Extrapair males were significantly less genetically similar to the female with whom they sired extrapair offspring compared to their within-pair female (paired t-test: t42= 2.94, P= 0.005, within-pair females: mean =−0.001, extrapair females: mean =−0.070, Fig. 4C). Extrapair females were not more heterozygous than within-pair females (paired t-test: t42=−0.40, P= 0.69, within-pair females: mean = 0.996, extrapair females: mean = 1.011).
GENETIC SIMILARITY, HETEROZYGOSITY, AND FITNESS
Approximately half of the variation in offspring heterozygosity was explained by parental genetic similarity, whereas maternal and paternal heterozygosity explained comparatively little (Table 4).
| Factor | r 2 | P | N |
|---|---|---|---|
| Heterozygosity offspring | |||
| Maternal heterozygosity | 0.11 | <0.001 | 166 |
| Paternal heterozygosity | 0.07 | <0.001 | 173 |
| Average parental heterozygosity | 0.15 | <0.001 | 167 |
| Parental genetic similarity | 0.51 | <0.001 | 167 |
Male heterozygosity showed a significant interaction with year on total fertilization success (Table 5A). A post hoc analysis, where we examined each year separately revealed a significant positive correlation between male heterozygosity and total fertilization success in 1998 (GLZ: W= 7.54, P= 0.006, estimate ± SE: 1.28 ± 0.47, N= 51), and a positive trend in 1999 (GLZ: W= 3.15, P= 0.076, estimate ± SE: 0.68 ± 0.38, N= 48), but no significant association in the two other years (both P > 0.21). Male heterozygosity was not significantly associated with the probability of losing paternity in his own nest, or the probability of gaining extrapair paternity (Table 5A).
| A) | Factor | N | Estimate±SE | Wald stat. | P |
|---|---|---|---|---|---|
| Response variable | |||||
| Probability of gaining extrapair fertilization | Heterozygosity | 235 | 0.22±0.93 | 0.05 | 0.82 |
| Age | 0.93±0.38 | 6.03 | 0.014 | ||
| Year | 6.71 | 0.082 | |||
| Probability of losing paternity | Heterozygosity | 172 | −1.05±0.92 | 1.3 | 0.25 |
| Age | 10.16 | 0.017 | |||
| Total fertilization success | Heterozygosity | 172 | 0.38±0.21 | 3.4 | 0.065 |
| Year | 9.48 | 0.024 | |||
| Heterozygosity×Year | 10.3 | 0.016 | |||
| Brood mean body mass | Heterozygosity | 118 | 0.11±0.05 | 6.28 | 0.012 |
| Brood mean PHA response | Heterozygosity | 111 | 0.31±0.17 | 3.40 | 0.065 |
| Age | 0.36±0.16 | 4.81 | 0.028 | ||
| Brood mean body mass | 0.05±0.02 | 4.85 | 0.028 | ||
| Prop. extrapair offspring | 0.17±0.08 | 4.83 | 0.028 | ||
| Year | 13.17 | 0.004 | |||
| Heterozygosity×Age | 4.15 | 0.042 | |||
| B) | Factor | N | Estimate±SE | Wald stat. | P |
|---|---|---|---|---|---|
| Response variable | |||||
| Laying date | Heterozygosity | 187 | 0.04±0.03 | 1.04 | 0.31 |
| Age | 0.12±0.04 | 12.23 | <0.001 | ||
| Year | 43.44 | <0.001 | |||
| Heterozygosity×Age | 8.38 | 0.004 | |||
| Clutch size | Heterozygosity | 181 | 0.23±0.89 | 0.07 | 0.79 |
| Age | 0.34±0.17 | 3.74 | 0.053 | ||
| Laying date | 0.17±0.05 | 9.77 | 0.002 | ||
| Year | 6.88 | 0.076 | |||
| Brood mean body mass | Heterozygosity | 124 | −0.01±0.04 | 0.06 | 0.80 |
| Year | 7.43 | 0.059 | |||
| Brood mean PHA response | Heterozygosity | 115 | 0.07±0.15 | 0.18 | 0.67 |
| Age | 0.29±0.16 | 3.57 | 0.059 | ||
| Brood mean body mass | 0.05±0.02 | 5.75 | 0.017 | ||
| Prop. extrapair offspring | 0.16±0.08 | 3.88 | 0.049 | ||
| Year | 13.96 | 0.003 | |||
| Heterozygosity×Age | 3.16 | 0.076 | |||
There was a significant positive relationship between male heterozygosity and the mean body mass at day 8 of the nestlings in his brood. Furthermore, male heterozygosity showed a significant interaction with male age on brood mean PHA response (Table 5A). A closer examination revealed a significant positive correlation between male heterozygosity and mean brood PHA response for older males (GLZ: W= 11.92, P < 0.001, estimate ± SE: 0.67 ± 0.19, N= 76), but no significant correlation for yearling males (GLZ: W= 0.65, P= 0.42, estimate ± SE: −0.23 ± 0.29, N= 35).
We tested whether the association between male heterozygosity and offspring PHA response was caused by genetic or environmental factors by rerunning the models using only extrapair offspring, which allowed us to include the heterozygosity of both the attending social male and the extrapair genetic father in the same analysis. Whereas the PHA response of extrapair offspring showed a significantly positive relationship with the heterozygosity of the social male, there was no such relationship with the heterozygosity of the genetic father. (GLZ: social male heterozygosity: W= 7.13, P= 0.008, estimate ± SE: 1.13 ± 0.42; genetic father heterozygosity: W= 2.20, P= 0.14, estimate ± SE: −0.82 ± 0.55; N= 30). Hence, the association between male heterozygosity and offspring PHA response is due to environmental (e.g., quality of territory or paternal care) rather than genetic effects. When looking at mean offspring body mass, neither social male nor genetic father heterozygosity showed a significant association when only including extrapair offspring (data not shown), and hence it was not possible to distinguish between a genetic or environmental effect.
There was a nonsignificant interaction between female heterozygosity and female age on brood mean PHA response. Among older females there was a tendency for a positive association between heterozygosity and brood mean PHA response (GLZ: W= 3.45, P= 0.063, estimate ± SE: 0.36 ± 0.19, N= 78), but not among younger females (GLZ: W= 0.98, P= 0.32, estimate ± SE: −0.24 ± 0.24, N= 37). Laying date was associated with a significant interaction between female heterozygosity and female age (Table 5B). Contrary to our expectations, this interaction was caused by a significant positive association for older females (GLZ: W= 11.83, P < 0.001, estimate ± SE: 0.14 ± 0.04, N= 120), that is, homozygous females laid earlier than heterozygous females. There was no such association for younger females (GLZ: W= 1.77, P= 0.18, estimate ± SE: −0.08 ± 0.06, N= 67). Neither clutch size nor brood mean body mass was associated with female heterozygosity (Table 5B).
To investigate “local” and “general” effects of heterozygosity (David 1998; Hansson and Westerberg 2002), we tested each microsatellite marker separately against each fitness variable. Of the eight markers ran for all four years combined, six showed a significantly positive single locus effect in at least one of the heterozygosity-fitness correlations (data not shown). Thus, none of the microsatellite loci made a particularly strong contribution to the heterozygosity-fitness correlations.
Discussion
Extrapair offspring had a significantly higher heterozygosity than their maternal within-pair half siblings. The increased heterozygosity appeared to be a result of extrapair mates being less genetically similar than within-pair mates, suggesting a selection for genetically dissimilar partners in the bluethroat. The previously found enhanced cell-mediated immunity in extrapair offspring was confirmed in this expanded dataset, and moreover, it seemed to be independent of the effect of increased heterozygosity. Thus, extrapair mating seems to entail multiple genetic benefits in the bluethroat.
The results of our half-sibling comparisons corroborate the conclusion of Johnsen et al. (2000) that females benefit by mating with a genetically compatible extrapair male rather than one with good genes in an absolute sense. First, the enhanced immune response of extrapair offspring was evident in both maternal and paternal half-sibling analyses, showing that it is the combination of male and female genotypes that produce the enhanced response rather than the male genotype alone. Second, heterozygosity depends more strongly on the relative genetic similarity of an individual's parents than on paternal heterozygosity per se (Table 4; Brown 1997; Charlesworth and Charlesworth 1999), and accordingly we found that the females were less genetically similar to the extrapair sires than their pair males in our dataset.
Extrapair offspring showed both an enhanced immunocompetence and an increased heterozygosity compared to their maternal half siblings (Fig. 1A). This could theoretically be due to a causal relationship between heterozygosity and PHA response. However, a correlation between the difference in PHA response and the difference in heterozygosity between half siblings did not reveal a significant relationship. In some broods, the extrapair offspring were more immunocompetent, in others they were more heterozygous, and in some broods they were both more immunocompetent and more heterozygous (Fig. 2A). Hence, there appears to be two independent genetic benefits of extrapair mating in this species. Moreover, in only a few broods, the extrapair offspring were both less heterozygous and less immunocompetent (Fig. 2A, lower left quadrant), indicating that the females in most cases obtained at least one of the genetic benefits.
In this study, we expanded the dataset in Johnsen et al. (2000) with two more years, and corroborated the effect of paternity on offspring PHA response when considering all four years combined. However, the difference between maternal half siblings was not significant when only considering the two most recent years alone. This may be attributed to the lower sample sizes in these two years, or possibly a genotype × environmental interaction. Garvin et al. (2006) found an effect of paternity on offspring PHA response in only one of two years in the common yellowthroat (Geothlypis trichas), and discovered that the effect was only evident in the coldest of the two years. Several studies have investigated PHA response among maternal half siblings, and failed to find a significant effect of paternity (Kleven and Lifjeld 2004; Kleven et al. 2006; Edly-Wright et al. 2007). Thus, it appears that this benefit may be both context and/or species dependent.
The higher level of heterozygosity among extrapair offspring in the present study is similar to the findings of Foerster et al. (2003) and Stapleton et al. (2007), who found that extrapair offspring were more heterozygous than their maternal half siblings in the blue tit and tree swallow, respectively. However, in those studies the increased heterozygosity among extrapair offspring was caused entirely by unknown sires, that is, males not caught by them during the field season, and most likely not breeding in their study areas. In the blue tit, this seemed to result from a local genetic structure in which genetic similarity decreased with increasing distance (Foerster et al. 2003, 2006). In our study however, local extrapair males increased both the heterozygosity as well as the immunocompetence among extrapair offspring (Fig. 3). In the bluethroat, most extrapair males are close neighbors (Johnsen et al. 2001, F. Fossøy, A. Johnsen, and J.T. Lifjeld. unpublished data), and they appear to be a sufficient sample for the females to obtain a genetically compatible male.
The difference in individual heterozygosity between paternal half siblings in the bluethroat was not significant, despite the fact that the males were significantly less genetically similar to the extrapair female than to their own pair female (Fig. 1B). This may in part be explained by the relatively lower sample size of paternal compared to maternal half siblings (N= 45 vs. N= 79), in combination with the difference in calculation of heterozygosity and genetic similarity. When calculating offspring heterozygosity, we only use the information from two alleles at each locus in one individual (Coltman et al. 1999), whereas for genetic similarity we use four alleles from two individuals at each locus, and furthermore, also control for the allele frequency for each allele in the population (Queller and Goodnight 1989). However, it is noteworthy that for both the maternal and paternal comparisons of offspring heterozygosity, the direction was positive in all four years (Figs. 1A.2 and 1B.2, respectively).
Heterozygosity may be an important determinant of fitness, either because genetically depauperate individuals are more vulnerable to the negative effects of deleterious recessive alleles or because heterozygous individuals are more fit than homozygous ones due to genetic overdominance (Charlesworth and Charlesworth 1987). We found that heterozygous males showed an increased fertilization success in some years and produced heavier and more immunocompetent offspring (Table 5). The latter association appeared to result from a “good parent” effect because it was the heterozygosity of the attending male, not the genetic father, which was important for offspring immunocompetence. We also investigated the contribution of each separate microsatellite marker to the heterozygosity-fitness correlations, and found no evidence of individual markers being responsible for the relationship. Hence, it is more likely that the heterozygosity-fitness correlations are caused by a genome-wide heterozygote advantage rather than a heterozygote advantage at a specific locus (David 1998; Hansson and Westerberg 2002). In other passerines, heterozygosity is found to correlate positively with a number of fitness-related variables (Hansson et al. 2001; Foerster et al. 2003; Cordero et al. 2004; Seddon et al. 2004). Hence, a high level of heterozygosity is likely to bring an increase in fitness and extrapair offspring should therefore perform better than their maternal half siblings. Contrary to our expectations, homozygous old females laid earlier than heterozygous old females. Assuming that laying early is positive for fitness, this may suggest that homozygous females had an advantage by getting an early start of breeding. However, the fact that old heterozygous females tended to produce offspring with higher immunocompetence suggests that any advantage of laying early may be offset by a disadvantage in terms of lower offspring quality for homozygous females.
Genetically similar pairs were more likely to produce extrapair offspring than less genetically similar pairs, which resulted in within-pair offspring from nonmixed paternity broods being significantly more heterozygous than within-pair offspring from mixed paternity broods (Fig. 4B). Furthermore, there appeared to be a directional choice of genetically dissimilar extrapair partners. How then are females able to select genetically dissimilar males? Either the females have to recognize the genetic similarity to their social male and potential extrapair males in the population and then decide whether to copulate extrapair or not, or else they have to “blindly” copulate with more than one male and rely upon a postcopulatory choice of sperm (Eberhard 2000; Birkhead and Pizzari 2002; Bernasconi et al. 2004). In a precopulatory female choice scenario, only females paired with less-compatible males are expected to engage in extrapair copulations, whereas in a cryptic postcopulatory choice scenario, all females may benefit by mating with more than one male to increase the probability of being fertilized by a compatible male. In the bluethroat, recent evidence suggests that most females copulate with extrapair males although not all of them produce extrapair offspring (Fossøy et al. 2006), lending support to the postcopulatory choice mechanism rather than a precopulatory choice based on phenotypic cues. We therefore hypothesize that the females copulate readily with extrapair males, in addition to their pair male, and that the least genetically similar male has the highest probability of fertilizing the eggs (i.e., a genetically loaded raffle sensu Ball and Parker (2003)). This does not require any a priori female knowledge of the genetic similarity to either the pair male or neighboring males. Males that are genetically dissimilar to their pair female will therefore have a high chance of obtaining paternity, whereby female extrapair copulations may not result in extrapair fertilizations. In addition, there may be a stochastic effects of sperm numbers (Parker 1990), as pair males are likely to have a sperm competition advantage through higher copulatory access. This could explain why most pair males sired one or more offspring in most broods. How the actual postcopulatory mechanism works is however unknown, but an intriguing possibility is that it results from an interaction between particular substances on the surface of sperm and egg (Vacquier 1998; Palumbi 1999; Evans 2000). Alternatively, selection might take place within the female reproductive tract (Birkhead and Brillard 2007).
In summary, we found a selection for genetically dissimilar mates in the bluethroat, resulting in extrapair offspring being more heterozygous than their within-pair half siblings. Moreover, this effect appeared to be independent of the enhanced immune response among extrapair offspring in this species. Our results therefore indicate that female bluethroats obtain two different compatible genes benefits via two different genetic pathways.
Associate Editor: K. Hughes
ACKNOWLEDGMENTS
This work was supported by several grants from the Norwegian Research Council. The immune experiment was approved by the Norwegian Animal Research Authority. We would like to thank G. Bjørnstad for laboratory assistance, and V. Andersen, R. Dahl, K. Fossøy, V. Fossøy, V. A. Larsen, O. Putot, H. Pärn, M. Snøtun, and C. Sunding for field assistance and R. V. Alatalo and P. O. Dunn for valuable comments.




