REPRODUCTIVE CONFLICTS AFFECT LABOR AND IMMUNE DEFENSE IN THE QUEENLESS ANT DIACAMMA SP. “NILGIRI”
Abstract
In many species of social Hymenoptera, totipotency of workers induces potential conflicts over reproduction. However, actual conflicts remain rare despite the existence of a high reproductive skew. One of the current hypotheses assumes that conflicts are costly and thus selected against. We studied the costs of conflicts in 20 colonies of the queenless ant Diacamma sp. “nilgiri” by testing the effects of conflicts on labor and worker immunocompetence, two parameters closely linked to the indirect fitness of workers. In this species, the dominant female is the only mated worker (gamergate) and monopolizes reproduction. We experimentally induced conflicts by splitting each colony into two groups, a control group containing the gamergate and an orphaned group displaying aggressions until a new dominant worker arises. Immunocompetence was assessed by the clearance of Escherichia coli bacteria that we injected into the ants. Time budget analysis revealed a lower rate of labor and especially brood care in orphaned groups, supporting the existence of a cost of conflicts on labor. Fifteen days after splitting, a lower immunocompetence was also found in orphaned groups, which concerned workers involved and not involved in conflicts. We propose that this immunosuppression induced by conflicts could stem from stress and not directly from aggression.
Animal societies can be viewed as harmonious and cooperative systems, however conflicts between members of the society can occur. These conflicts result from divergent interests between individuals of the same sex mainly over reproduction. In most vertebrate societies, dominance interactions that can include aggressive behaviors lead to a hierarchy where dominant individuals try to monopolize reproduction (Emlen 1995; Clarke and Faulkes 1997; Mock and Parker 1997). Such conflicts can be found in primitively eusocial insects (Gadagkar 1991; Keller and Vargo 1993; Peeters 1997). In highly eusocial insects, these conflicts are partly solved by the extreme division of reproductive labor with only the queen caste monopolizing reproduction. The worker caste, instead, performs all the other tasks of the colony, which includes foraging, brood care, and maintenance of the nest. Because of the haplodiploid system of sex determination of Hymenoptera (haploid males are produced by arrhenotokous parthenogenesis whereas diploid females result from fertilized eggs), relatedness asymmetries between colony members generate specific conflicts between queen and workers (Bourke and Franks 1995; Crozier and Pamilo 1996). In particular, conflicts over the production of males by workers or the optimal colony sex ratio have largely been investigated as they provide elegant tests of the kin selection theory (Bourke and Chan 1999). However in many cases, even though conflicts are predicted, no evidence of manipulation can be detected, indicating that the conflicts are already resolved. For example, eggs laying by workers rarely occurs in monogynous colonies, even though this is predicted by kin selection theory (Ratnieks 1988).
Several hypotheses are proposed to explain why actual conflicts are often absent in situations in which they should be expected. One of the leading hypotheses suggests that conflicts are costly and thus pacific solutions are selected for (Ratnieks and Reeve 1992; Bourke and Franks 1995). For example, the cost of worker reproduction (West-Eberhard 1975; Cole 1986) could favor the spread of self-restraint or worker policing alleles in populations, resulting in the increase of social harmony (Ratnieks 1988). Furthermore direct aggressions between colony members appear to be very rare in insect societies, which indirectly suggests that they should be associated with high costs. Fighting behaviors are described between queens after pleometric foundation (Röseler 1991; Choe and Perlman 1997), between workers and eventually queens for male production in stingless bees (Peters et al. 1999), or between workers for dominance and reproductive status in some formicoxenine and queenless ponerine ants (Heinze et al. 1994; Monnin and Peeters 1999). In the queenless ponerine ants, the queen caste does not exist and workers have retained the ability to reproduce leading to basic conflicts over reproduction. In the monogynous queenless species, with a singly mated reproductive worker (called a gamergate), aggressions are just used for the establishment of the gamergate in orphaned colonies. Thereafter workers restrain their reproduction as long as the gamergate keeps a correct level of oogenesis, which can be detected by a fertility signal based on cuticular hydrocarbons (Monnin et al. 1998; Cuvillier-Hot et al. 2004a, b). This switch from aggressive to chemical regulation of reproduction strongly suggests that aggressions are costly.
Even though costs of conflicts are often assumed to be of major importance for understanding the evolution of conflicts and how they are solved, few studies have estimated such costs. An energetic cost of worker aggressions was observed in orphaned colonies of Pachycondyla obscuricornis, through a higher metabolic rate measured by the total CO2 emission of the colony (Gobin et al. 2003). As the time budget of a worker is limited, workers involved in aggressive behaviors could also invest less time in labor, which would decrease colony efficiency. Such a decrease of workload in aggressive workers has been observed in different species (Cole 1986; Schmid-Hempel 1998; Monnin and Peeters 1999; Monnin and Ratnieks 1999). Direct costs on the quality of individuals have still remained unexplored. In this study we proposed to address this question by investigating potential costs of conflicts on immune defense. Although this approach is new, immune defense could be an interesting trait to consider for two reasons. First, the level of immune defense is known to affect the probability of resisting a pathogen infection (Adamo 2004). It should therefore be linked to worker survival and colony productivity, all the more in social insects, for which living in groups composed of closely related individuals facilitates disease transmission. Second, it is now generally assumed by ecological immunologists that immune defense is costly and integrating immunity as a life-history trait has been revealed to be useful in understanding the evolution of life-history strategies in insects (Lochmiller and Deerenberg 2000; Rolff and Siva-Jothy 2003; Schmid-Hempel 2005).
The invertebrate immune system is based on both cellular and humoral components and infection stimulates a range of diverse defensive responses (Gillespie et al. 1997). Hemocytes attach to invading organisms and then isolate them by phagocytosis or by forming an organized multicellular capsule around them. These responses are often associated with proteolytic activation of the phenoloxydase zymogene, present in the hemolymph. Another component of insect immune response to pathogens is the synthesis by fat body and hemocytes of a variety of antimicrobial proteins and peptides, which are secreted in the hemolymph. Insect immunity thus relies on complex interactions between humoral and cellular components, which should be considered when trying to assess the relative strength of the immune system (Adamo 2004).
In this study, we investigated costs of aggressive reproductive conflicts in a queenless monogynous species Diacamma sp. “nilgiri,” both on working efficiency and on quality of individuals, assessed by their level of immune defense. We studied an immune response involving several components, by measuring the clearance of Escherichia coli bacteria that we injected into the ants. To assess the cost of conflicts, aggressive interactions were induced by splitting each colony into two groups, a control group (with gamergate) and an orphaned group (without gamergate). Behavioral studies allowed us to test for an effect of conflicts on workload in orphaned groups. The existence of a cost of conflicts on immune defense and its origin within orphaned groups was also investigated. Reproductive conflicts could have two kinds of costs. First, a stress response may be induced for all individuals of the orphaned groups, which could negatively affect immune defense as observed in many species (Lackie 1988; Apanius 1998). A lower immune response should thus be expected in most workers of orphaned groups. Second, aggression by itself could represent a cost. In such a case, a decrease of immune defense in orphaned groups would especially affect individuals involved in aggressive interactions.
Materials and Methods
STUDIED SPECIES
We conducted the study on an Indian population of Diacamma which is highly related to Diacamma ceylonense and referred to as “nilgiri” (Baudry et al. 2003). All the species of the genus Diacamma are queenless and have one singly mated worker called a gamergate. In all species, except in Diacamma sp. “nilgiri,” the conflicts over reproduction are reduced by a peculiar behavior (L. Cournault and C. Peeters, pers. comm.; Peeters et al. 1992) in which the gamergates bites off a pair of thoracic appendages (called gemmae) of all emerging workers (Fukumoto et al. 1989; Peeters and Higashi 1989; Peeters and Billen 1991). By doing so, she prevents them from mating and reproducing sexually (Peeters and Higashi 1989), therefore removing any potential conflict over the reproduction of females. Interestingly, in Diacamma sp. “nilgiri,” in which mutilation does not occur, the regulation of reproduction is similar to the one found in many monogynous queenless species. In an orphaned colony, aggressive interactions are used to determine a hierarchy and the future gamergate. In Diacamma sp. “nilgiri,” these aggressions can include antennal boxing, biting, immobilizations (several workers holding another worker for hours), thoracic attacks (repeated attacks on gemmae), and sting smearing (Peeters et al. 1992, L. Cournault and C. Peeters, pers. comm.). Cuvillier et al. (2004a) demonstrated, in a different queenless species, that once the gamergate is established, aggressions are replaced by chemical fertility signaling. Aggressive interactions can therefore be observed after the death of a gamergate or after colony fission. Even though the details of this mode of colony foundation are not known, it implies that after fission one of the groups of workers will not have a gamergate, and might thus show aggressive behaviors.
ANTS COLLECTION AND REARING
Twenty colonies of Diacamma sp. “nilgiri” were collected in February and November 2004 near Gundlupet, Tamil Nadu (mean initial colony size ± SE: 278.9 ± 15.0). The colonies were reared in the laboratory in plaster nests at 25°C with 12 h light–12 h dark cycle and fed ad libitum on Tenebrio molitor pupae and crickets. Workers in this species are monomorphic, with a body size of about 1.5 cm and very few intracolonial size variation (Karpakakunjaram et al. 2003). All ants were individually marked with spots of colored paint, and each colony was observed before the start of the experiment to identify the gamergate, the foragers, and the nurses.
EXPERIMENTAL GROUPS
Aggressive behavior is known to occur in orphaned groups whereas it is not observed when the gamergate is present (Peeters et al. 1992). To induce social conflicts, we therefore split each initial colony into two groups: the control group containing the gamergate and the orphaned group without the gamergate. We randomly allocated the workers to the two groups, while ensuring that they were identical in size and composition with respect to the proportion of foragers and nurses. The brood was also equally shared. This process marked the start of the experiment (day zero). The size of these groups varied among colonies from 52 to 80 ants (mean group size ± SE = 70.2 ± 2.2; see Table 1). If the size of the initial colony exceeded 160 individuals, a maximum of 80 ants was used in each group.
| Colony | N workers | Total number of aggressions | N days | N dead workers | Behavioral scan | |
|---|---|---|---|---|---|---|
| Control g. | Orphaned g. | |||||
| 1 | 80 | 1104 | 8 | 2 | 13 | − |
| 2 | 80 | 1363 | 8 | 5 | 3 | |
| 3 | 80 | 970 | 8 | 6 | 1 | − |
| 4 | 80 | 606 | 8 | 5 | 5 | |
| 5 | 52 | 220 | 8 | 9 | 2 | + |
| 6 | 75 | 496 | 8/15 | 6/7 | 7/10 | |
| 7 | 67 | 1015 | 8/15 | 3/3 | 8/12 | + |
| 8 | 67 | 767 | 8/15 | 8/8 | 2/2 | |
| 9 | 72 | 1478 | 8/15 | 5/7 | 4/4 | + |
| 10 | 81 | 360 | 8/15 | 2/3 | 1/3 | |
| 11 | 82 | 325 | 8/15 | 1/2 | 4/7 | + |
| 12 | 78 | 37 | 8/15 | 0 | 1/1 | |
| 13 | 70 | 377 | 8/15 | 10/11 | 4/8 | + |
| 14 | 78 | 867 | 15 | 0 | 7 | |
| 15 | 57 | 431 | 15 | 1 | 2 | − |
| 16 | 53 | 753 | 15 | 5 | 6 | |
| 17 | 62 | 435 | 15 | 6 | 4 | + |
| 18 | 58 | 58 | 15 | 7 | 6 | |
| 19 | 68 | 720 | 15 | 3 | 3 | + |
| 20 | 63 | 705 | 15 | 3 | 7 | |
BEHAVIORAL OBSERVATIONS
After splitting, we estimated the intensity of aggressions in orphaned groups by counting the number of aggressive interactions during a continuous 30-min observation period twice daily until the end of the experiment. Donor and receiver were recorded. Antennal boxing and sting smearing were rarely observed. Aggressive behavior began on the first or second day after colony splitting, and lasted for about eight days. Furthermore, for 14 colonies, scans of the orphaned and control groups were performed twice a day during the first eight days of the experiment. Each scan consisted in recording the behavior performed by each individual. Ants were considered resting when they remained totally motionless or when they moved only antennas. We also recorded working behaviors, comprising of brood care, foraging, and nest maintenance. As the time spent on nest maintenance was low, we included it with foraging in the category “working behaviors others than brood care” along with nest provisioning. Other behaviors were also recorded, including social interactions, grooming, moving around the nest and eating, but were not individually considered in the analysis.
IMMUNOLOGICAL MEASUREMENTS
We tested the effect of conflicts on immunocompetence at two different times: eight days after splitting, when the aggressions were at the highest level in most colonies, and 15 days after splitting, when very few aggressions were observed. The immunocompetence measurement was performed eight days after splitting for five colonies, 15 days after splitting for seven colonies, and both eight days and 15 days after splitting (on different individuals) for eight colonies (see Table 1). The immune response was evaluated by measuring the resistance to bacterial infection (see below). To perform the test on the same day for the orphaned and control groups of a given colony, the immune response was measured on a maximum of 40 ants in each group. To ensure a representative distribution of ants according to their involvement in conflicts, ants were categorized by the number of aggressions received (0, 1–4, 5–9, 10–19, 20–49, 50–100, >100) and the proportion of each class was reproduced in the subsample.
The antibacterial response was measured as the persistence of a bacterial infection with E. coli strain CIP 103470 (Pasteur Institute Collection, Paris), after injection in the hemolymph, as described in Gorman and Paskewitz (2000). The resistance of an organism to a pathogen depends on different parameters, including the existence of behavioral adaptations, the level of genetic variation in factors that determine pathogen intrusion and recognition, and the efficiency of the immune response, which corresponds to immunocompetence. Indeed, a variation of resistance to a pathogen between individuals is not always related to differences in the capacity of the immune system and caution is required when interpretating the results (Apanius 1998). In our experiment, defense mechanisms other than immune system should not interfere: first, the behavioral and cuticular lines of defense are not involved in resistance because the pathogen is directly introduced in the hemolymph. Second, it is unlikely that E. coli and ants have coevolved because E. coli is a general and ubiquitous pathogen. Third, ants from a given colony are very closely related genetically, thus the problem of genetic variation of resistance based on individual differences in pathogen recognition should not be important.
Bacterial solutions were prepared by diluting an overnight culture in an LB culture medium to a concentration of 300 bacteria/μL. Bacteria were counted using a neubauer hemocytometer. Ants from orphaned and control groups from a given colony were injected on the same day with the same bacterial solution. Prior to injection we cold-anesthetized ants and cleaned the sternites with 70% ethanol. One microliter of the bacterial solution was then injected ventrally through the intersegmental membrane between the third and fourth abdominal segments using a 10-μL syringe connected with a flexible capillary to a 0.3-mm-diameter dental needle. Injected ants from each group were kept together in a plastic box with a water supply, separate from the rest of the colony. Ten hours later, most ants had survived (97.7%) and the hemolymph was sampled using a disposable graduated capillary tube through a hole performed in the abdomen. Hemolymph was sampled from each ant. As much liquid as possible was removed (this manipulation is lethal), and the volume, varying from 0.3 to 1.5 μL, was diluted 100-fold in an LB culture medium. From this solution, 30 μL were spread on LB agar plates containing tetracycline at a concentration of 10 μg/mL (the E. coli strain used is resistant to tetracyclin). The plates were incubated overnight and then scored for the number of E. coli colonies. The level of immune response for each ant was then characterized by the bacterial count, a low bacterial count indicating a high immune response and vice versa. Ants that died during immunological manipulation or for which no hemolymph could be sampled were excluded from the analysis (in total 3.4% of manipulated ants). The concentration of bacteria injected and the time between injection and hemolymph sampling were chosen to allow a high survival of the ants but providing a sufficiently large variation in the number of bacteria between individuals.
DATA ANALYSIS
For each colony, the level of aggressiveness in control and orphaned groups was compared using a Mann–Whitney test on the mean number of aggressions per individual. To test for the effect of conflicts on behavior and immune defense, analyses were carried out using mixed models, with treatment (control/orphaned) as fixed factor and with colony and interaction treatment by colony as random factors. For behavioral data, the dependant variable was the ratio of the number of scans for which the individual was observed performing a given behavior to the total number of scans, and the binomial distribution was used (lmer function in R 2.4 for Windows). For immune defense data, the bacterial count (number of bacteria in 30 μL of 100-fold diluted hemolymph) was the dependant variable, following a negative binomial distribution (glmmADMB function in R 2.4 for Windows). Effects were tested by comparing two models, one having the term of interest removed, using the likelihood ratio test (LRT). To compare model 1 and model 2, the LRT statistic was calculated as 2 [log(L2) − log(L1)] and follows a χ2 distribution. The degrees of freedom was the difference in the numbers of parameters between the models. In all tests the interaction terms were removed when not significant.
Concerning immune defense data, given that we always plated the same volume of LB-hemolymph mixture, the number of bacteria counted reflected the concentration of bacteria in hemolymph. For such a measure it would be relevant to control for the global level of hydration of ants. These data were not available but we included in the models the volume of hemolymph that we were able to remove from ants as it might positively correlate to the total volume of hemolymph present in the body. Moreover, the colonies tested 15 days after splitting included eight colonies for which at least half of the workers were removed eight days after splitting (see Table 1). To control for a possible disturbance induced by the reduction of the colony size, the interaction treatment by size reduction was initially included in the model.
To test for a direct cost of aggressions on immune defense, we performed an analysis within orphaned groups using a mixed model with the bacterial count as dependant variable. As above, we used a negative binomial distribution for the bacterial counts. The independent variables were the volume of hemolymph removed from ants and the number of aggressions received or given by ants and the colony as random factor. The effect of the interaction factor number of aggressions by colony was not considered because our data were not appropriate to test such an effect because the range of aggressions experienced by ants largely differed among orphaned groups.
Results
AGGRESSIONS AND TIME ACTIVITY BUDGETS
The number and pattern of aggressions varied strongly among the orphaned groups (see Table 1), but for each colony, aggressiveness was significantly higher in orphaned than in control group (Mann–Whitney analysis on the mean number of aggressions per individual, all P < 0.05). Splitting the colonies into two groups was thus a successful way to induce aggressions in the orphaned groups as predicted. For colonies in which immunocompetence was only measured eight days after splitting observations were stopped at this date, whereas they were performed until 15 days for other colonies. However this should not have highly influenced the total of aggressions counted because most aggressions occurred during the first eight days. Indeed the total number of aggressions counted in orphaned groups for colonies observed for eight days (total ± SE: 709.4 ± 129.4, n= 5) was even higher than in for colonies observed for 15 days (total ± SE: 562.1 ± 105.1, n= 15).
In the models analyzing behavioral data, the effect of the interaction treatment by colony (considered as a random effect) was never significant, and was therefore removed. Individuals spent less-time working in orphaned groups than in control groups (17.7% of reduction in total labor,  , P= 0.0039; see Table 2 and Fig. 1). More precisely, brood care was significantly affected (23.7% of reduction,
, P= 0.0039; see Table 2 and Fig. 1). More precisely, brood care was significantly affected (23.7% of reduction, 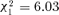 , P= 0.0145) whereas the decrease in nest maintenance and foraging was not significant (11.2% of reduction,
, P= 0.0145) whereas the decrease in nest maintenance and foraging was not significant (11.2% of reduction,  , P= 0.221). No difference in resting rate was found between control and orphaned groups (
, P= 0.221). No difference in resting rate was found between control and orphaned groups (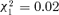 , P= 0.88).
, P= 0.88).
| Frequency (%, ±SE) | ||
|---|---|---|
| Control group | Orphaned group | |
| Total labor | 35.6±0.9 | 29.3±0.8 |
| Brood care | 17.7±0.7 | 13.5±0.6 |
| Others | 17.9±0.9 | 15.9±0.8 |
| Resting | 29.2±0.7 | 28.8±0.7 |
| Aggressive interactions | 0.2±0.04 | 5.9±0.4 |
| Others | 35.1±0.6 | 35.9±0.6 |

Percentage of scans (± SE) where workers from control (white bars) and orphaned groups (black bars) were recorded performing a given behavior for each colony. (A) Total labor; (B) Brood care; (C) Resting.
The time spent on aggressive behaviors was quite low (5.9%), and therefore not sufficient to explain the lower working activity in orphaned groups. This was checked by calculating the frequency of each behavior by each individual after having removed the data of the scans involving an aggressive act. As expected, the same qualitative results were found (data not shown), indicating that the difference in workers time budget between the two groups cannot simply be explained by the occurrence of aggressive acts in orphaned groups. The social conflicts in orphaned groups therefore induced disturbance that decreased the working effort.
EFFECTS OF CONFLICTS ON IMMUNE RESPONSE
Data on antibacterial response were collected for a total of 1920 ants from 20 colonies. The level of immune response of each ant was characterized by the bacterial count 10 h after injection, a low bacterial count indicating a high immune response and vice-versa. The results suggested a lower bacterial count in orphaned groups eight days after splitting, although the effect is only marginally significant ( , P= 0.042, n= 999; main statistical results are reported in Table 3; see also Fig. 2). On the other hand, a significantly higher bacterial count in orphaned groups was detected 15 days after splitting (69.6% of increase in bacterial count,
, P= 0.042, n= 999; main statistical results are reported in Table 3; see also Fig. 2). On the other hand, a significantly higher bacterial count in orphaned groups was detected 15 days after splitting (69.6% of increase in bacterial count, 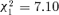 , P= 0.008, n= 921). This indicated a lower resistance to infection for groups displaying conflicts. The volume of hemolymph removed from ants had no significant effect on the bacterial count (see Table 3). Note that there was no effect of treatment on the volume of hemolymph sampled (linear mixed model on hemolymph volume, eight days after splitting, treatment: F1, 12= 0.23, P= 0.64, random factors, colony: Z-value = 2.16, P= 0.016, colony by treatment: Z-value = 1.34, P= 0.090; 15 days after splitting, treatment: F1, 14= 0.36, P= 0.56, random factors, colony: Z-value = 1.76, P= 0.039, colony by treatment: Z-value = 1.52, P= 0.064). The interaction treatment by group size-reduction was not significant (
, P= 0.008, n= 921). This indicated a lower resistance to infection for groups displaying conflicts. The volume of hemolymph removed from ants had no significant effect on the bacterial count (see Table 3). Note that there was no effect of treatment on the volume of hemolymph sampled (linear mixed model on hemolymph volume, eight days after splitting, treatment: F1, 12= 0.23, P= 0.64, random factors, colony: Z-value = 2.16, P= 0.016, colony by treatment: Z-value = 1.34, P= 0.090; 15 days after splitting, treatment: F1, 14= 0.36, P= 0.56, random factors, colony: Z-value = 1.76, P= 0.039, colony by treatment: Z-value = 1.52, P= 0.064). The interaction treatment by group size-reduction was not significant ( , P= 0.19) which showed that the possible disturbance induced by the removal of 40 individuals eight days after splitting did not influence the effect of treatment at 15 days. The effect of colony on bacterial count can be assessed by the variance component associated with this random factor (variance parameter ± SD, eight days: 1.30 ± 0.54; 15 days: 0.76 ± 0.29). In our models the confidence intervals of this parameter do not overlap 0 (95% confidence interval, eight days: [0.41–2.19]; 15 days: [0.28–1.24]), suggesting that the variance associated with the colony is significantly higher than 0 both at eight and 15 days. This is not surprising because individuals from the same colony share a common environment as well as 75% of their genotype (in a monogynous and monoandrous colony).
, P= 0.19) which showed that the possible disturbance induced by the removal of 40 individuals eight days after splitting did not influence the effect of treatment at 15 days. The effect of colony on bacterial count can be assessed by the variance component associated with this random factor (variance parameter ± SD, eight days: 1.30 ± 0.54; 15 days: 0.76 ± 0.29). In our models the confidence intervals of this parameter do not overlap 0 (95% confidence interval, eight days: [0.41–2.19]; 15 days: [0.28–1.24]), suggesting that the variance associated with the colony is significantly higher than 0 both at eight and 15 days. This is not surprising because individuals from the same colony share a common environment as well as 75% of their genotype (in a monogynous and monoandrous colony).
| Effect tested | Models compared | LRT | ||||
|---|---|---|---|---|---|---|
| Fixed terms/random terms | Log(L) | npar | χ2 | df | P | |
| 8 days | ||||||
| Treatment | 1. T+V/Col | −6157.34 | 6 | 4.12 | 1 | 0.042 |
| 2. V/col | −6159.40 | 5 | ||||
| Volume | 1. T+V/T(Col)+Col | −6157.08 | 7 | 1.32 | 1 | 0.25 |
| 3. T/T(Col)+Col | −6157.74 | 6 | ||||
| Treatment(col) | 1. T+V/T(Col)+Col | −6157.08 | 7 | 0.52 | 1 | 0.47 |
| 5. T+V/Col | −6157.34 | 6 | ||||
| 15 days | ||||||
| Treatment | 1. T+V/Col | −5046.34 | 6 | 7.10 | 1 | 0.008 |
| 2. V/col | −5049.89 | 5 | ||||
| Volume | 1. T+V/T(Col)+Col | −5046.35 | 7 | 0.24 | 1 | 0.62 |
| 3. T/T(Col)+Col | −5046.47 | 6 | ||||
| Treatment(col) | 1. T+V/T(Col)+Col | −5046.35 | 7 | 0.02 | 1 | 0.89 |
| 5. T+V/Col | −5046.34 | 6 | ||||

Comparison of mean bacterial count (± SE) in control groups (white bars) and orphaned groups (black bars). (A) Eight days after splitting. (B) 15 days after splitting.
If aggressions per se were directly responsible for the decrease of immune response in orphaned groups, a positive relation between the level of aggressions and the amount of decrease of immune response in orphaned groups should be expected. However the difference in bacterial count between the two treatments within colonies was not dependent on the total number of aggressions in orphaned groups, neither eight days after splitting (linear regression: F1,11= 0.74, P= 0.41) nor 15 days after splitting (F1,13= 0.51, P= 0.49).
In the same line of reasoning, we also tested whether all ants from orphaned groups were equally affected by conflicts, or whether only those really involved in conflicts suffer specific costs. To do so, we performed the analysis of bacterial counts as for complete groups (with a mixed model, using negative binomial distribution), keeping in orphaned groups either the ants not suffering aggressions or the ants directly suffering aggressions. Any worker was likely to receive one or two aggressions accidentally and thus we considered only ants that received at least three aggressions in this latter group. Eight days after splitting the treatment was not significant for ants not suffering aggressions (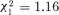 , P= 0.281, n= 799), and close to the significance for the ants suffering aggressions (
, P= 0.281, n= 799), and close to the significance for the ants suffering aggressions ( , P= 0.052, n= 703). Fifteen days after splitting, the effect of treatment was significant both when considering ants not receiving aggressions (
, P= 0.052, n= 703). Fifteen days after splitting, the effect of treatment was significant both when considering ants not receiving aggressions ( , P= 0.016, n= 744), and ants suffering aggressions (
, P= 0.016, n= 744), and ants suffering aggressions ( , P= 0.028, n= 635) with again a higher bacterial count in orphaned than in control groups. Therefore the cost of conflicts on immunocompetence does not appear to be due to a direct effect of aggressive interactions. Note that similar results were found whatever the number of aggressions considered as a threshold.
, P= 0.028, n= 635) with again a higher bacterial count in orphaned than in control groups. Therefore the cost of conflicts on immunocompetence does not appear to be due to a direct effect of aggressive interactions. Note that similar results were found whatever the number of aggressions considered as a threshold.
Individuals that gave and received aggressions might not be the same, making it pertinent to conduct the same analysis also considering the number of aggressions given. There was a positive correlation between the number of aggressions received and given, both eight days after splitting (r2= 0.13, P < 0.0001) and 15 days after splitting (r2= 0.15, P < 0.0001). Not surprisingly, the analyses based on the number of aggressions given gave qualitatively similar results to the ones based on aggressions received (see above). There was no effect of treatment eight days after splitting (considering nonaggressive ants: 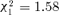 , P= 0.075, n= 808; considering aggressive ants:
, P= 0.075, n= 808; considering aggressive ants:  , P= 0.18, n= 694) whereas 15 days after splitting workers from orphaned groups had a higher bacterial count (considering nonaggressive ants:
, P= 0.18, n= 694) whereas 15 days after splitting workers from orphaned groups had a higher bacterial count (considering nonaggressive ants:  , P= 0.0062, n= 776; considering aggressive ants:
, P= 0.0062, n= 776; considering aggressive ants:  , P= 0.081, n= 603).
, P= 0.081, n= 603).
Within orphaned groups, there was no significant correlation between the number of aggressions and the bacterial count neither eight days after splitting (aggressions received: 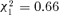 , P= 0.42; aggressions given:
, P= 0.42; aggressions given:  , P= 0.37), nor 15 days after splitting (aggressions received:
, P= 0.37), nor 15 days after splitting (aggressions received:  , P= 0.153; aggressions given:
, P= 0.153; aggressions given: 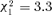 , P= 0.069).
, P= 0.069).
Discussion
AGGRESSIONS AND TIME ACTIVITY BUDGETS
The splitting of colonies was followed by a period of conflicts in orphaned groups. The number of aggressions in these groups was highly variable, which confirmed previous observations made on this species (Peeters et al. 1992; L. Cournault and C. Peeters, pers. comm.). The age distribution of individuals within the orphaned groups is probably a major factor determining the intensity of reproductive conflicts because young individuals are more prone to seek access to reproduction.
Workers in orphaned groups spent less-time working. The difference was quite low for nest maintenance and foraging, in accordance with a study on the social wasp Polistes dominulus where foraging was not affected by conflicts following queen removal (Strassmann et al. 2004). However, the reduction was more important for brood care. Because at a given time in a colony a large percentage (around 30%) of workers are resting they may represent a reserve that could be mobilized if necessary. We could have then expected a lower percentage of inactive workers in orphaned groups. However, our results did not confirm such a role of reserve because no difference was found in the percentage of inactive workers between groups, even if the time spent on working behaviors was lower in orphaned groups.
Two nonexclusive hypotheses can be put forward to explain the decrease in labor, and especially brood care, in orphaned groups. First of all, dominant individuals could realize a trade-off between working and fighting for time and metabolic reserves. In colonies of Temnothorax allardycei, Cole (1986) analyzed the time budget of the three top ranking ants and observed a negative relation between dominance activity and brood care. However, working rate was not related to aggressions in colonies of bumblebees (Foster et al. 2004). Additionally, a recent study on hover wasps demonstrated that helpers adjust their working effort according to the probability of attaining breeding status themselves (Field et al. 2006). The authors experimentally removed a high-ranking worker and found that subordinates who consequently rose in the hierarchy worked less afterwards. The evolutionary basis of this response should lie in a trade-off between working effort, which increases the indirect component of their fitness, and future direct fecundity and survival. A similar phenomenon could occur in queenless ants, even if the hierarchy is not always well defined. In our species, removing the gamergate increases the probability for the workers to inherit the nest, and could lead them to invest less in collective labor, at least temporally. A second hypothesis would be that conflicts between workers competing for reproduction may disturb peaceful workers and prevent them from working (Cole 1986). In accordance with this hypothesis, aggressions occurred most of the time close to the brood area and fighting ants frequently induced movements in nearby quiet ants. Working ants may also waste some time in submissive behavior to the dominant individuals patrolling.
Whatever the mechanisms leading to a reduced workload in orphaned groups, the likely consequence of a disturbance of brood care is a delay in the brood development or the death of part of the brood (not measured here). This should result in a decrease of colony productivity and represent a cost of conflicts. A cost of conflicts was detected in P. obscuricornis at the colony level by Gobin et al. (2003). A higher metabolic rate, measured by total CO2 emission, was observed in orphaned groups displaying conflicts, thus demonstrating an energetic cost. Both ours and their results strongly suggest a cost of conflicts at the colony level that could negatively affect productivity.
COSTS OF CONFLICTS ON IMMUNE RESPONSE
The technique used here to measure the level of immune defense is a test of resistance to a pathogen. However it is often argued that the immune response differs depending on the type of pathogen and that a variation in resistance to infection by a particular pathogen could result from a change in allocation of resources to different elements of the immune system (Adamo 2004). Similarly, a decrease in maintenance of the immune system may remain undetected if it affects a component of the immune system little involved in the resistance to the tested pathogen. As a consequence it would be relevant to associate different measurements of immunity. However, most immune defense measurements require a sample of hemolymph, and applying them to the same individuals is not possible in organisms like ants in which the volume of hemolymph is limited. To circumvent such problems, we used an immune response, the clearance of a generalist bacteria, that involves several components of the immune system.
Eight days after splitting, a trend of a higher immunocompetence in orphaned groups was detected. The effect of treatment remained close to the significance when, in orphaned groups, only ants suffering aggressions were included, but not when these ants were excluded. Such increase in the antibacterial response could first correspond to a proximate effect of physical harm caused by aggressive interactions because the immune system may be partly activated if the cuticle is damaged (Brey et al. 1993; Plaistow et al. 2003). This effect could also result from a prophylactic increase of immune defense to face infections that could be associated with cuticle injuries (Barnes and Siva-Jothy 2000; Adamo et al. 2001; Calleri et al. 2007). Anyway, our results clearly showed an absence of immunosuppression in orphaned groups on the eighth day.
Fifteen days after splitting, the immune response was lower in workers from orphaned groups. The differences found between orphaned and control groups from the same colony could be partly due to the distribution of workers during splitting, as age and status of workers may influence their immune defense. However as ants were randomly distributed between groups this should not result in the pattern found, which is a lower immunocompetence in most orphaned groups. The intensity of immunosuppression was not related to the level of aggressions in the orphaned groups. For instance, a lower immunocompetence was found in some orphaned groups with a low number of aggressions (e.g., colony 12) even when workers involved in aggressions (giving or receiving aggressions) were excluded from the analysis.
All these results suggest that aggressions alone do not directly account for the cost of conflicts measured in orphaned groups. Instead, we propose that a conflicts-derived stress response mediates the immunosuppression found in most colonies. In Diacamma sp. “nilgiri,” conflicts frequently induce avoidance or submission reactions in surrounding workers and could represent a stress factor in orphaned colonies for most of the workers including those not involved in the hierarchy. Moreover, removing the gamergate could itself represent a stressful situation for ants even if the intensity of conflicts remains low. Many studies on social insects have clearly showed that the presence of the reproductive can easily be perceived by workers, either directly by specific pheromones produced by the queen (Sledge et al. 2001; Hannonen et al. 2002) or the gamergate in queenless species (Monnin et al. 1998; Tsuji et al. 1999; Cuvillier-Hot et al. 2004a), or indirectly by the presence of the brood (Endler et al. 2004).
Much is known about stress in vertebrates, with many studies arguing that stress could lead to an immunosuppression (Apanius 1998). In invertebrates, research on stress is relatively recent and few studies have been performed until now. However some experiments performed on molluscs (Lacoste et al. 2001a, b) and crustaceans (Le Moullac et al. 1998; Perazzolo et al. 2002; Pascual et al. 2003) strongly suggest that invertebrates also present a typical stress response whose basic characteristics are relatively similar to those found in vertebrates (Ottaviani and Franceschi 1996). Moreover in vitro and in vivo experiments in molluscs have also revealed that a stress response could exert an inhibitory effect on immune function and increase susceptibility to pathogen infection (Stefano and Salzet 1999; Lacoste et al. 2002; Malham et al. 2003). During a stress response, immunity could be temporarily down-regulated to make nutrients available for other organismal processes that have a higher priority (Sheldon and Verhulst 1996). Indeed, immune defense has been shown to be costly in many studies on invertebrates (Rolff and Siva-Jothy 2003; Schmid-Hempel 2005) including social insects (Moret and Schmid-Hempel 2000). An alternative hypothesis proposes that, during a stress response, the immune system could be down-regulated to prevent hyperactivation and ensuing autoimmune response, which are more susceptible to occur in a context of high physical workload and production of stress proteins (Raberg et al. 1998).
In our study, the immunosuppression was qualitatively the same whatever the level of aggressions given or received by ants, suggesting that the conflicts were as stressful for dominants as for subordinates. However, our data appeared insufficient to address such questions because we do not know the level of immune response of the individuals before being involved in conflicts. In social vertebrates, comparative studies have shown that the relative levels of glucocorticoids (indicative of the level of stress) in dominants and subordinates vary among species. Differences in glucocorticoids concentrations between dominants and subordinates were found in some species, but not consistently (Clarke and Faulkes 1997; Creel 2001; Goymann and Wingfield 2004; Sands and Creel 2004; Creel 2005; Poisbleau et al. 2005). Concerning immune defense, higher quality of dominant individuals compared to subordinates has been reported in some vertebrates and invertebrates studies (Zuk and Johnsen 2000; Koskimäki et al. 2004; Rantala and Kortet 2004; Hawley et al. 2006). We did not find such relation in Diacamma sp. “nilgiri” because the immunocompetence of workers within orphaned groups was not dependant on the number of aggressions they gave or received. Using different measures of immune defense is required to confirm this result because in vertebrate and invertebrates species a higher immune response has been found in dominants for some immune assays but equivalent or even weaker for others (Ahtiainen et al. 2006; Hawley et al. 2007).
In conclusion, our study provides arguments in favor of the existence of a decreased immune defense in orphaned groups probably linked to the stress response generated by the absence of the reproductive and not to a direct cost of aggressions. Moreover the immunosuppression was not detected at eight days after splitting, when aggressiveness is still high, but was observed on the fifteenth day, when conflicts are over. This suggests that the effect of the stress induced by conflicts on immune defense is not instant but is slightly delayed. In addition, the behavioral study confirmed that conflicts affect worker labor and especially brood care, potentially leading to a decrease in colony productivity. In queenless ants, once dominance relationships stabilize, conflicts stop, and are replaced by a chemical fertility signaling (Monnin et al. 1998; Cuvillier-Hot et al. 2004a, b). Our results support that this limitation of conflicts to the period of hierarchy formation could have evolved because of their costs. In species where workers are fertile, maintaining dominance interactions in presence of the reproductive female allows workers to rapidly start reproduction if she dies. Such a mode of reproduction regulation would be selected only if the benefits outweighs the costs, which is more likely in species where workers can produce both males and females. Indeed, physical aggressions of workers in the presence of gamergates are common in ponerine ant species in which workers can mate. In Diacamma sp. “nilgiri,” the benefits of maintaining a hierarchy appear to be insufficient to balance the costs of conflicts. Even though different studies have applied the principles of ecological immunology to social insects (Traniello et al. 2002; Rolff and Siva-Jothy 2003; Vainio et al. 2004; Evans and Pettis 2005; Schmid-Hempel 2005; Baer et al. 2006), our study is, to our knowledge, the first to have investigated the costs of conflicts on immune defense.
Associate Editor: N. Wedell
ACKNOWLEDGMENTS
We thank S. Baratte for his help in collecting colonies in the field; F. Rineau and J. B. Caillau for their contribution to behavioral observations; and A. Lenoir, T. Monnin, and C. Peeters for their helpful comments on the manuscript. This study was supported by the French Ministry of Research ‘Action concertée incitative jeunes chercheurs’ (No. 5183).




