PARALLEL EVOLUTIONARY PATTERNS IN MULTIPLE LINEAGES OF ARCTIC ARTEMISIA L. (ASTERACEAE)
Abstract
Early observers of plant evolution in the Arctic have noted a floristic similarity with temperate alpine regions and a predominance of high ploidy levels. The aim of our study was to survey these and other traits in multiple closely related but independently evolved lineages of Artemisia. Our phylogenetic study was based on 133 taxa using 3′-ETS and ITS, and on data on morphology, karyology, distribution, and ecological preferences. We compared Arctic lineages with sister groups and tested whether patterns were significantly different. We found: (1) Artemisia has independently adapted to Arctic habitats 13–18 times; (2) There were no ecological preferences of putative progenitors that might determine the colonization success in the Arctic, although most sister groups were centered in steppe habitats; (3) Plant height was distinctly reduced in Arctic lineages; (4) Arctic lineages contained no more polyploids than their respective sister groups or taxa from other habitats; (5) Enlarged flower heads have evolved repeatedly, probably for better pollinator attraction. This strategy could be a substitute for polyploidy, which is typical in other Arctic taxa. Stronger pollinator attraction should result in better outcrossing and higher heterozygosity in the offspring, which is among the main effects of polyploidy.
The Arctic is the natural end point of many ecological gradients and evolutionary research on Arctic environments has established several general and long-standing evolutionary hypotheses for organisms. Mammals from cold regions, for example, are generally larger and have a relatively smaller surface area than their southern relatives (Bergmann's rule; Bergmann 1847), and extremities and other exerted body parts (e.g., ears and limbs) are comparatively small, giving better protection from cold (Allen's rule; Allen 1877). Similar general rules for higher plants of the Arctic are rare and our aim was to find common evolutionary patterns in a plant genus with high specific diversity in the Arctic.
The best-known general pattern in Arctic plants is probably a higher proportion of polyploidy relative to more southerly areas (Hagerup 1932; Johnson and Packer 1965). A striking example is the Svalbard flora (latitude 80°N) where 78% of the taxa are (often highly) polyploid (Brochmann et al. 2004). Polyploidization (reviewed in Stebbins 1984, 1985; Levin 2002; discussed with regard to Artemisia L. in McArthur and Sanderson 1999) creates permanently heterozygous lineages. This increased heterozygosity might help species survive a greater array of future evolutionary challenges or to facilitate niche differentiation in Arctic plants. Several alternative hypotheses (Brochmann et al. 2004; Meyers and Levin 2006) for elevated ploidy levels in the Arctic exist (often based on chance effects) but their relative importance has not yet been evaluated. One of our aims in this study was to map the geographical distribution of polyploidy in Artemisia and eventually evaluate different explanations in a phylogenetic framework. For example, repeated independent transitions to polyploidy upon arrival in the Arctic in a single genus would rule out chance explanations.
THE ORIGIN OF THE ARCTIC FLORA
Two general hypotheses have been suggested for the geographical and ecological origin of the Arctic flora (summarized in Murray 1995; Abbott and Brochmann 2003): (1) Some taxa may have evolved more or less in situ by gradual adaptation to cooling climate from late Tertiary forests or neighboring habitats. (2) Arctic taxa may have their ancestors in more southerly alpine regions that share some macroecological patterns with the Arctic and, therefore, (A) alpine taxa may have migrated along mountain ridges, for example, the Rocky Mountains or Far Eastern mountains northward to the Arctic (e.g., Yurtsev 1962). (B) Alternatively, during cold stages of the Pleistocene Arctic and alpine vegetation zones came into close contact and could have mutually exchanged taxa. This latter theory is well supported, for example, the glacial or postglacial migration routes in Eurasia have been roughly determined for several alpine/Arctic species with currently disjunct ranges (e.g., Schönswetter et al. 2003, 2006). In contrast, the evidence of older migrations or of other geographical regions as potential source areas for Arctic lineages is rather limited (Abbott and Brochmann 2003). For our study group Artemisia we therefore address the following questions: Where do the sister groups of the Arctic lineages occur? Have the Arctic taxa evolved preferentially from taxa of certain vegetation types or biomes? Which morphological changes occurred upon the arrival in the Arctic compared with non-Arctic sister groups? Was adaptation to Arctic habitats simple, occurring in many different lineages independently, or were the many Arctic species the outcome of one or few major radiations?
THE STUDY GROUP ARTEMISIA
We have chosen Artemisia from the Asteraceae–Anthemideae–Artemisiinae as our study group, because with 33 species reported it ranks among the most diverse genera of the Arctic, exceeded only by a few other genera such as Carex L., Oxytropis DC, or Salix L. This is valuable because a potentially high number of independent Arctic lineages avoids the n= 1 problem and allows to conduct statistical tests. Artemisisa comprises altogether ca. 450 species and has a circumpolar and northern hemispherical distribution with few species in South America and Africa (Ling 1994b). It is especially diverse in mountains, steppe regions, and semideserts of Central Asia, but about 50 species each are also reported for Europe and North America (Tutin et al. 1976; Shultz 2006). The delineation of subgenera within Artemisia traditionally rests on capitulum characters such as number of flowers, distribution of uni- and bisexual flowers, head shape, hairy or glabrous receptacle, and indumentum, but none of the earlier classifications proposed was fully corroborated in recent molecular studies (cf. Torrell et al. 1999; Watson et al. 2002). These and other molecular phylogenetic studies published to date analyzed the internal transcribed spacer (ITS) regions of nuclear rDNA for some 60 species and few segregate genera (Kornkven et al. 1998; D'Andrea et al. 2003; Vallès et al. 2003). Despite the discrepancy with molecular studies we informally use here a slightly modified comprehensive classification developed by Ling in a series of papers (Ling 1991, 1992, 1994a, 1995a, b) to facilitate comparison with older literature.
Artemisia comprises annuals, perennials, and woody bushes and many species are clearly wind pollinated (e.g., Korobkov 1981; Wang 2004). However, there is evidence for a transition to insect pollination because of colorful capitula and sticky pollen in some species (Hesse 1979; Wagenitz 1987; Vallès et al. 2001). Self-compatibility has been observed in the few species tested thus far (Estes 1969; McArthur and Durant 1994). Chromosome number in diploids is most often 2n= 18 or 16, the highest number known is 2n= 144 (Pellicer et al. 2007), and aneuploidy is frequent. Within several species, different ploidy levels have been observed that were considered to rest on autopolyploidy (e.g., McArthur and Sanderson 1999). However, based on characters such as intermediate morphology and geographical distribution, Ehrendorfer (1964) suggested that several Eurasian polyploids were in fact allopolyploids. Although much cytological work has been done in Artemisia (reviewed by McArthur and Sanderson 1999; Vallès and Garnatje 2005) definite evidence for allopolyploidy is scarce (but see Clausen et al. 1940; Estes 1969; Persson 1974). The distribution of ploidy levels in Artemisia on a large geographical scale has not been analyzed, but appears especially interesting in view of the general hypotheses on plant evolution in the Arctic as outlined above.
Materials and Methods
TAXON SAMPLING
Our primary objective was to study all 33 Arctic Artemisia species, however, A. aleutica Hultén and A. arktisibirica Korobkov could not be included. Several subspecies confined to Arctic habitats were additionally sampled and are usually treated as separate taxa in the following. We further sampled most species from Mongolia and the majority of Russian or Siberian species to achieve good representation of areas geographically close to the Arctic. This set of taxa was complemented with ITS sequence data available from European Molecular Biology Laboratory (EMBL) (http://srs.ebi.ac.uk; segregate genera and North American, European, and South Asian material) and some more of our own material (see online Supplementary Appendix S1). ITS sequence entries in EMBL with poor sequencing quality (more than 10 unknown positions) were not used in this study as they substantially lowered the signal/noise ratio of the data for phylogenetic reconstruction. Notably missing are species from minor sections native to Southern Asia. Ajania fastigiata (C. Winkl.) Poljakov, Chrysanthemum mongolicum Y. R. Ling, and Chrysanthemum naktongense Nakai also from the subtribe Artemisiinae were chosen as outgroups following Vallès et al. (2003). Plants were identified using standard floras (Poljakov 1961; Hultén 1968; Tutin et al. 1976; Korobkov 1987, 1992; Krasnoborov 1997; Shultz 2006) and voucher details are given in the online Supplementary Appendix S1. In total, our molecular sample consisted of 133 taxa.
CHOICE OF DNA SEQUENCE MARKERS
We supplemented the stock of available sequenced ITS regions of nuclear ribosomal DNA with data from our new taxa. We also tested the chloroplast regions trnT-L spacer, trnL-F spacer, and rpl16 intron (primers from Shaw et al. 2005), but these proved to be rather conserved and even combining them did not provide a sufficient number of variable characters. In contrast, the external transcribed spacer (ETS) region of nuclear ribosomal DNA was highly variable in the 12 species tested. However, the first two-thirds (5′-region) contained a multitude of minor to very large indels (up to 200 bp) and repetitive duplications. Only the 3′-region could be aligned reliably. It contained slightly less variation than the ITS region, and was selected as a second marker.
MOLECULAR TECHNIQUES AND PHYLOGENETIC ANALYSIS
Total genomic DNA was isolated from herbarium and silicagel-dried leaf material using DNA binding columns (Macherey and Nagel, Düren, Germany). ITS primers A, B, C, and D were taken from Blattner (1999) and external ETS primers 1F and 2L-18S from Baldwin and Markos (1998). The 3′-ETS region was amplified with our internal primer ETS 615F (GGG GAT GTT GTC CGT AAA GG) and ETS 2L-18S using standard PCR protocols (annealing at 55°C). After column purification (Macherey and Nagel) the PCR primers and the ET Terminator Kit were used for sequencing on a MegaBACE 1000 capillary sequencer (both GE Healthcare, Little Chalfont, U.K.). The software Sequencher 4.6 (Genecodes Corporation, Ann Arbor, MI) was used to edit and combine forward and reverse strand chromatograms and to align sequences manually. It was not necessary to clone sequences from polyploid taxa in vivo, due to absence or low frequency of double peaks (when compared with chromatograms from diploid specimen). Potentially informative indels for both spacers from unambiguously aligned regions were coded separately and included in the final parsimony analysis.
Three datasets (ITS, ETS, and the combined dataset) were analyzed using PAUP* b10 (Swofford 2002) under the maximum parsimony criterion. Standard parameters used were: Multrees, 100 random taxon additions, maxtrees = 100,000, and tree-bisection-reconnection (TBR). Branch support was evaluated with 100 bootstrap replicates, the closest taxon addition option, and maxtrees = 5000. A Bayesian analysis of the combined dataset (without coded indels) was performed using MrBayes version 3.1.2 (Huelsenbeck and Ronquist 2006) with initial settings: nst = 6, rates = invgamma, ngen = 3,000,000, nchains = 4, samplefreq = 100. Tree-building parameters (after checking the treespace using the SUMP command) were: burnin = 3000, contype = halfcompat. The branch lengths of the Bayesian tree were visualized using TreeView version 1.6.6 (Page 1996).
DATA ON MORPHOLOGY, DISTRIBUTION, ECOLOGY, AND KARYOLOGY
We collected data on growth form, plant height, head diameters, and ecological preferences from the following sources: Poljakov (1961), Hultén (1968), Tutin et al. (1976), Korobkov (1981, 1987, 1992), Krasnoborov (1997), Shultz (2006), and Ling et al. (unpubl. ms.). The growth form of perennial Artemisiae is sometimes difficult to ascribe to either shrubs, subshrubs, or perennial herbs, due to frequent transitions. True perennial herbs that persist during unfavorable seasons by herbaceous, soft and fleshy organs are rare. Most perennials form (to varying extent) woody parts that may be situated below ground or form aerial parts as shrubs or subshrubs. In case of doubt or ambiguity in the literature we made double entries in our data matrix. When a range of head diameters was given we used the average. Because plant heights often depend on seasonal and environmental conditions, we used the maximum values given in the literature as an indication of plant heights under optimum field conditions.
Presence/absence data for each taxon were recorded for two different ecological classifications separately, that is, vegetation type and habitat. Vegetation types were: tundra, forest, forest margins, meadow, steppe, semidesert, and desert. Habitats were: mountains, hillsides, rocky slopes, coastal rocks, seashore, saline/alkaline soils, riverbanks, and ruderal sites. The geographical distribution of species in Eurasia was scored following the divisions of Meusel and Jäger (1992; cf. Fig. 1): Siberia, East Asia, continental Central Asia (comprising Mongolia and Northern China; main precipitation in summer), Middle Asia (Turanian region; main precipitation during the winter), South-West Asia (the oriental region), Europe in a broad sense, including floristically similar North Africa and extending to the Ural Mountains, and most of Africa with Macaronesia. North America south of the Arctic was divided into the sectors used by Shetler and Skog (1978), but Alaska was treated as separate due to its floristic relatedness with the Far East of Asia. The Arctic in our study is the northern most part (usually north of the tree line) of several of these zones as phytogeographically defined by Meusel and Jäger (1992) and Yurtsev (1994). Data on chromosome numbers were obtained from the IPCN database (http://mobot.mobot.org/W3T/Search/ipcn.html) and many other sources specified in the online Supplementary Appendix S1.
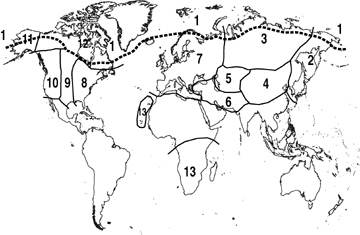
An outline of the distribution of Artemisia and the floristic regions used in our analysis. Additionally, there are a few taxa of Artemisia known from South America. Floristic regions are simplified from Shetler and Skog (1978), Meusel and Jäger (1992), and Yurtsev (1994). The numbers also refer to the columns 1–13 in Figure 3. 1 = The Arctic region represents a combination of the Arctic sections of regions 2, 3, 7, 11, and 12 above the dashed line, 2 = East Asia, 3 = Siberia, 4 = Central Asia, 5 = Middle Asia, 6 = South West Asia, 7 = Europe, 8 = East North America, 9 = Central North America, 10 = West North America, 11 = Alaska, 12 = North North America, 13 = Macaronesia and Africa.
DEFINING THE UNITS OF COMPARISON: ARCTIC VERSUS NON-ARCTIC TAXA
The precise distribution of many species of Artemisia is insufficiently known and some are very widespread. After several tests we decided to rank them under four types of distribution. (1) The Arctic s. str. group consists of species that have been basically recorded from the Arctic only. They may occur sporadically out of Arctic habitats but only in the adjacent floristic regions East Asia, Siberia, Alaska, or North North America. Artemisia norvegica Fr. with its occurrence in Scotland, Norway, and the Polar Ural region is additionally part of our Arctic s. str. group. (2) The Arctic s. l. group consists of species recorded at least once in the Arctic zone, but may also occur in other floristic regions than defined for the Arctic s. str. groups. This group also comprises very widespread taxa such as ruderal A. vulgaris L. By definition the Arctic s. str. taxa are part of this Arctic s. l. group. The other contrasting groups are termed (3) non-Arctic s. str. for species that are not growing exclusively in Arctic habitats and (4) non-Arctic s. l. for species that have never been recorded from the Arctic.
We next defined the closest non-Arctic relatives of Arctic (s. str. or s. l.) lineages using the molecular phylogeny (exact definitions of sister groups are given in the online Supplementary Appendix S2). We made some conventions to maximize the number of comparisons. Generally, we used all branches with Bayesian support > 0.5. In polytomies the Arctic lineages were considered as monophyletic and all non-Arctic taxa were regarded as the sister group. Subspecies were mostly treated as independent taxa. We are aware that the inclusion of rather weakly supported branches may have decreased phylogenetic accuracy. Therefore, we also took a more conservative approach, examining sister group relationships between Arctic and non-Arctic taxa under different levels of boot-strap support (>50%, >70%) only. The more conservative approach lowered the number of sister group comparisons included for statistical analysis, but the results remained qualitatively similar. In our view, these findings justify including weakly supported branches of the Bayesian tree to increase statistical power.
STATISTICAL ANALYSIS OF EVOLUTIONARY TRENDS IN ARCTIC TAXA
To evaluate whether the colonization of Arctic habitats was correlated with repeated and, therefore, probably adaptive responses in Artemisia, we conducted several statistical tests. All procedures were performed manually and we followed the formulas of Fowler and Cohen (1990) and the critical tables of test statistics provided therein. Because we sometimes tested two variables on almost the same dataset (head size and plant height; vegetation type and habitat) we applied sequential Bonferroni corrections (Holm 1979). The Arctic s. l. and Arctic s. str. calculations were, however, from different datasets and there was no need to correct them. When there were multiple character states in a sister group, for example, often there are several preferred habitat types, we selected the state or several states that were most frequent in that clade. Double and missing data entries, and equal values between sister groups explain the different numbers of items (N) used for subsequent statistical tests. To compare the maximum plant heights of Arctic taxa and their sister groups we defined maximum height classes (0–20 cm = class 1, 20–40 cm = class 2, and so forth) and applied Wilcoxon's test for matched pairs (two tailed), which orders the class differences between sister groups and evaluates whether the distribution of differences is significantly skewed toward one group. The same test was also applied for head diameters. For the comparison of ploidy levels between Arctic lineages and their sister groups we had to apply the less-powerful sign test because the differences are qualitative only (higher versus lower ploidy level). When one clade was heterogeneous in ploidy we took the lowest level known because this is probably the primitive state of an originating lineage and, therefore, the state probably present at the lineage splits to its sister group.
We also wanted to test whether certain ecological preferences of progenitors were a precondition for the successful colonization of the Arctic. As a proxy, we assumed the ecology of the non-Arctic progenitor of an Arctic taxon to be that of the current ecological preferences of the sister group. We collated the presence data of ecological classes across all non-Arctic sister groups (separately for vegetation types and habitats). The observed occurrences found in the actual sister groups were compared with the number of occurrences one would expect if the sister groups of the Arctic lineages were chosen at random from the non-Arctic taxa. Therefore, we calculated the relative frequency of ecological preferences across all non-Arctic taxa (separately for sister groups of the Arctic s. str. and s. l. groups). We then employed a χ2 analysis to test whether certain ecological preferences are relatively over-represented in the sister groups or whether this could simply reflect the frequency of ecological classes present in our sampling.
Results
PHYLOGENETIC ANALYSIS OF ITS AND ETS
Our complete ITS dataset consists of 133 taxa, 47 of which were taken from EMBL. Sequences of 13 more taxa were also already published but we verified them here (and used our own data), and 73 taxa were new for ITS. The length of the ITS region (incl. 5.8S rDNA) varied from 488 bp to 643 bp. The pairwise distances (Kimura-2-parameter) within Artemisia ranged from 0 to 12.7%. The alignment was 661-bp long and slightly ambiguous for three short regions. Including these regions did not alter the resulting topologies, so we used them in the final calculations. Thirteen indels were coded separately and included in parsimony calculations. The parsimony analysis resulted in more than 100,000 trees (saved and swapped to completion due to memory limitations) with 634 steps. The backbone of the strict consensus tree (not shown) was essentially congruent with the results of previous studies (Watson et al. 2002; Vallès et al. 2003) with numerous supraspecific taxa being polyphyletic. The complete ETS dataset consists of 86 taxa (unfortunately, many taxa from Watson et al. 2002 and Vallès et al. 2003 were not available). The length of the 3′-ETS region used varied from 368 bp to 423 bp. The pairwise distances (Kimura-2-parameter) within Artemisia ranged from 0 to 11.4%. The alignment was unambiguous and 423-bp long. One indel was coded in addition and included in parsimony calculations. The parsimony analysis resulted in more than 100,000 trees (saved and swapped to completion) with 183 steps. The strict consensus tree (not shown) contained less taxa but was congruent with the ITS tree when only nodes were compared that received bootstrap support >50% in separate analyses of the one or the other dataset. Therefore, both datasets were combined, missing ETS sequences were treated as unknown data, and all further results refer to the combined dataset only.
The parsimony analysis of the combined dataset resulted in more than 100,000 tress (saved and swapped to completion) with 846 steps (not shown). The Bayesian analysis of the combined dataset (see Fig. 2) was congruent in most parts but better resolved than the strict consensus parsimony tree. A comparison of branch support shows this pattern consistently because all branches with bootstrap support (see Fig. 2, below branches) receive high Bayesian support (see Fig. 2, above branches) and there are several branches with Bayesian support only. We have indicated in Figure 2 that the topology does not support the traditional classifications in several details. For example, subgenus Seriphidium was shown to consist of two independent groups and three of the four sections analyzed within subgenus Artemisia were polyphyletic. The placement of some segregate genera within Artemisia also points to shortcomings of the traditional taxonomy. Details of character evolution and the major relationships within Artemisia, however, were already discussed in some previous studies. Only the placement of A. comata Rydb. close to species growing in geographical proximity is rather odd considering that from a morphological point of view it clearly belongs to the A. arctica/norvegica group. We have rechecked our identification and the sequencing results and for the time being we conclude that the misleading rDNA region in A. comata probably results from introgression. We did not find evidence for hybridization in other taxa. The exact identification of one individual of A. cf. leucophylla (Turcz. ex Besser) Pamp., was unusually difficult and should be considered with caution. Consequently, A. comata and A. cf. leucophylla were excluded from all later sister group comparisons.
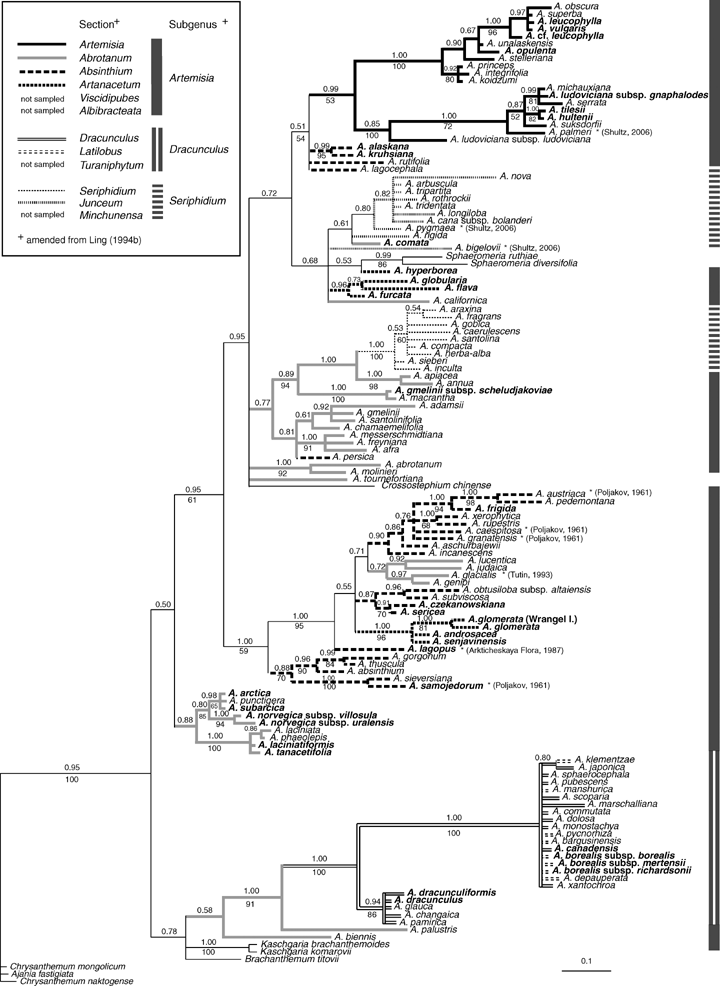
Result of the Bayesian and the parsimony bootstrap analyses and a comparison with the traditional classification (Bayesian support > 0.50 above and parsimony bootstrap support > 50% below branches). A slightly modified and informal version of the traditional classification by Ling (mainly 1991, 1992, 1994a, 1995b) was used for most species. The topology shows that the traditional system is not in accordance with molecular data as shown before (e.g., Watson et al. 2002; Vallès et al. 2003). Minor exceptions are denoted with an asterisk, and are classified differently according to the references appended. Taxa in bold occur in Arctic habitats (Arctic s. l. group).
MAPPING THE GEOGRAPHICAL DISTRIBUTION
The sister taxa to Artemisia and basal branches in the genus are from Asia, thus it is most parsimonious to assume an Asian origin. In Figure 3, columns 1–13, we have indicated in which major floristic regions the sampled taxa occur. Depending on the exact mapping (polytomies and ambiguous parsimony optimizations) there have been 16–22 migrations from Eurasia into America, among which are 14 species of putative Asian origin presently distributed in both continents. There have been two to five possible migrations from America into Asia. Among our 34 taxa (including subspecies) recorded from Arctic habitats (our Arctic s. l. group; see Figs. 2 or 3, taxa in bold) there are about five very widespread, sometimes ruderal species, for example, A. vulgaris L. or A. frigida Willd., that grow even in far distant regions to the South and both in Eurasia and North America. Our Arctic s. l. group consisted of 34 taxa (including subspecies but excluding A. comata and A. cf. leucophylla), which evolved in about 18 independent lineages (see Fig. 3, in bold) and our Arctic s. str. group consisted of 21 taxa in 13 lineages (see Fig. 3, gray boxes).
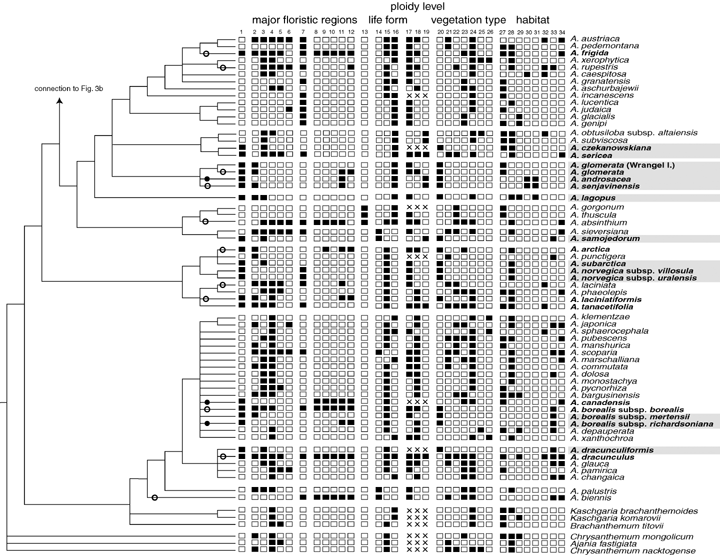
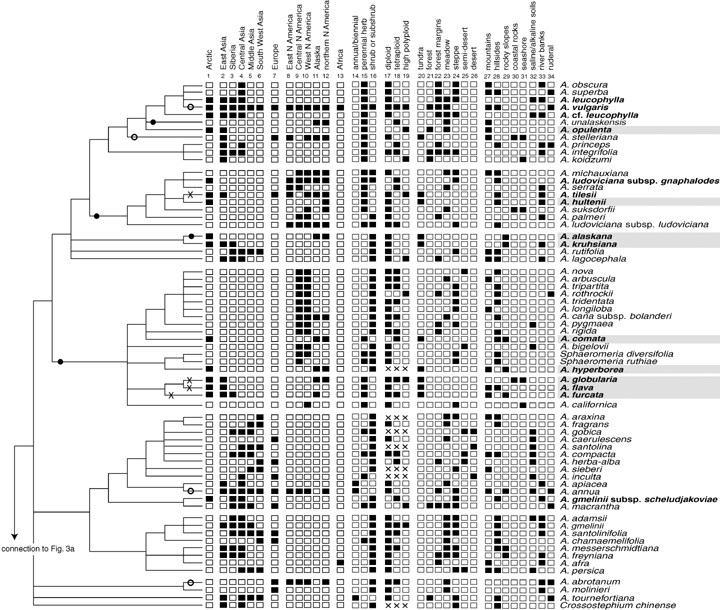
Mapping the geographical distribution, life form, ploidy levels, and ecological preferences on the phylogeny. The data were compiled from various sources detailed in the Materials and Methods section and the online supplementary Appendix S1. The floristic regions used are outlined in Figure 1. Column 1 is a joint region and indicates whether a taxon occurs in the Arctic part of the major floristic regions or not. Additionally, it is indicated which taxa we regard as members of the Arctic s. l. and the Arctic s. str. groups (bold and bold with gray boxes, respectively). Only A. comata and A. cf. leucpophylla were not included (see text). Filled circles on branches indicate possible migrations from Eurasia to North America, open circles indicate intraspecific disjunctions between Eurasia and North America possibly with an Eurasian origin, crosses indicate migrations or disjunctions that may have proceeded in the opposite direction. Some migrations could be optimized differently, for example, for the A. glomerata-clade or in polytomies. This could change the direction and true number of migration events.
GROWTH FORMS, FLOWERING HEADS, AND PLANT HEIGHT
Figure 3, columns 14–16, shows the distribution of growth form characteristics along the phylogeny, with double entries in columns 15 and 16 in case of uncertainties. Most taxa are perennial and the eight annual species included here seemed to have originated at least five times. Only minor clades of the phylogeny were distinguished by a uniform habit.
Head diameters are given in the online Supplementary Appendix S1. Average diameters ranged from 1.5 mm, e. g., in A. gobica (Krasch.) Grubov to about 11 mm in A. norvegica Fr. The minimum, maximum, and the average values for each geographical group are shown in Figure 4A. Both the Arctic s. str. and s. l. groups have mean head diameters twice as large as for the non-Arctic taxa. Our Wilcoxon's test for matched pairs was designed to test whether this was a phylogenetic constraint or a repeated and therefore possibly adaptive response to the colonization of northern latitudes. The differences between the average of all Arctic s. str. or s. l. lineages and their respective sister groups were significant to highly significant. This was the case for the Arctic s. l. lineages even after sequential Bonferroni correction (Table 1).
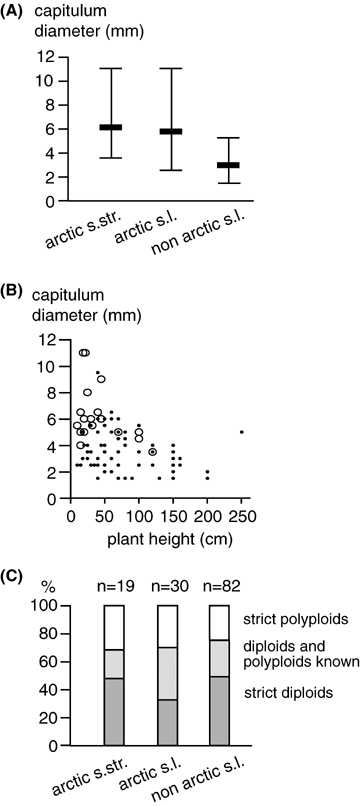
(A) Average diameters of capitula in different geographical groups of Artemisia. The minimum, maximum, and average values are shown. (B) Head diameters plotted against maximum plant height for Arctic s. str. (open circles) and non-Arctic taxa (dots). (C) Proportional distribution of ploidy levels among geographical groups of Artemisia. All three figures are based on literature data summarized in the online Supplementary Appendix S1.
| N | Arctic larger | Equal | Arctic smaller | df | Smallest sum | Level of significance | |
|---|---|---|---|---|---|---|---|
| Head diameter | 11 | 7 | 1 | 3 | 10 | 7 | P<0.05 |
| Arctic s. str./sister group | PBf<0.1 | ||||||
| Head diameter | 15 | 13 | 0 | 2 | 15 | 3 | P<0.001 |
| Arctic s. l./sister group | PBf<0.001 | ||||||
| Plant height | 12 | 2 | 2 | 8 | 10 | 5.5 | P<0.05 |
| Arctic s. str./sister group | PBf<0.1 | ||||||
| Plant height | 18 | 6 | 1 | 11 | 17 | 36 | 0.1>P>0.05 |
| Arctic s. l./sister group | 0.1>PBf>0.05 |
The maximum plant heights (see online Supplementary Appendix S1) are generally lower in Arctic than in non-Arctic plants, yet the Arctic plants have larger heads (Fig. 4B). Consequently, the overall Pearson correlation coefficient in our sample was negative (PCC =−0.328, P= 0.022, N= 88), but this measure has to be taken with care, because it did not consider phylogenetic constraints (Felsenstein 1985). Our Wilcoxon's test for matched pairs was significant for a difference in plant height between Arctic s. str. lineages and their sister groups and marginally nonsignificant for Arctic s. l. lineages (Table 1). Sequential Bonferroni corrections also lowered the significance in the Arctic s. str. case to marginally nonsignificant.
THE DISTRIBUTION OF POLYPLOIDY
In Figure 3, columns 17–19, we have attached the ploidy levels reported in the literature to the phylogenetic tree (see online Supplementary Appendix S1 for detailed chromosome numbers). For 50 taxa only diploid individuals are known. There are 32 taxa in which diploids and polyploids have been observed, and there were 20 strictly tetraploid and nine strictly higher polyploid taxa. There are no major groups in the tree, which consist of tetra- or polyploid taxa only and polyploidy occurs in all groups. Figure 4C shows the proportion of taxa with different ploidy levels, which belong to geographical groups outlined before. The pattern for Arctic s. str. and for non-Arctic taxa is rather similar although there are slightly less strictly polyploids in the latter. The most important difference between the geographic groups seems to be that the Arctic s. l. group contains more taxa with several ploidy levels. The ploidy levels of Arctic taxa s. l. and s. str. are compared with their respective sister groups in Table 2. Both sign tests were nonsignificant. It must be noted that this test was not very powerful because the number of transitions between sister groups was rare (three and five cases for Arctic s. str. and arctica s. l., respectively) but the high number of equal ploidy levels between sister groups (8 and 11 cases, respectively) was not expected.
| Higher in Arctic group | Equal | Lower in Arctic group | Level of significance | |
|---|---|---|---|---|
| Arctic s. str. | 2 | 8 | 1 | P>0.05 |
| Arctic s. l. | 3 | 11 | 2 | P>0.05 |
VEGETATION TYPE AND HABITAT PREFERENCES
The collection of vegetation types and habitat preferences (see Fig. 3, columns 20–34) posed some problems because the floristic works used differed in their terminology. Nevertheless, all taxa could be affiliated with vegetation types that are widely recognized. There are some clusters in our tree where species have similar preferences, for example, clades with all or most species occurring in steppes. Conversely, even rather special habitat types such as salt marshes or deserts have been colonized repeatedly by most major lineages. In Table 3, we have shown the preferred vegetation types of the sister groups of the Arctic taxa. Most sister groups were centered in the steppe vegetation type (eight or 13). These observed values of the actual sister groups were compared with the distribution of vegetation types found in our complete sampling of non-Arctic taxa (rows with expected values). The χ2 analysis to test the differences between actual sister groups and the complete sampling of non-Arctic taxa was highly significant for the Arctic s. str. case (in bold) even after Bonferroni correction, but not for the Arctic s. l. comparisons. Tundra taxa in the actual sister groups of the Arctic s. str. taxa are greatly over-represented (three observed but only 0.4 expected based on randomly chosen non-Arctic s. str. sister groups), exerting by far the greatest influence on the significant test statistic.
| Sister-groups of | Tundra | Forest | Forest margins | Meadow | Steppe | Semidesert | Desert | χ2 | Level of significance at 6 df |
|---|---|---|---|---|---|---|---|---|---|
| Arctic s. str. observed | 3 | 1 | 3 | 0 | 8 | 0 | 0 | ||
| Arctic s. str. expected | 0.4 | 1.5 | 2.5 | 3.5 | 5.5 | 0.7 | 0.6 | 22.1 | P<0.01 |
| PBf<0.01 | |||||||||
| Arctic s. l. observed | 0 | 4 | 8 | 3 | 13 | 0 | 0 | ||
| Arctic s. l. expected | 0 | 2.6 | 4.7 | 6.6 | 11.0 | 1.7 | 1.3 | 8.39 | P>0.1 |
The habitat preferences of the sister groups of the Arctic taxa are shown in Table 4. Most sister groups grew in hillsides as their preferred habitat (10 and 12 cases). These observed values were compared with the distribution of habitats found in all non-Arctic taxa. It turned out that the habitats of the actual Arctic s. str. and s. l. sister groups mirrored those of our sampling because the χ2 test statistic did not indicate a significant difference between observed and expected values.
| Sister groups of | Mountains | Hillsides | Rocky slopes | Coastal rocks | Seashore | Saline/alkaline soils | Riverbanks | Ruderal | χ2 value | Level of significance at 7 df |
|---|---|---|---|---|---|---|---|---|---|---|
| Arctic s. str. observed | 5 | 10 | 0 | 0 | 0 | 0 | 3 | 1 | ||
| Arctic s. str. expected | 4.2 | 5.7 | 0.9 | 0.3 | 0.4 | 2.1 | 2.9 | 2.3 | 7.8 | P>0.1 |
| Arctic s. l. observed | 9 | 12 | 0 | 0 | 0 | 2 | 4 | 5 | ||
| Arctic s. l. | 7.1 | 9.9 | 1.5 | 0.6 | 0.9 | 3.7 | 4.3 | 3.9 | 5.1 | P>0.1 |
Discussion
KARYOLOGICAL PATTERNS IN ARCTIC ARTEMISIA
Much in contrast to data from other plant groups (e.g., Brochmann et al. 2004) and our initial expectations, the number of polyploid species in Arctic habitats was about as high as in other regions (Fig. 4C). This concept is based on absolute species numbers and may be biased by inherited ploidy levels but even when we used multiple sister group comparisons as a phylogenetic correction there was no significant difference in ploidy level between Arctic and non-Arctic lineages (Table 2). Comparable phylogenetic corrections have not been attempted within other Arctic genera yet and, therefore, our results seem to contradict the Arctic polyploidy hypothesis. However, in our view, Artemisia may just be an interesting exception, and does not overturn the concept in general, which is based on a great number of unrelated taxa with elevated ploidy levels in the Arctic (which may be considered as a type of phylogenetic correction at a higher taxonomic level). We will discuss the issue of the lack of increase in proportion of polyploid taxa in Arctic Artemisia, in combination with other results, further below.
There are two more karyological findings that need to be explained are: First, why was the proportion of species with varying ploidy level in the Arctic s. l. group higher than in the other geographical groups (see Fig. 4C)? Our explanation is that the taxa, which are added to the Arctic s. str. group to yield the Arctic s. l. group, are widespread and differing ploidy levels are more likely to originate or persist in species with many populations. Moreover, widespread species may be analyzed more often by researchers and the chance to detect differing or rare ploidy levels will thus be higher. In conclusion, this observation seems an artifact at least in the context of Arctic versus non-Arctic lineages. Second, following Ehrendorfer (1964) we initially hypothesized that Artemisia could have evolved much like Achillea L. (also part of the Anthemideae), which went through repeated cycles of hybridization, allopolyploidization, and diversification (Ehrendorfer 1959; Guo et al. 2005). Moreover, allopolyploid origins have already been proven for many other Arctic polyploids (Brochmann et al. 1992; Brysting et al. 2004). However, in the modern karyological literature on Artemisia (see online Supplementary Appendix S1) allopolyploidization was never reported. We also did not observe heterogeneous sequencing reactions in polyploid species that is another piece of evidence, albeit weak, that the polyploids of Artemisia, regardless whether they are from Arctic or non-Arctic habitats, are in fact autopolyploids (cf. Wendel et al. 1995; Blattner 2004).
MORPHOLOGICAL PATTERNS IN ARCTIC ARTEMISIA
The survey of life-form characteristics indicates repeated changes in life forms across the genus (see Fig. 3, column 14–16). Remarkably, one species of the Arctic s. str. group (A. samojedorum) was an annual, which is rarely encountered in the Arctic flora perhaps because of competition from perennials or the very short and fluctuating growth conditions. In summary, Artemisia seems to have switched between life forms rather frequently regardless of the geographical region colonized by the different taxa.
In contrast, we found evidence for a latitudinal cline in plant height. Arctic taxa are marginally significantly smaller than closely related non-Arctic species (Table 1, Fig. 4B). Other very small plants were often from (semi) desert habitats, but typically possessed narrow flowering heads. Small absolute plant size in the Arctic may be adaptive because microclimatic conditions are better near the ground surface, or it gives protection under low snow cover. This difference in height seems a rather simple and fundamental observation, but, in fact, we did not find this relationship previously tested in the literature on Arctic plants, although we would predict that it will also hold as a general pattern in other taxa. Unfortunately, our approach of using the largest plant size reported does not completely exclude the modifying influence of regional climate that could be tested in common garden experiments only.
The third morphological character tested was the size of flowering heads. The prominent difference between Arctic and non-Arctic taxa (see Fig. 4A) received rather high statistical support in multiple sister group comparisons (Table 1). It seems that enlarged capitulum size has been a necessary and independently evolved adaptation for the survival and proliferation of most Arctic lineages. The biological meaning of enlarged head diameters in Artemisia remains to be tested in the field, but we can propose three different ideas why this might be important. First, larger heads carry more seeds per head and this could replace more numerous smaller heads in plants of greater height with higher branching order. However, this argument can be doubted because an inverse relationship was not found in other small species, for example, from semidesert habitats. Second, large heads may collect and retain more heat, which could be valuable for reproductive processes in cold regions. Third, it could be connected with pollinator attraction. Larger heads are usually also more colorful (e.g., A. norvegica heads are bright yellow) and they may supply a suitable landing platform for insects and send stronger optical signals than smaller heads. Judging from the capitula size alone, insect pollination would dominate in the Arctic over wind pollination, which is typically assumed for Artemisia taxa from more southerly regions. Unfortunately, for most species it is not known in detail whether they are wind- or insect-pollinated and transitions seem to occur (McArthur and Durant 1994).
We prefer the third option because similar pollination problems seem to be important in alpine plants that are much better known. These problems are synchronous flowering of plant communities due to a short vegetation period and poor abundance and diversity of pollinators. For temperate alpine plants it is a long-standing observation that they invest in larger and more colorful flowers for better pollinator attraction at the expense of other organs relative to their lowland counterparts (e.g., Miller et al. 1994; Kudo and Molau 1999; Kühn et al. 2006). This seems to parallel our findings in Arctic Artemisia. In contrast to this, it has to be noted, however, that a trend from insect- to wind-pollination has been observed in tropical alpine taxa (e.g., Berry and Calvo 1989). Interestingly, there also is an intermediate possibility, for example, it has been shown for Salix that transitional pollination systems (insect and wind) are beneficial especially under unpredictable weather conditions as found in the Arctic (Totland and Sottocornola 2001). These conflicting findings in different groups may depend on genetic constraints, life form, length of the vegetation period, average weather stability, density of compatible mates, or abundance of pollinators. These issues are not fully resolved yet, but the most unequivocal result of the present study is the repeated response of the pollination syndrome to the colonization of the Arctic. Accordingly, it seems to have been important for Arctic Artemisia to secure outcrossing over selfing (the few species tested yet are capable of selfing), as better outcrossing would increase levels of genetic diversity within populations.
ARE LARGER HEADS AN ALTERNATIVE STRATEGY TO POLYPLOIDY?
Of the different explanations in the literature for the prevalence of polyploidy in Arctic plants, two are adaptive and emphasize different aspects of genetic diversity: mobilization and recombination of genetic diversity prior to speciation; and conservation of heterozygosity and redundant genes for adaptive challenges in the long run. Both hypotheses would imply generally low levels of genetic diversity in Arctic taxa, which could be due to repeated founder events (Brochmann et al. 2004) or difficulties with cross-pollination (Totland 1993; Strathdee and Bale 1998), although present levels of genetic diversity in Arctic plants seem perfectly normal (e.g., Abbott et al. 1995; Bingham and Ranker 2000). Other theories involve more indirect chance effects: repeated large-scale range movements should have been especially prevalent in the Arctic during the Quaternary and may have lead to higher hybridization and subsequent polyploidization rates (Brochmann et al. 2004); high group age (=many lineages have already gone extinct) in polyploid complexes should inevitably result in generally high ploidy levels because polyploidization is usually regarded as an unidirectional process (Meyers and Levin 2006); finally, high ploidy levels may be inherited by chance from non-Arctic progenitors, and thus this may be a phylogenetic constraint that is not necessarily adaptive (at least for individual lineages).
Artemisia is a major exception to the rule of elevated ploidy levels in the Arctic, but we have shown that its particular and repeated adaptive response to Arctic environments seemingly was the evolution of mixed pollination systems or greater pollinator attractiveness, which probably helps to maintain genetic diversity in populations. In this sense Artemisia is not an exception from other Arctic taxa. Instead it could be regarded as an example for a different strategy for the same evolutionary objective, which is consistent with the adaptive explanations of polyploidy only. In consequence, our findings indirectly support the adaptive explanations for polyploidy in other Arctic plants.
The key question as to why Artemisia has repeatedly adapted to the Arctic environment in a manner unlike that of other plant taxa might be that, in comparison, adaptation of Artemisia to the Arctic may not be very challenging, as suggested by the many independent Arctic lineages recovered. There was presumably no need for a maximum initial pulse of genetic variability or the permanent maintenance of heterozygosity provided by polyploidy. The less drastic effect of improved outcrossing could have been sufficient. This admittedly speculative hypothesis needs to be tested further, but would smoothly integrate three striking findings of this study: multiple and uncomplicated colonizations of the Arctic; significantly enlarged flowering heads; and geographical indifference of ploidy levels in which difference would follow the rule.
ECOLOGICAL PRECONDITIONS FOR THE SUCCESSFUL COLONIZATION OF THE ARCTIC
Arctic habitats established gradually as late as the late Tertiary by cooling climate when most other habitat types already existed in the northern hemisphere (Matthews and Ovenden 1990; Mai 1995). Therefore, Arctic habitats should more likely be biogeographical sinks than sources of lineages, and we employed the ecology of sister groups to infer potential ecological preferences of the progenitors of the Arctic lineages. Most sister groups of Arctic Artemisia (s. str. and s. l.) occur in steppes or on hillsides (Tables 3 and 4), suggesting such site preferences as a good prerequisite to successfully colonize the Arctic. This finding above the species level agrees with an analysis of the distribution of East Asian plant species, where many species were distributed both in the Arctic and in (cold) steppe vegetation (Yurtsev 1962). However, three of four χ2 tests showed that almost the same result would have been obtained if sister groups were randomly chosen from the non-Arctic species of our sample. Consequently, Artemisia species from all vegetation types or habitats had the same probability of colonizing the Arctic as steppe or hillside taxa, the latter are just most abundant in our sampling (and probably also in the genus).
The fourth χ2 test on the proportion of vegetation types for the sister groups of the Arctic s. str. lineages was significant, because the tundra vegetation type was found in the actual sister groups more often than expected (see Table 3 in bold). In this context, however, the significant result is an artifact because Arctic s. l. taxa that already occur in Arctic habitats such as tundra are often part of the Arctic s. str. sister groups.
In view of the many examples reported for intraspecific Arctic/alpine disjunct plant distributions (e.g., Meusel and Jäger 1992; Schönswetter et al. 2003), a strong bias toward mountain habitats was also expected among the preferred habitats of the sister groups of Arctic Artemisia. In fact, this pattern was observed quite often (five and nine instances; Table 4) and seems to support this theory, but again it was about as often as would be expected from a random sample. Whether the relative frequency of Arctic/alpine disjunctions is higher in young, postglacial intraspecific disjunctions compared with old and potentially preglacial disjunctions of now separate taxa (as studied here) is an interesting but open question.
BIOGEOGRAPHICAL CONSIDERATIONS
The closest extant relatives of Artemisia (Watson et al 2002; Vallès et al. 2003) and the taxa from lower branches of the phylogeny grow in Asia (see Fig. 2), in agreement with the oldest unambiguous pollen records of Artemisia reported from the Oligocene of western China (fossil data reviewed in Wang 2004). Following our reconstruction of migration events, different Artemisia lineages reached North America no less than about 17–22 times (see Results and Fig. 3), and these gave rise to more than 50 species (Shultz 2006). There appear to have been two to four migrations from North America to Asia (including intraspecific disjunctions), but these were not followed by radiations. Many species of Artemisia are common to both continents and therefore may have spread only recently, for example during the last glacial maximum across the Beringian land bridge (Clark and Mix 2002; other examples in Hultén 1937; Yurtsev 1962; Abbott et al. 2000). The spread into North America across Beringia would fit the series of the earliest pollen records from the Miocene of North America proceeding from West to East (Graham 1996). Exchanges of lineages between Eurasia and North America via Iceland and Greenland (Hultén 1958; Hagen et al. 2001; Abbott and Brochmann 2003) appear unlikely for most of Artemisia.
There is a conspicuous richness of Artemisia species (eight species from four independent lineages) in outmost northeastern Asia, a well-known center of Arctic plant diversity (Hultén 1937; Krascheninnikov 1946). It is also an important center of genetic diversity within Vaccinium uliginosum L. (Alsos et al. 2005) and other organisms (Hewitt 2004). Although situated far in the North, large parts of this area remained unglaciated during the Quaternary (Andersen and Borns 1997). Therefore, “Beringia” (in the sense of Hultén 1937, including western parts of Alaska) might have served as a glacial refuge also for Artemisia. However, for Artemisia this region also seems to have been the evolutionary center of two small radiations of three endemic species each. Thus, it could additionally be characterized as a minor evolutionary hot spot for Artemisia in the Arctic and, therefore, possibly also for other taxa.
Associate Editor: J. Feder
ACKNOWLEDGMENTS
We would like to thank the keepers of the herbaria in Halle (HAL), Saint Petersburg (LE), and Novosibirsk (NS, NSK) for allowing us to extract DNA from voucher specimens. We thank the German Academic Exchange Service (DAAD) for a grant to NVT and Mrs. B. Hildebrandt for excellent technical assistance. We are also grateful to J. L. Feder, two anonymous reviewers, and E. J. Jäger who gave valuable comments on the manuscript, and to C. Muir for help with the English.




