HOST SHIFTS AND THE BEGINNING OF SIGNAL DIVERGENCE
Abstract
Divergence between populations adapting to different environments may be facilitated when the populations differ in their sexual traits. We tested whether colonizing a novel environment may, through phenotypic plasticity, change sexual traits in a way that could alter the dynamics of sexual selection. This hypothesis has two components: changes in mean phenotypes across environments, and changes in the genetic background of the phenotypes that are produced—or genotype × environment interaction (G × E). We simulated colonization of a novel environment and tested its effect on the mating signals of a member of the Enchenopa binotata species complex of treehoppers (Hemiptera: Membracidae), a clade that has diverged in a process involving host plant shifts and signal diversification. We found substantial genetic variation and G × E in most signal traits measured, with little or no change in mean signal phenotypes. We suggest that the expression of extant genetic variation across old and novel environments can initiate signal divergence.
Ecological and sexual selection may act synergistically during speciation. When differences in sexual traits arise as populations adapt to different environments, the resulting reproductive isolation can facilitate divergence (Rundle and Nosil 2005). Further, sexual selection can foster rapid evolution (West-Eberhard 1983; Kirkpatrick and Ravigné 2002); and, if sexual traits are condition related, mate choice may facilitate adaptation (Lorch et al. 2003). Thus, once sexual traits differ between populations, further divergence is facilitated. But how do differences in sexual traits appear? Hypotheses about early divergence in sexual traits often rely on mutation, drift, or natural selection (Kaneshiro 1980; Wells and Henry 1998; Boughman 2002; Coyne and Orr 2004; Rundle and Nosil 2005) to create the initial differences. Here we focus on the hypothesis that colonizing a new environment may change sexual traits (and, potentially, sexual selection dynamics) due to the expression of extant genetic variation and phenotypic plasticity.
The presence of genetic variation (Andersson 1994; Bakker and Pomiankowski 1995; Roff 1997; Bakker 1999) and phenotypic plasticity (Schlichting and Pigliucci 1998; West-Eberhard 2003, 2005) in fitness-related traits in natural populations has important implications for the course of evolution. With genetic variation, populations have the potential to quickly respond to selection. When plasticity changes the mean phenotypes expressed across environments, selection on developmental regulation can foster divergence through the process of genetic accommodation (West-Eberhard 2003, 2005; Suzuki and Nijhout 2006), and plasticity-induced changes in mean sexual phenotypes may contribute to reproductive isolation. Another factor that can have important consequences for divergence is genotype × environment interaction (G × E), which refers to genetic variation in reaction norms. When reaction norms differ so much that they cross among environments, the fitness ranking of genotypes may change (Lynch and Walsh 1998). This gives G × E the potential to maintain genetic variation under selection if there is high gene flow among environments (Gillespie and Turelli 1989; Ellner and Hairston 1994; Sasaki and De Jong 1999; Turelli and Barton 2004; Byers 2005). By the same logic, with low gene flow across environments G × E can promote divergence (Etges 2002; Greenfield and Rodríguez 2004). Because the genetic backgrounds of expressed phenotypes differ across environments, G × E sets the stage for differences in the response to selection, even if selection does not change. For example, developing in novel environments can change the genetic correlations among traits (Service and Rose 1985; de Jong 1990; Stearns et al. 1991; Bégin and Roff 2001, 2004), and such changes can influence divergence through selection or drift (e.g., Badyaev and Hill 2000; Phillips et al. 2001; Kause et al. 2001; Steppan et al. 2002; Bégin and Roff 2003, 2004; Roff 2004). Consequently, phenotype differences may appear, promoting divergence.
Phenotypic plasticity and G × E are widespread in sexual traits (e.g., Eberhard and Gutiérrez 1991; Emlen 2001; Qvarnström 2001; Greenfield and Rodríguez 2004; Etges et al. 2007; Mills et al. 2007; see also Mackay and Anholt 2007 for G × E in behavioral traits), suggesting that both factors may contribute to early divergence. This hypothesis predicts that colonizing a novel environment should result in (1) changes in mean phenotypes, and/or (2) G × E with reaction norm crossovers and changes in genetic correlations. These predictions are best tested with an organism in which divergence in sexual traits and colonizing new environments are known to influence speciation.
We tested the hypothesis that phenotypic plasticity and G × E contribute to signal divergence after a host shift in a plant-feeding insect. The Enchenopa binotata complex of treehoppers (Hemiptera: Membracidae) is a clade of 11 sympatric species in eastern North America, each specializing on a different host plant. Speciation in this group has involved host plant shifts that generate divergent ecological selection, as well as reproductive isolation due to plasticity in life-history timing and host fidelity (Wood and Guttman 1983; Wood 1993). These species are also isolated behaviorally (Rodríguez et al. 2004). Pair formation is facilitated by male–female duets with plant-borne vibrational signals, and signal differences largely mirror host plant usage (Hunt 1994; Rodríguez and Cocroft 2006; Cocroft et al. 2007, unpubl. ms.). Females exert mate choice through their decision to respond to a male, and preference changes have caused signal divergence (Rodríguez et al. 2004, 2006). Because both host shifts and signal evolution have contributed to speciation in the complex, it is important to understand the immediate effects of a host shift on signal variation. In this study we experimentally shifted one species onto a novel host, to partition signal variation between components for full-siblings families (as a proxy for genotype), host plant species (environment), and G × E. Our findings highlight the importance of G × E for early divergence.
Materials and Methods
REARING DESIGN
Experiments were performed during 2003–2005. We used a full-sibling split-family design (Lynch and Walsh 1998). We chose the E. binotata species that lives on Ptelea trifoliata (Rutaceae) plants (species in the complex are currently being described; they can be recognized by their host usage, nymphal morphology and coloration, and adult signals). We reared the insects on their native host (P. trifoliata) and on the host used by another species in the complex: Robinia pseudoacacia (Fabaceae). Robinia is a relatively benign host: members of the complex from other hosts have high survivorship on it, relative to other hosts (Wood 1993). This allowed us to rear the large sample required by our design. We collected 69 mated females in the field (Boone County, Missouri) during the summer, and allowed them to lay eggs on potted host plants (Ptelea), placing each female on an individual plant. The broods overwintered as eggs on those plants. When the nymphs eclosed in the spring, we divided them between fresh Ptelea and Robinia plants, placing half of each family on a different plant. Rearing was conducted at an outdoor facility at the University of Missouri-Columbia.
This experiment was not designed to evaluate the effect of variation within rearing plant species. We attempted to remove this source of variation by homogenizing plant size (within species), vigor, and phenology. Because Ptelea bud earlier in the spring than Robinia, we brought Robinia plants into the greenhouse when Ptelea plants began to sprout leaves. Robinia plants remained in the greenhouse until they reached a similar stage of leaf development as Ptelea in outside conditions. By the time nymphs were transferred to the plants, both hosts were at the stage in which nymph eclosion would occur in nature.
For each family, we placed 30 nymphs on each plant (Ptelea and Robinia), with only one group of 30 nymphs per plant. We transferred nymphs as first or second instars, allowed them to develop to adulthood, and recorded the males when they were 2- to 3-week old, at the peak of their signaling activity. Seven of 69 females that we collected laid too few eggs (<60) to allow their progeny to be reared on the two hosts. From the remaining 62 broods, we randomly selected 27 for the experiment. Eight of these 27 families had low or no survivorship on one or the other hosts, reducing our sample to 19 families. We recorded the signals of 1–14 males for each half family (mean = 7, mode = 9). This variation in sample size was due to low survivorship of some families on one or the other host plant.
To contrast the effect of developing on different host plants to that of simply signaling on them, we reared 19 control males on Ptelea (their natal host), and as adults induced them to signal on Ptelea and Robinia plants, as in Sattman and Cocroft (2003).
SIGNAL RECORDING AND ANALYSIS
We recorded male signals on the stems of potted plants (ca. 50 cm tall). For the full-sibling experiment, we recorded males on the plant species on which they were reared (Ptelea or Robinia), using a single plant individual for all males reared on a given species. A different individual plant was used each year, but year had no effect on signal variation. Sattman and Cocroft (2003) also found no differences between samples of males recorded on different individual host plants of the same species. Control males were recorded in 2004 on the Ptelea and Robinia plant individuals used for recording the full-sibling families; the order of the plants was switched from male to male.
The recording plant was placed on a vibration isolation table (Vibraplane, Kinetic Systems, Boston, MA) to minimize noise from building vibrations; we isolated the plant from the table with shock-absorbing sorbothane (Edmund Scientifics, Tonawanda, NY). We placed males one-by-one at a specified location on a plant stem, and induced them to signal by broadcasting a recording of a male–female duet through a loudspeaker. We recorded male signals with a laser vibrometer (Polytec CLV 1000 with a CLV M030 decoder module; Polytec Inc., Auburn, MA), focusing the laser beam on a piece of reflective tape (ca. 2 mm2) affixed to the stem. Males were within a few centimeters of the reflective tape when recorded. The laser beam was approximately perpendicular to the stem, with the source ca. 50 cm from the plant. The laser signal was high-pass filtered (Krohn-Hite 3202; Krohn-Hite Corporation, Brockton, MA) at 60 Hz. The output was sent to a Macintosh G4 computer through an Edirol UA–5 USB interface (Roland Corporation, Japan) and recorded with SoundEdit 16 version 2 (Macromedia, Inc., San Francisco, CA) at a 44.1-kHz sampling rate. We monitored male signaling with a Radio Shack MPA-45 amplifier connected to an RCA loudspeaker and a Hameg HM 203–7 20 MHz oscilloscope (Hameg Instruments, Mainhausen, Germany). Air temperature was maintained at 25.2 ± 1.6°C (mean ± SD).
Enchenopa males often produce bouts of several signals, and some of the signal features measured here change slightly throughout the bout (Cocroft et al., unpubl. ms.). We standardized our measurements by taking them from the fifth signal in a bout (for males that produced fewer than five signals, from the last signal in a bout). This represents a compromise between the benefits of having a landmark present in most of the recorded signals (73% of the males produced bouts with five signals or more) and the benefits of measuring high-amplitude signals (signal amplitude increases throughout the bout, often leveling around the fifth signal). When more than one bout was available for each male, we chose the one with the highest amplitude.
We analyzed variation in seven signal traits that differ among species: number of signals per bout, interval between signals within a bout, length of the whine component of the signal, dominant frequency, number and length of pulses, and pulse rate. Females choose males on the basis of variation in most signal traits, especially frequency and whine length; there is no preference for the interval between signals, and we have not tested for preferences for pulse length (Rodríguez et al. 2006). We conducted this analysis in SoundEdit. Signal traits influenced by recording temperature were corrected to 25°C.
STATISTICAL ANALYSIS
Our aim was to test for two forms of host shift-induced plasticity in signals: changes in mean phenotypes and G × E. The full-sibling family design allowed us to partition variation among components for families, rearing plant species, and their interaction. The family component provides an estimate of broad-sense heritability (H2; Lynch and Walsh 1998). Because H2 is influenced by additive and nonadditive variation (including maternal effects), it does not provide an accurate predictor of the short-term capacity to respond to selection (Lynch and Walsh 1998). However, rather than precise estimation of genetic parameters, our purpose was to test for host shift-induced changes in mean phenotypes and G × E. Our sample size of ca. 9 males per family and 19 families (see above) is more than sufficient to minimize the sampling variance for H2 (Lynch and Walsh 1998, p. 545).
We used mixed-model ANOVAs to test the effect of family (random factor), rearing plant species (fixed effect), and their interaction. (For the control males, we tested the effect of recording plant species as a fixed effect, and of individual male identity as a random factor.) We conducted separate tests for each signal trait. We estimated H2 for a full-sibling design with unequal family sizes (Roff 1997, p. 41). The test for H2 > 0 is provided by F=MSfamily/MSresidual (Fry 1992). The test for the effect of rearing plant is an indication of change in mean phenotypes across rearing plants. To evaluate G × E we first tested for a family × rearing plant interaction. If the interaction was significant—indicating G × E (Schlichting and Pigliucci 1995; Hunt et al. 2004)—we inspected the reaction norm plots to assess crossover prevalence.
Because our tests indicated the presence of G × E (see below), we evaluated host shift-induced changes in genetic correlations among signal traits. We estimated genetic correlations with Pearson's product-moment correlation coefficients among family means on each host plant (Roff 1997). With n= 19 families, statistical power is adequate (1 −β > 0.80) only for r≥ 0.60 (Cohen 1988; Zar 1999); power to detect changes in correlations and in correlation matrices is even lower (Stearns et al. 1991; Roff 1997, pp. 101–117; Steppan et al. 2002). We thus present a qualitative description of changes in genetic correlations. First, we assessed matrix similarity on a scatter plot of the correlations between any two traits on the rearing plants. With equal matrices the intercept should be 0 and the relationship 1:1 (Roff 1997, p. 107). Significance testing is invalidated because the elements in each vector are not independent (Roff 1997: 107), so we focused on the magnitude and intercept of the correlation. Second, we used an effect size criterion (Cohen 1988) to evaluate changes in genetic correlations. We calculated the absolute magnitude of the changes in the genetic correlations, and categorized those magnitudes into bins of < 0.2; 0.2–0.5; and > 0.5.
Our trait-by-trait analysis allowed us to focus on signal traits that influence female preferences and differ among species in the E. binotata complex (especially frequency and whine length; Rodríguez et al. 2004, 2006; Cocroft et al. 2007). A drawback of this approach is that we conducted multiple statistical tests on the same data, increasing the likelihood of spurious significance (Rice 1989). However, corrections for multiple tests can seriously reduce statistical power (Moran 2003; Nakagawa 2004). To deal with both concerns, we follow Moran (2003) in using the number of significant tests in a table as an indication of the strength of the evidence against the null hypothesis, and compare the outcome with that of the sequential Bonferroni correction (Rice 1989). Our findings are not changed by this correction and provide post hoc support for our approach: host shift-induced changes in genetic correlations (see below) suggest that signal traits may evolve independently across hosts.
Results
For the control males, recording plant species influenced four of seven signal traits; importantly, frequency was not among the traits affected (Table 1). There was a trend for control males to produce fewer, shorter, and more closely spaced signals on the foreign host; similar behavior was seen in a previous study and interpreted as an aspect of host fidelity (Sattman and Cocroft 2003). Otherwise there was little effect of signal structure. In any case, this low number of significant tests (2/7) argues against a prominent effect of inducing males to signal on different plant species (Moran 2003). Likewise, after a sequential Bonferroni correction, only the effect on pulse rate remains significant. We conclude that inducing males to signal on a foreign host has minimal effects on signal variation. This result provides a baseline for evaluating the effect of the experimental host shift (Fig. 1; black lines and symbols).
| Recording plant species (F, P) | Individual male (F, P) | |
|---|---|---|
| Signals per bout | 4.25, 0.054 | 0.93, 0.56 |
| Signal interval | 5.70, 0.028 | 0.85, 0.63 |
| Whine length | 4.40, 0.05 | 0.70, 0.77 |
| Frequency | 2.04, 0.17 | 1.70, 0.14 |
| Pulse number | 0.49, 0.49 | 0.96, 0.54 |
| Pulse length | 0.93, 0.35 | 1.71, 0.13 |
| Pulse rate | 20.4, 0.0003 | 3.23, 0.008 |
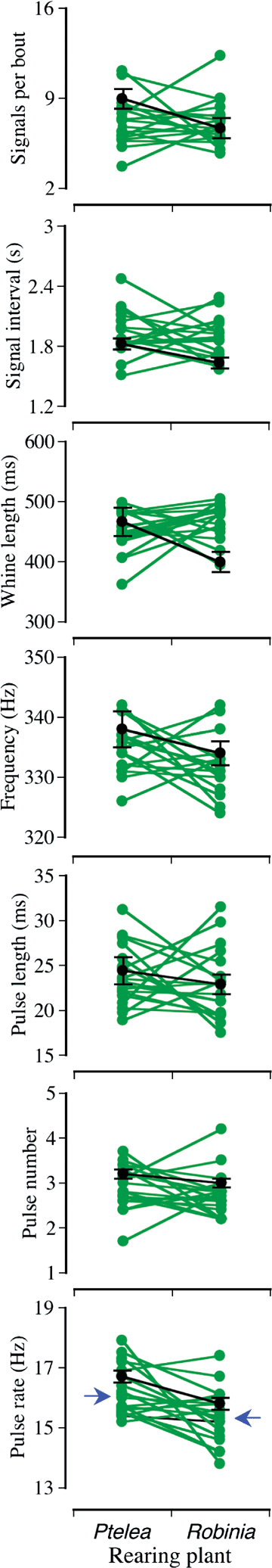
Signal variation according to family, rearing plant, and their interaction. y-axes span the range of variation in the population from which the dams were collected. Green symbols and lines show the reaction norms of 19 full-sibling families reared on Ptelea and Robinia plants; green symbols indicate the family mean on each plant. Blue arrows indicate the mean trait value for each plant when there was a significant host plant effect. Black symbols and lines indicate the mean ± SE trait values for control males reared on Ptelea plants and recorded on Ptelea and Robinia.
We found significant H2 in all but one signal trait, and family × rearing plant interactions in all traits (Table 2). This high number of significant tests is consistent with a prominent effect of genotype and G × E on signal variation (Moran 2003), and with the outcome of the sequential Bonferroni correction—after which all interactions remain significant and only one H2 changed to marginally significant (Table 2). Inspection of the reaction norms confirms the prevalence of crossovers for all traits (Fig. 1; green lines and symbols). By contrast, rearing plant had a significant effect only for pulse rate; although significant after the sequential Bonferroni correction, this effect was of very small magnitude (Table 2; Fig. 1). This pattern remained even when we repeated the test with all 27 families (i.e., including those that had no survivorship on one host): there was still no effect of rearing plant for any signal trait except for a small effect on pulse rate (data not shown). We thus conclude that developing on novel host plants had little effect on the mean signal phenotypes.
| Source of variation (df num, df denom) | MS | F, P | Test for H2>0: F, P | H2 | |
|---|---|---|---|---|---|
| Signals per bout | Family (18, 18) | 18.22 | 1.26, 0.32 | 2.32, <0.001 | 0.17 |
| Host plant (1, 25. 97) | 1.04 | 0.08, 0.78 | |||
| Interaction (18, 231) | 14.49 | 1.84, 0.022 | |||
| Residual | 7.86 | ||||
| Signal interval | Family (18, 18) | 201,219 | 0.78, 0.70 | 1.82, <0.025 | 0.11 |
| Host plant (1, 24.08) | 35,946.1 | 0.16, 0.69 | |||
| Interaction (18, 230) | 259,771 | 2.35, 0.002 | |||
| Residual | 110,566 | ||||
| Whine length | Family (18, 18) | 3,026.94 | 0.39, 0.97 | 0.75, >0.75 | −0.04 |
| Host plant (1, 25.58) | 14,323.8 | 2.12, 0.16 | |||
| Interaction (18, 231) | 7,794.58 | 1.93, 0.014 | |||
| Residual | 4,033.97 | ||||
| Frequency | Family (18, 18) | 106.6 | 0.92, 0.57 | 1.78, <0.025 | 0.10 |
| Host plant (1, 25.56) | 261.8 | 2.60, 0.12 | |||
| Interaction (18, 231) | 115.8 | 1.94, 0.014 | |||
| Residual | 59.8 | ||||
| Pulse number | Family (18, 18) | 1.36 | 1.28, 0.30 | 4.12, ≪0.001 | 0.36 |
| Host plant (1, 22.47) | 0.88 | 1.02, 0.32 | |||
| Interaction (18, 231) | 1.06 | 3.18, <0.0001 | |||
| Residual | 0.33 | ||||
| Pulse length | Family (18, 18) | 51.80 | 0.66, 0.80 | 1.71, 0.025 | 0.10 |
| Host plant (1, 23.50) | 29.49 | 0.45, 0.51 | |||
| Interaction (18, 230) | 78.18 | 2.58, 0.0006 | |||
| Residual | 30.29 | ||||
| Pulse rate | Family (18, 18) | 4.84 | 1.70, 0.14 | 5.32, ≪0.001 | 0.48 |
| Host plant (1, 22.33) | 26.54 | 11.37, 0.0027 | |||
| Interaction (18, 227) | 7.85 | 3.14, <0.0001 | |||
| Residual | 0.91 | ||||
The plot of genetic correlations across rearing plants had an intercept of zero, but the correlation was low (r= 0.29, P= 0.20; Fig. 2A). This suggests that the matrices were changed by the host shift. Closer inspection shows that changes in the correlations were common and large. There were changes in all 21 correlations: nine of those changes were changes in sign, and most of the changes were of intermediate (0.2–0.5) or large magnitude (>0.5; Fig. 2B). The signal traits for which females show the strongest preferences were involved in these changes. Five of the six genetic correlations involving signal frequency changed, with three changes in sign. And all six correlations involving whine length changed (including the correlation between frequency and whine length), with two changes in sign (Table 3).
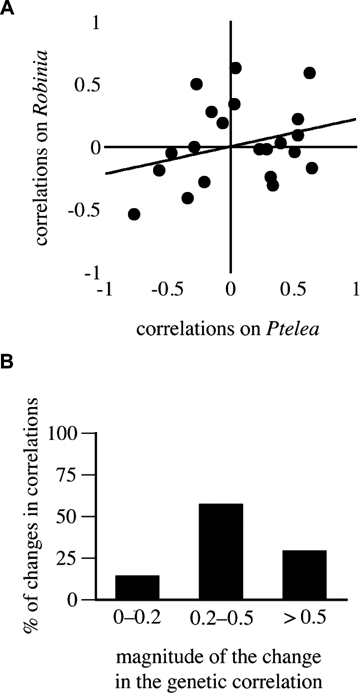
Changes in genetic correlations among signal traits across host plants. (A) Relationship between correlations on each host plant. (B) Magnitude of the changes in the genetic correlations.
| Signals per bout | Signal interval | Whine length | Frequency | Pulse number | Pulse length | |
|---|---|---|---|---|---|---|
| Families on Ptelea | ||||||
| Signal interval | −0.77, <0.0001 | |||||
| Whine length | −0.29, 0.23 | 0.34, 0.15 | ||||
| Frequency | 0.32, 0.18 | −0.47, 0.04 | −0.06, 0.81 | |||
| Pulse number | 0.54, 0.017 | −0.57, 0.011 | 0.03, 0.89 | 0.65, 0.0024 | ||
| Pulse length | −0.27, 0.27 | 0.51, 0.026 | 0.54, 0.018 | −0.34, 0.16 | −0.15, 0.53 | |
| Pulse rate | 0.23, 0.35 | −0.21, 0.38 | 0.04, 0.88 | 0.40, 0.09 | 0.63, 0.004 | 0.29, 0.23 |
| Families on Robinia | ||||||
| Signal interval | −0.55, 0.01 | |||||
| Whine length | −0.01, 0.96 | −0.32, 0.18 | ||||
| Frequency | −0.25, 0.30 | −0.06, 0.81 | 0.18, 0.47 | |||
| Pulse number | 0.21, 0.39 | −0.20, 0.41 | 0.33, 0.17 | −0.18, 0.46 | ||
| Pulse length | 0.49, 0.03 | −0.05, 0.85 | 0.08, 0.73 | −0.42, 0.07 | 0.27, 0.27 | |
| Pulse rate | −0.03, 0.90 | −0.29, 0.23 | 0.62, 0.0047 | 0.02, 0.94 | 0.58, 0.009 | −0.03, 0.91 |
Discussion
Host shifts and signal evolution are central to speciation in Enchenopa (reviewed in Cocroft et al. 2007). We simulated a host shift to examine how signal divergence may begin. The host shift-induced G × E with reaction norm crossovers in all signal traits measured, and changed genetic correlations between traits. For male Enchenopa, developing on a nonhost had a very different effect than simply being induced to signal on a nonhost, which had little impact (this article; Sattman and Cocroft 2003; see also Cocroft et al. 2006). As a consequence of G × E, attractive males will differ across host plants in their genetic backgrounds and genetic correlations among signal traits. Thus, even if females were to prefer the same signal values across hosts (a question that remains to be addressed), they will favor different genotypes on different hosts, setting the stage for divergent responses to selection. The potential for divergence would be even greater with G × E in preferences as well as signals, because differences in the distribution of preferences and signals across environments would change the patterns of sexual selection. These patterns might remain unchanged if preferences and signals showed similar forms of G × E, such that the same preferences genotypes favored the same signal genotypes across environments, but this possibility is unlikely (Rodríguez and Greenfield 2003).
Divergence will depend importantly on the genetic variation present. Our estimates of H2 encompass additive and nonadditive genetic variation, and maternal effects (Lynch and Walsh 1998), so they are not good quantitative predictors of the short-term response to selection. They do, however, indicate that Enchenopa signals have the potential to diverge rapidly. Some features of our experimental design may have reduced the likely impact of maternal effects. First, we excluded broods consisting of < 60 eggs, because we needed to start the experiment with 60 nymphs per family (30 for each host). This probably excluded broods produced by dams in poor condition, reducing a potential source of maternal effects. Second, all dams were placed on their native environments (Ptelea plants), where they laid eggs and where their broods eclosed; then, half of all nymphs were reared on the same environment as their mothers (Ptelea plants). Thus, except for the nymphs reared on the novel host, all individuals were reared in the same environment as their lineage evolved on, which is likely to reduce the expression of maternal effects (Roff 1997; Lynch and Walsh 1998). Nevertheless, we anticipate that maternal effects, as well as additive and nonadditive genetic variation, contributed to the outcome that similar phenotypes are produced by different genetic backgrounds across environments.
Changes in genetic correlations among signal traits suggest that nonadditive components of variation may play an important role in early divergence. Our finding of G × E indicates that different genes influenced the same signal traits on different host plants. The host shift may thus have altered the patterns of pleiotropy influencing the expression of signal traits. This finding agrees with work emphasizing the role of nonadditive components of genetic variation, including epistasis, in adaptation and divergence (e.g., Wade 2000; Carroll et al. 2003; Carroll 2007).
Our host shift caused few changes in mean signal phenotypes, and in no case did trait values extend beyond the range of variation in the population from which the dams were collected. These results suggest that host shift-induced changes in mean signal phenotypes may play a minor role in promoting signal novelty. However, we chose the novel host not only because it is used by a species in the E. binotata complex, but also because it presents a benign environment that allowed rearing the required sample size. Other hosts may present a more challenging developmental environment, with the potential to result in mean differences in phenotypes. However, this effect on adult signals may in turn be harder to detect because of higher mortality during development. We thus do not argue against novel environments as a source of changes in mean phenotypes. Instead, we emphasize that, even in the absence of such effects, G × E may promote divergence.
The mode of speciation in Enchenopa—host plant shifts—is common in herbivorous insects (Berlocher and Feder 2002). Host shifts, in turn, are a good example of ecological speciation (Schluter 2001; Rundle and Nosil 2005). Our findings may thus represent an important factor in the beginning of divergence. We suggest that G × E in sexual traits at the start of the colonization of a novel environment may cause immediate changes in sexual selection dynamics, not only facilitating divergence, but also initiating it.
Associate Editor: S. Steppan
ACKNOWLEDGMENTS
We thank G. Höbel, M. Rausher, S. Steppan, and four anonymous reviewers for comments on the manuscript, and B. Sonderman for logistic support. Financial support was provided by NSF Grant IBN 0318326 to RBC and RLR, and by a University of Missouri–Columbia LSUROP fellowship to LMS.




