LOCAL ADAPTATION AND COGRADIENT SELECTION IN THE ALPINE PLANT, POA HIEMATA, ALONG A NARROW ALTITUDINAL GRADIENT
Abstract
Alpine environments are particularly susceptible to environmental changes associated with global warming but there is potential for alpine plants to adapt to warming if local adaptation occurs and gene flow allows genotypes adapted to low altitudes to colonize higher altitude sites. Here we examine the adaptive potential of a common alpine grass, Poa hiemata, within the restricted alpine habitat of Australian mountains, across a narrow altitudinal gradient replicated in three areas. Grasses at high altitude sites had shorter leaf lengths and larger circumferences than those at lower sites. Transplant experiments with clonal material and plants grown from seed indicated that these differences were partly genetic, with environmental and genetic factors both contributing to the differences between altitudes. Differences in altitudinal forms were also evident in a common garden experiment. Plants showed a home-site advantage in terms of survival. A fitness analysis indicated that at high altitude sites, selection favored plants with short leaves and larger circumferences, whereas these traits were selected in the opposite direction at the low altitude sites. These findings indicate cogradient selection and potential for both plastic and genotypic shifts in response to climate change in P. hiemata.
It is not yet clear whether the majority of plant populations are able to evolve to maintain current distributions under global climate change (Jump and Penuelas 2005). Some recent studies have indicated substantial adaptive potential as reflected by high heritability estimates for traits likely to be selected (Billington and Pelham 1991; Savolainen et al. 2004). However even where there is heritable variation in some traits, this does not guarantee a response to selection if rates of climate change are too rapid or trait interactions constrain evolutionary responses (Billington and Pelham 1991; Bennington and McGraw 1996; Roff 1996; Etterson and Shaw 2001; Blows and Hoffmann 2005). Moreover there is very little information on adaptive potential in environments that are particularly threatened by climate change such as alpine areas.
Local character variation can indicate the potential of plants to adapt to environmental conditions that vary geographically. Such variation is found in many species and arises from direct effects of environment on the phenotype or from underlying genetic variation. Phenotypic variation may exhibit cogradient variation (CoGV) or countergradient patterns (CnGV). CoGV arises when the genetic and environmental components of variation act in the same direction, and/or when selection changes the reaction norm in the same direction as the environmental effect on a trait's expression (Antonovics 1976; Sultan 1987; Eckhart et al. 2004). CnGV occurs when genetic influences on a trait oppose those of the environment and/or when phenotypic variation is not adaptive (Eckhart et al. 2004). For instance, imagine a situation in which tall plants occur at the bottom of a mountain and small plants at its apex. When seeds or clones from these gradient ends are reciprocally transplanted, CoGV is present if plants originating from the bottom are relatively tall regardless of where they are planted, but nevertheless tallest when planted to their home site at the bottom of the mountain (reaching the same height as plants found there naturally). CnGV is present if plants originating from the bottom and transplanted to the top are smaller than those found growing there naturally, and vice versa for plants originating from the top.
One environment particularly threatened by climate change is the alpine area (Beniston 2003). This environment is locally variable as small changes in altitude can lead to large changes in temperature, humidity, exposure, and other types of changes (Korner 1999; Hovenden and Vander Schoor 2004). Selective pressures affecting plants in this zone can therefore change over a relatively small scale. Beginning with the classic studies of Clausen and colleagues (Turesson 1922; Clausen et al. 1941), there is substantial evidence for variation in plants along altitudinal gradients. Sometimes this variation is largely genetically based, but in other cases it is mostly environmentally based. Recent examples include the genetic and environmental contributions to altitudinal variation in both vegetative and reproductive investment traits in the moss Pogonatum dentatum (Hassel et al. 2005), mostly environmental factors contributing to physiological altitudinal differences but mostly genetic factors contributing to morphological altitudinal differences in a Hawaiian tree species Metrosideros polymorpha (Cordell et al. 1998), and both genetic and environmental factors contributing similarly to altitudinal variation in both leaf length, width, and area in Tasmanian Nothofagus (Hovenden and Vander Schoor 2004). Characterization of spatial patterns in selection along altitudinal gradients will help illuminate how selection will be altered by climate change (Etterson 2004).
Although local adaptation in alpine and subalpine regions has been tested and established in a number of cases (Morecroft and Woodward 1996; Stanton and Galen 1997; Gauthier et al. 1998; Volis et al. 2002; Stinson 2004), there is little information on the nature of traits under selection and the relative importance of CoGV or CnGV. Reciprocal transplant experiments can be used to identify the form of selection, by characterizing the nature of phenotypic variation and adaptation between populations along environmental gradients and the nature of selection on traits (Etterson and Shaw 2001; Kawecki and Ebert 2004). These approaches have rarely been applied to comparisons in alpine regions. Moreover, when local adaptation is suggested along a gradient, it is often unclear if this reflects altitudinal variation, because gradients are often not replicated.
In this study we consider transplants across three comparable environmental gradients to examine local adaptation in an alpine grass, Poa hiemata, from Victoria, Australia. By considering multiple gradients we test the relative importance of altitude versus other factors in determining phenotypic differences among locations. We use a combination of direct measures, transplants based on seed and clonal material as well as a common garden experiment to evaluate the relative importance of CoGV and CnGV and selection responses at range margins. We focus on P. hiemata because it is a predominant perennial tussock-forming grass in tall alpine herbfields within alpine and subalpine zones and because it is used widely in restoration efforts.
The Victorian Alps contain more than 10% of the recorded flora in Australia, and almost 60% of this flora is endemic (McDougall 2003). This region is vital as catchment for the supply of high-quality water, nature conservation, biodiversity resources, tourism, recreation, and education (Wahren et al. 1994). Of the many threats to the Australian Alps, warming and drying associated with climate change are likely to have the most widespread and disastrous impacts (Williams et al. 2006). In alpine areas around the world, it is expected that warming will allow invasion from lower elevations and create pressure for upward migration of alpine species (Woodward 1987; Jump and Penuelas 2005). Upward encroachment of subalpine woodland species into alpine herbfields has already been documented in Australia and overseas (Grabherr et al. 1994; Lloyd and Fastie 2003). Alpine areas in Australia are particularly restricted in terms of altitudinal retreat compared with other such areas around the world, as there are no areas above the alpine zone; the potential impacts on communities in Australia could therefore be greater than elsewhere (Brereton et al. 1995; Hughes 2003).
We address the following main questions. (1) Is there altitudinal variation in growth characteristics in P. hiemata? (2) What proportion of the phenotypic differences across environments is genetic? (3) Do patterns of phenotypic plasticity reflect cogradient versus countergradient responses? (4) How does selection on traits vary across environments? (5) What is the strength of selection on traits? (6) What does the observed pattern of variation mean for future adaptive potential of Australian Poa?
Materials and Methods
SPECIES AND SITES
Poa hiemata (soft snow-grass) is widespread within alpine and subalpine zones generally above 1500 m, and is common on the Bogong High Plains (Alpine National Park) in Northeastern Victoria and also the Kosciuszko Alpine National Park in New South Wales. Plants are bisexual, exhibit both panicle inflorescences, hermaphrodite spikelets and grow vegetatively through rhizomatous shoots, and only flower once per season (Vickery 1970). The genus of Poa is large with over 200 species in temperate-to-cold climates; 34 native Australian species are recognized (Vickery 1970).
East-facing altitudinal transects around 1 km long were placed along three mountains within the Bogong High Plains, Alpine National Park, Victoria, Australia. These were selected on the basis of their similar species composition, the fact that they covered an altitudinal range of approximately 1700–1880 m a.s.l. (typical of high alpine ranges in Australia), and a similar aspect. The sites were at Mount Cope (high altitude, 147˚16′E 36˚55′S, 1847 m, to low altitude, 147˚17′E 36˚55′S; 1700 m), Mount Nelse Central (high altitude, 147˚20′E 36˚50′S, 1880 m to low altitude, 147˚20′E 36˚49′S; 1780 m) and New Country Spur (high altitude, 147˚21′E 36˚50′S, 1877 m to low altitude, 147˚21′E 36˚49′S; 1746 m) (Fig. 1). Populations of P. hiemata were continuously distributed along each of the transects; beyond both the high and low altitude extremes of the transects they became fragmented as range margins were approached. Voucher specimens at high and low altitudes for each transect were collected for P. hiemata and submitted to the Victorian Herbarium.
Location of the three altitudinal transects, New Country Spur (A), Mount Nelse Central (B), and Mount Cope (C) within the Alpine National Park, Victoria, Australia. Contour lines are every 30 m (Source: Melbourne University Library Electronic Map Collection).
One of the environmental variables that was quantified along the transects was temperature; averages from data loggers placed at 100-m intervals along each transect between November and April indicated that over the snow-free growing season, mean temperature at the low altitude sites was consistently 1°C (or more) higher compared to the cooler high altitude sites. However other variables are also likely to differ along these gradients; these include depth and duration of snow, wind regime, soil conditions and many others (Korner 1999).
ALTITUDINAL VARIATION IN GROWTH FORM
To examine the growth form of P. hiemata along each transect, we measured the leaf length and plant circumference of 440 plants per transect. At 100-m intervals along the transect, 40 plants were randomly selected from a 50-m2 area (using randomly assigned coordinates within the area). Two measures per plant were taken for longest leaf length and one for circumference. Length was measured to the nearest millimeter with a ruler and circumference to the nearest millimeter with a graduated string. Only green living leaves were measured for length. Preliminary assessments on a set of 40 plants indicated that both leaf length and circumference could be measured with a high degree of repeatability (r= 0.99 for length, r= 0.97 for circumference).
RECIPROCAL TRANSPLANT EXPERIMENTS
For the transplants, vegetative and seed materials were collected within 100-m2 areas at the high and low altitude ends of each transect using random sampling (randomly assigned coordinates within the area). Vegetative clones were established in January 2005 from young plants (< 5 cm high, clump size < 20 cm diameter) to minimize the effects of early environment on phenotypic variation. Whole plants were divided by hand into small clumps with a diameter < 1 cm. These were placed individually in 140-mL square propagation tubes (50 mm wide) in a shadehouse at Melbourne University and maintained at ambient conditions. They were grown in a soil mix involving a ratio of four parts ground medium-grade pinebark to one part coarse mined sand, with several additives (per m3): 4000 g Debco Green Jacket® No.2 (N:P:K of 16.5:14.1:9.6), 1500 g Saturaid®, and dolomite (1000 g/m3). The air filled porosity (AFP) of this mix was 24%, similar to alpine soils. Plants were manually watered every 2 days (to container capacity) or less often depending on rainfall.
Seed was collected from field transects in January and February 2005. It was weighed and tested for viability (which was always > 40%) before being dried at 30°C for several hours and placed in cold storage (4–5 °C) for later use. In August 2005, a sample of seed from high and low altitude sites of each transect was sown in a heated glasshouse at Melbourne University. Seedlings were initially raised in a mix of five parts medium-grade pinebark, five parts fine pinebark, one part coarse sand, and one part sieved peat. Additives (per m3) were 1500 g Saturaid (Debco, Tyabb, VIC, Australia) and 750 g dolomite. Seeds were covered with vermiculite (to retain moisture) in 56-cell trays (cell size 36 × 38 × 65 mm). After three to four weeks, germinated seedlings were transferred to the same soil mix used for the clonal material in 140-mL square propagation tubes. Watering in the glasshouse was automatic and was adjusted to water to container capacity once a day or less often depending on climatic conditions. The controller in the glasshouse was a Richdel 6 station with irrigation spray nozzles (Cameron mist nozzles # 03, Cameron Irrigation Co., Ltd., Littlehampton, West Sussex, U.K.), which delivered water at 112 L per hour at 100 KPA. Insecticide (Pyrethrum, Yates Australia, Padstow NSW, Australia): active constituents 0.3 g/L pyrethrins, 1.2 g/L piperonyl butoxide) was applied when needed to provide aphid control, generally every month. Clonal and seed material were also treated with Mancozeb fungicide (Kendon Chemical and Manufacturing Co. Pty. Ltd., Thornbury, VIC, Australia) (active constituent—800 g/kg mancozeb) to treat any outbreaks of disease when needed (at a rate of 15 g per 10 L of water—10 L spray applied per 10 m2, repeated every 10 days during susceptible periods).
Both seeds (after germination) and clonal material were grown for ∼3 months to establish root systems in the pots but before roots were crowded (i.e., when roots were just starting to emerge from the propagation tubes). Alpine plants store most of their reserves (carbohydrates) underground as an adaptation for survival during winter periods, so an adequate root system is essential for successful establishment (Mooney and Billings 1960; Korner 1999). At this stage plants were ∼5- to 10-cm high and their circumference was ∼5–6 cm.
Vegetative clones were planted in the field in April 2005 (autumn) and seedlings in November 2005 (spring). Clonal material and seedlings from each transect-altitude combination were planted at each of the high and low altitude sites at the three transects (including a home site control). This gave a total of 3 (transect) × 2 (altitude) × 15 (replicate) × 3 (transect of origin) × 2 (altitude of origin) = 540 transplants for clonal material and the same number for the seedlings. The planting density (∼15 plants per m2) approximated the mean density of P. hiemata at the source sites at the time when source material was collected. To assist with successful initial establishment of seedlings, they were planted into 1 m2 coconut-fiber matting that provided biodegradable mulch. Seedlings were watered for the first few weeks to help establishment as 2005 and 2006 were particularly dry seasons. As many aspects of regional and range adaptation are related to variation in plant size (Geber and Eckhart 2005), leaf length (average of 2 longest leaves per plant) and plant circumference of all replicates were measured three times during the snow-free period in early December, mid-to-late January and late February/early March for two years. Plant survival was also scored. Measurements on both clonal material and seedlings were taken from December 2005.
COMMON GARDEN EXPERIMENT
In August 2005, a random sample of viable seed (from storage) was also taken from high-, mid- and low-altitude sites and grown in a glasshouse at Melbourne. This experiment involved a total of 3 (transect) × 3 (altitude) × 25 (replicate) = 225 plants. Seeds were first germinated in the seed raising mix (as above), and after ∼3–4 weeks they were transferred to the soil mix used in the propagation tubes (as above). Plants were measured (leaf length, circumference) after 1.5 years to assess the development of differences in growth form of the plants. The watering system and insecticide/fungicide treatments were as outlined for the transplants. Temperatures in the glasshouse varied from 10 to 40 °C during the 1.5 years that plants were grown.
ANALYSES
Comparing altitudes
Linear regression was used to examine changes in plant growth form with altitude at all three sites. Polynomial terms for altitude were also included in the regressions but these were never significant and are not presented. To compare sites, we undertook ANOVAs on the entire dataset and included terms for the sites as well as altitude. Post hoc Tukey b tests were used to examine which sites differed significantly for plant morphology. All analyses were carried out with SPSS for Windows version 14.
For the transplant experiments, we undertook four-way ANOVAs with (A) altitude of origin, (B) transect of origin, (C) transect planted, and (D) altitude planted as the factors and treating each surviving plant as a datapoint. The altitude and transect terms were treated as fixed rather than random effects because we chose altitudes near the extremes of the P. hiemata range, and because the transects were selected for the convenience and accessibility rather than being chosen randomly. Leaf length and plant circumference data did not deviate from normality according to Anderson-Darling tests and were therefore not transformed. To analyze survival data, we computed the mean percent survival of plants (out of 15) for a particular treatment combination at each site. These data did not deviate from normality and not transformed prior to analysis. Because there were no replicate datapoints for survival data (plants from each treatment at a site were pooled to estimate survival), we pooled three-way interactions in the ANOVAs on survival to obtain an error estimate.
To assess whether patterns of altitudinal variation corresponded to countergradient or cogradient selection, we graphically compared all “away” responses from the reciprocal transplant experiments (seedlings and clonal material placed at an altitude different to their origin) with the phenotypic response of both traits by local plants across each transect separately. To directly compare genetic (G) and environmental (E) effects on the differences in altitudinal variation, we computed differences between treatments separately for each transect. We computed E by averaging the absolute mean difference between plants from the high site grown at the low versus high site and plants from the low site being grown at the low versus high site. We computed G by averaging the absolute mean difference between plants from the high and low sites being grown at the high site, and plants from the high and low sites being grown at the low site. These values were then expressed as a percentage of combined difference (G + E) for each site.
In the common garden experiments, the effects of transect and altitude of origin on leaf length and circumference were examined using two-way ANOVAs with Post hoc Tukey b tests to examine which transects differed significantly for plant morphology.
Fitness and growth form
Survival data from plants measured for leaf length and circumference at the beginning of the growing season were used to assess whether leaf length and circumference influenced plant fitness. This analysis was undertaken with trait data collected in February 2006 before winter, and survival data collected in December 2006 in the middle of the growing season. Trait measures from late 2005 were not used as plants were still recovering from snow burial in May–October 2005.
We used both linear and binary logistic regression analyses to infer the strength and direction of selection at each high/low altitude site for each transect following Janzen and Stern (1998). We assessed the effects of the two traits on survival simultaneously in multiple regressions as well as individually. For the logistic regressions, we standardized coefficients by the standard deviation and then determined probabilities for individual datapoints from the regressions. These probabilities (P) were then multiplied by 1 −P; the average values across all datapoints were multiplied by the regression coefficients to obtain selection gradients. In these analyses of selection at a particular altitude and transect, we combined data across all plants growing at that site regardless of their origin, to obtain adequate sample sizes for analyses.
When significant (P < 0.05) associations between traits and survival were detected, we examined the shape of the fitness relationship using a nonparametric method (cubic spline) as implemented in the program glms40 (Schluter 1988; Schluter and Nychka 1994). Bootstrap routines were used to generate confidence intervals of the fitness relationships although these should be interpreted cautiously (Schluter 1988; Schluter and Nychka 1994).
By combining individuals from different origins in these analyses, we ignored ways in which plants might differ between locations (apart from circumference and leaf length) that might influence their survival. To directly assess selection on circumference and length, plants from the same population should ideally be assessed, although in our experimental design there were too few datapoints for such an analysis. Nevertheless we did consider whether plants from the same site/transect combination planted at a particular site showed consistent patterns in their regression slopes, to test whether there were consistent within-population associations between survival and leaf length/circumference.
Results
ALTITUDINAL VARIATION IN GROWTH FORM
Linear regression analyses indicated highly significant changes in leaf length and circumference with altitude. Regressions for circumference were significant for the New Country (R2= 0.307, F1, 438= 194.09, P < 0.001), Nelse Central (R2= 0.367, F1, 438= 254.31, P < 0.001), and Cope (R2= 0.188, F1, 438= 102.07, P < 0.001) transects. Regressions for leaf length were also significant for the New Country (R2= 0.599, F1, 438= 656.11, P < 0.001), Nelse Central (R2= 0.455, F1, 438= 366.56, P < 0.001), and Cope (R2= 0.500, F1, 438= 438.36, P < 0.001) transects. With increasing altitude, mean length of leaves decreased and circumference increased, a pattern consistent across all three transects (Fig. 2). Circumference values tended to be more variable than those for leaf length as reflected by larger standard errors.
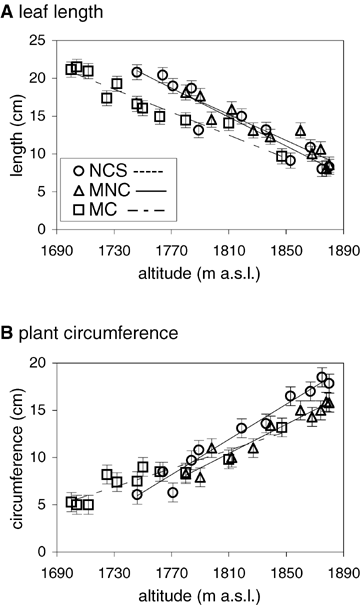
Linear regression of leaf length (A) and plant circumference (B) against altitude (m a.s.l.) for Poa hiemata. Values are mean (± SE mean) at each of the three sites. Key: NCS (New Country Spur), MNC (Mount Nelse Central), and MC (Mount Cope).
Despite the consistent altitudinal patterns across transects, there are also some differences in the intercept of the altitudinal relationships for both traits (Fig. 2). In an ANCOVA with altitude as a covariate, there was a significant difference between transects for leaf length (F2, 1314= 54.09, P < 0.001) and circumference (F2, 1314= 83.13, P < 0.001). This reflected the lower leaf lengths at Cope across the entire altitudinal range and relatively greater circumference of the New Country plants (Fig. 2). For circumference, there was also a difference in the slope of the altitudinal relationships as reflected by a significant interaction between altitude and transect (F2, 1314= 24.13, P < 0.001).
TRANSPLANTS
Survival
The four-way ANOVAs indicated a highly significant (P < 0.001) interaction between altitude of origin and altitude where plants were transplanted for both the seedling and clonal data (A × D interaction; Table 1). Means from interaction plots (Fig. 3) indicate that genotypes tended to survive better at the same altitude from which they originated regardless of transect. This is reflected in the consistent improvement in survival in all three high altitude populations when transplanted to high altitude sites (six out of six instances in the overall data from the seedling and clonal material; Fig. 3) and similarly the consistent improvement (six out of six) when low altitude populations were transplanted to low altitude versus high altitude sites.
| Source | Clonal transplants | Seedling transplants | ||||
|---|---|---|---|---|---|---|
| df | MS | F | df | MS | F | |
| Altitude of origin (A) | 1 | 51.3 | 0.20 | 1 | 169.3 | 1.41 |
| Transect of origin (B) | 2 | 41.7 | 0.16 | 2 | 1062.4 | 8.86** |
| Transect planted (C) | 2 | 2279.6 | 9.15** | 2 | 512.1 | 4.27* |
| Altitude planted (D) | 1 | 742.0 | 2.97 | 1 | 40.7 | 0.34 |
| A × B | 2 | 161.5 | 0.64 | 2 | 50.3 | 0.42 |
| A × C | 2 | 28.1 | 0.11 | 2 | 79.0 | 0.66 |
| A × D | 1 | 5051.0 | 20.28*** | 1 | 3015.1 | 25.14*** |
| B × C | 4 | 207.3 | 0.83 | 4 | 120.6 | 1.01 |
| B × D | 2 | 216.1 | 0.86 | 2 | 124.4 | 1.04 |
| C × D | 2 | 3810.6 | 15.30*** | 2 | 406.9 | 3.39 |
| Error | 4 | 32.8 | 16 | 119.9 | ||
- *P < 0.05;**P < 0.01;***P < 0.001
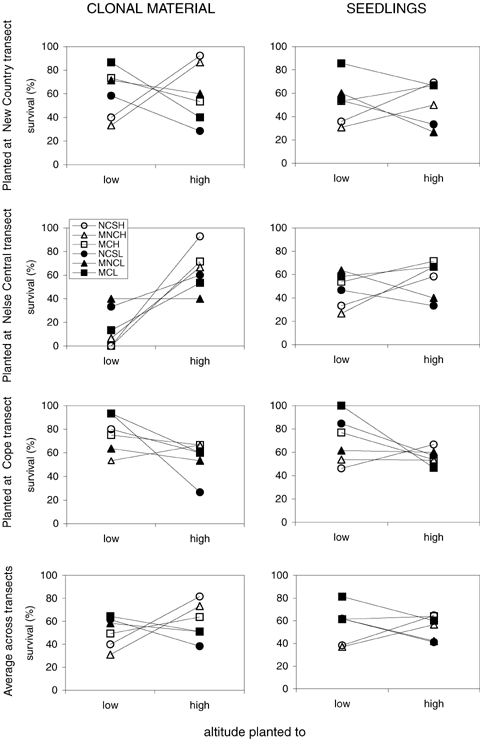
Interaction plots for fitness (mean survival (%)) from reciprocal transplant of Poa hiemata clonal (left column) and seedling (right column) experiments. Rows indicate sites planted to including a combined response of all three transects. The altitude plotted is provided on the x-axis. Key depicts transect and altitude of origin of transplants respectively: NCSH (New Country Spur high), MNCH (Mount Nelse Central high), MCH (Mount Cope high), NCSL (New Country Spur low), MNCL (Mount Nelse Central low), and MCL (Mount Cope low).
Circumference
The four-way ANOVA (Table 2) indicated a significant effect of altitude of origin (A) in both the seedling and clonal data. High altitude genotypes maintained a larger circumference regardless of altitude planted to when compared to low altitude genotypes (Fig. 4), suggesting genetic differentiation between high and low altitude genotypes regardless of environmental conditions.
| Source | Clonal transplants | Seedling transplants | ||||
|---|---|---|---|---|---|---|
| df | MS | F | df | MS | F | |
| Altitude of origin (A) | 1 | 125.02 | 54.61*** | 1 | 57.74 | 43.56*** |
| Transect of origin (B) | 2 | 17.61 | 7.69** | 2 | 14.40 | 10.87*** |
| Transect planted (C) | 2 | 26.61 | 11.62*** | 2 | 12.50 | 9.43*** |
| Altitude planted (D) | 1 | 113.51 | 49.59*** | 1 | 58.92 | 44.44*** |
| A × B | 2 | 2.57 | 1.12 | 2 | 6.61 | 4.99** |
| A × C | 2 | 0.26 | 0.11 | 2 | 2.07 | 1.56 |
| A × D | 1 | 7.55 | 3.30 | 1 | 6.42 | 4.84* |
| B × C | 4 | 6.04 | 2.64* | 4 | 5.70 | 4.30** |
| B × D | 2 | 4.93 | 2.16 | 2 | 17.12 | 12.91*** |
| C × D | 2 | 77.28 | 33.76*** | 2 | 21.70 | 16.37*** |
| Error | 427 | 2.29 | 253 | 1.33 | ||
- *P < 0.05;**P < 0.01;***P < 0.001
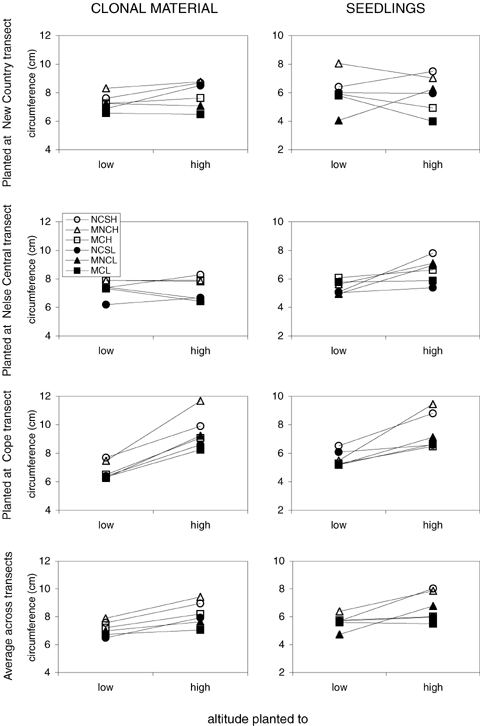
Interaction plot for mean plant circumference (cm) from reciprocal transplant of Poa hiemata clonal (left column) and seedling (right column) experiments. Rows indicate sites planted to including a combined response of all three transects. The altitude plotted is provided on the x-axis. Key depicts transect and altitude of origin of transplants respectively: NCS (New Country Spur high), MNCH (Mount Nelse Central high), MCH (Mount Cope high), NCSL (New Country Spur low), MNCL (Mount Nelse Central low), and MCL (Mount Cope low). Error estimates (± SE mean) ranged within 0.16–0.87 (clonal), and 0.21–0.46 (seedling) when planted to NCS; 0.25–0.50 (clonal) and 0.11–0.41 (seedling) when planted to MNC; 0.22–0.69 (clonal) and 0.14–0.45 (seedling) when planted to MC.
Environmental effects on plant circumference were evident from the effects of transect planted (C) and altitude planted (D) for both clonal and seedling data, reflecting plasticity for this trait. (Fig. 4). Plants placed at low altitudes had a smaller circumference than those placed at higher altitudes, consistent with differences in the altitudinal transect. There were also significant interactions between C and D in the two datasets, suggesting that environmental effects of altitude depended on the transect involved.
Leaf length
The ANOVAs indicated significant overall differences depending on whether the plants originated from high or low altitude sites (Table 3). Plants from high altitudes had shorter leaf lengths than those from lower altitudes regardless of where they were transplanted (Fig. 5). These patterns were evident in both the clonal and seedling material. The transect from which plants originated also influenced leaf length in the vegetative clone transplants but not the seedlings (Table 3). Leaf lengths of Cope plants grown from clonal material tended to be longer than those from other sites (see Fig. 5—average across transects). There were no significant interactions involving altitude of origin, suggesting that genotypes from different altitudes expressed different phenotypes regardless of where they were transplanted. However for the clonal material there were interactions between transect of origin and both altitude and transect at which the plants were placed (B × C, B × D) (Table 3).
| Source | Clonal transplants | Seedling transplants | ||||
|---|---|---|---|---|---|---|
| df | MS | F | df | MS | F | |
| Altitude of origin (A) | 1 | 468.13 | 102.57*** | 1 | 86.68 | 29.13*** |
| Transect of origin (B) | 2 | 58.57 | 12.83*** | 2 | 8.31 | 2.79 |
| Transect planted (C) | 2 | 5.05 | 1.10 | 2 | 7.35 | 2.47 |
| Altitude planted (D) | 1 | 1036.20 | 227.04*** | 1 | 13.22 | 4.44* |
| A × B | 2 | 11.01 | 2.41 | 2 | 2.32 | 0.78 |
| A × C | 2 | 11.36 | 2.49 | 2 | 4.15 | 1.40 |
| A × D | 1 | 16.11 | 3.53 | 1 | 1.16 | 0.39 |
| B × C | 4 | 77.57 | 16.99*** | 4 | 3.98 | 1.34 |
| B × D | 2 | 45.25 | 9.91*** | 2 | 14.11 | 4.74* |
| C × D | 2 | 138.76 | 30.40*** | 2 | 1.66 | 0.56 |
| Error | 415 | 4.13 | 251 | 2.98 | ||
- *P < 0.05;**P < 0.01;***P < 0.001
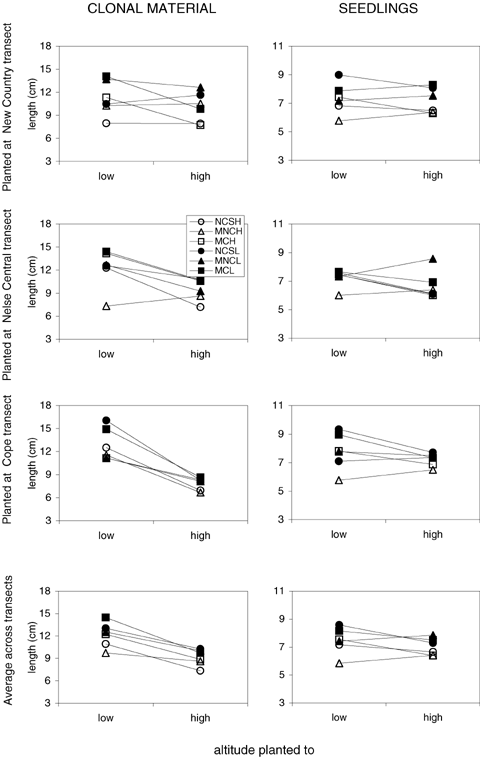
Interaction plot for mean leaf length (cm) from reciprocal transplant of Poa hiemata clonal (left column) and seedling (right column) experiments. Rows indicate sites planted to including a combined response of all three transects. The altitude plotted is provided on the x-axis. Key depicts transect and altitude of origin of transplants respectively: NCS (New Country Spur high), MNCH (Mount Nelse Central high), MCH (Mount Cope high), NCSL (New Country Spur low), MNCL (Mount Nelse Central low), and MCL (Mount Cope low). Error estimates (± SE mean) ranged within 0.32–0.84 (clonal) and 0.09–0.73 (seedling) when planted to NCS; 0.36–0.50 (clonal) and 0.12–0.60 (seedling) when planted to MNC; 0.48–0.82 (clonal) and 0.30–0.64 (seedling) when planted to MC.
Environmental effects were indicated by a significant effect of altitude where plants were grown in both the clonal and seedling transplants (Table 3). This reflected a relatively longer leaf length in plants grown at the lower altitudes. Differences in leaf lengths were similar when plants were grown at the different transects, although there was a transect by altitude planted interaction for the clonal material (C × D).
Comparing genetic and environmental effects on altitudinal differences
To directly compare the effects of genetic and environmental factors on altitudinal differences, we computed the mean difference between plants grown in their home and away sites separately for each transect. Both factors contributed to altitudinal differences, although this varied between transects, and environmental effects tended to be larger at Cope (Table 4). Overall, altitudinal differences in circumference had a genetic component of 33%, whereas differences in leaf length had a genetic component of 60%.
| Trait | Source | Transect | |||||
|---|---|---|---|---|---|---|---|
| NCS | MNC | MC | |||||
| Clonal | Seedling | Clonal | Seedling | Clonal | Seedling | ||
| Circumference | G | 26 | 63 | 66 | 19 | 18 | 8 |
| E | 74 | 37 | 34 | 81 | 82 | 92 | |
| Length | G | 84 | 61 | 59 | 69 | 31 | 58 |
| E | 16 | 39 | 41 | 31 | 69 | 42 | |
Countergradient or cogradient patterns
To examine these patterns, we plotted the mean length and circumference values of material taken from a high altitude and planted to a low altitude and vice versa, and then compared them to the phenotypes of local plants occurring naturally (Fig. 6). If countergradient variation was present, we would expect material from the low altitude transplanted to the high altitude to fall below the line joining the phenotypic variation for length (see Loman 2003). If CoGV occurs, the opposite patterns are expected. Clearly the clonal responses showed CoGV at all transect sites (Fig. 6). Note that for Cope, the low-to-high altitude response fell on the line for natural field variation line, so both lines are not visible. Seedling responses also showed CoGV at all sites, except that the low-to-high altitude mean response fell below the line for natural variation at Cope.
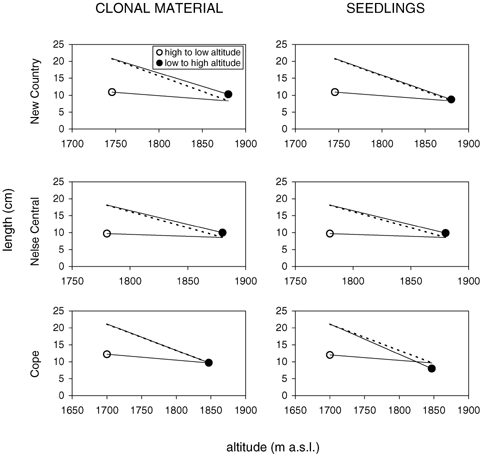
Cogradient variation for leaf length in Poa hiemata vegetative clones (left column) and seedlings (right column). Observed variation in the field is given by the dashed lines, and mean response of phenotypes after reciprocal transplanting is given by the solid lines when the material originated from a high altitude site and was planted at a low altitude (open circles) or vice versa (closed circles). Transplants at the three transects (New Country, Nelse Central, and Cope) are presented separately.
Comparisons of natural field variation in circumference with reciprocal transplant values for clonal material and seedlings also revealed CoGV at all sites (Fig. 7). However, natural variation between altitudes tended to be steeper than heritable variation in the seedling and clonal transplants, which reflects the fact that there were environmental as well as heritable differences between the altitude sites.
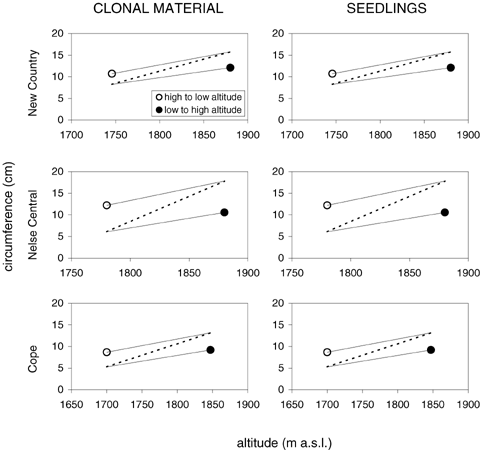
Cogradient variation for plant circumference in Poa hiemata vegetative clones (left column) and seedlings (right column). See Figure 6 legend for explanation.
GROWTH FORM AND RELATIVE FITNESS
Logistic regression analysis on survival data revealed contrasting selection gradients for the length and circumference traits at the two altitudes. For clonal data obtained from low altitudes, plants with a long leaf length had a higher probability of survival, as reflected by positive coefficients, although none of these coefficients was individually significant (Table 5). In contrast, the negative coefficients for plant circumference suggest the opposite pattern. At a high altitude, coefficients for leaf length were negative, suggesting that plants with shorter leaves had a higher survival, a pattern that was significant in two of the three individual comparisons. Conversely, coefficients for circumference were positive (significantly in two cases), reflecting higher survival for plants with large circumferences.
| Altitude | Trait | Transect | Clonal data | Seedling data | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| α | SE | P | C | βavggrad | α | SE | P | C | βavggrad | |||
| Low | Length | NCS | 0.390 | 0.077 | 0.117 | −0.665 | 0.083 | 1.51 | 0.227 | 0.001 | −3.07 | 0.256 |
| MNC | 0.179 | 0.098 | 0.569 | −2.37 | 0.023 | 1.28 | 0.272 | 0.002 | −3.81 | 0.234 | ||
| MC | 0.487 | 0.097 | 0.106 | −0.548 | 0.261 | 1.67 | 0.342 | 0.001 | −4.60 | 0.187 | ||
| Circumference | NCS | −0.122 | 0.114 | 0.628 | 1.06 | −0.027 | −1.64 | 0.395 | <0.001 | 8.10 | −0.255 | |
| MNC | −0.198 | 0.208 | 0.526 | −0.727 | −0.026 | −1.40 | 0.383 | <0.001 | 7.14 | −0.230 | ||
| MC | −0.346 | 0.214 | 0.268 | 3.09 | −0.150 | −1.02 | 0.512 | 0.008 | 7.60 | −0.128 | ||
| High | Length | NCS | −1.22 | 0.136 | <0.001 | 5.68 | −0.201 | −0.563 | 0.192 | 0.070 | 3.31 | −0.102 |
| MNC | −0.808 | 0.148 | 0.011 | 4.84 | −0.133 | −0.619 | 0.185 | 0.028 | 3.14 | −0.125 | ||
| MC | 0.312 | 0.123 | 0.206 | −0.615 | 0.070 | −0.510 | 0.201 | 0.061 | 3.20 | −0.103 | ||
| Circumference | NCS | 0.650 | 0.190 | 0.027 | −2.35 | 0.124 | 0.821 | 0.267 | 0.017 | −1.64 | 0.141 | |
| MNC | 0.295 | 0.168 | 0.298 | −0.117 | 0.053 | 1.81 | 0.403 | <0.001 | −5.94 | 0.274 | ||
| MC | 0.625 | 0.109 | 0.018 | −1.76 | 0.132 | 1.73 | 0.360 | <0.001 | −5.53 | 0.269 | ||
The same pattern was evident in the seedling data (Table 5). Coefficients were consistently different between altitudes and in the opposite direction for leaf length and circumference. For the seedling data, most (7/9) coefficients were individually significant at the 5% level. Patterns of survival selection are therefore consistent with those observed for the clonal material even though these experiments cover different time points.
Fitness curves were computed to examine changes in survival with different trait values and are presented in Figure 8 for cases in which coefficients were significant. These indicate that selection tended to be directional and monotonic for both traits. The only exception appears to be leaf length at Nelse Central where individuals with intermediate leaf lengths tended to have a higher fitness.
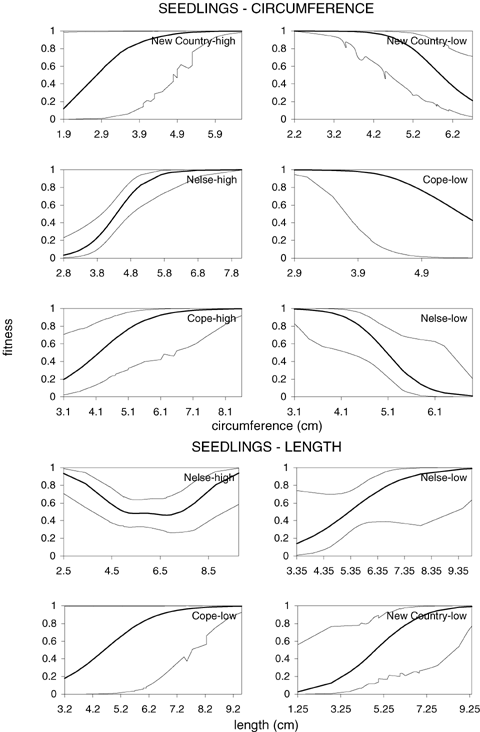
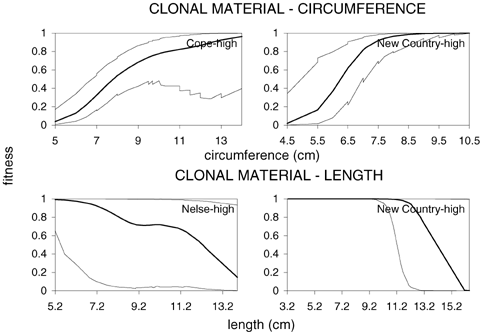
Association between survival and circumference (upper row of graphs) and leaf length (lower row of graphs) for significant regressions. Dashed lines are ± one SE of fitness associations (solid line) detected in the clonal and seedling experiments. Codes at upper-right side of graphs indicate transect (New Country, Nelse Central or Cope) and altitude planted (high = high altitude, low = low altitude).
To assess selection at the population level, we also computed regression coefficients for the individual populations to test for consistent associations between survival and both leaf length and plant circumference. Coefficients could not be computed in cases in which all plants from a treatment survived or died. For the clonal data collected at a high altitude, 7 of 14 (50%) of slopes for leaf length were negative. For plant circumference 10 of 14 (71%) had a positive slope. At low altitude, for leaf length, 8 of 12 (66%) of regressions had a positive slope, whereas 7 of 12 (58%) were negative for this trait. Overall the expected patterns were therefore present in 32 of 52 comparisons (64%). For the seedling data at high altitude, for leaf length 12 of 15 (80%) showed a negative response. For circumference 15 of 15 (100%) showed a positive response. At low altitude, for leaf length 15 of 15 (100%) were positive, and for circumference 15 of 15 (100%) were negative.
Therefore for the seedling data 57 of 60 (95%) patterns were in the expected direction. These analyses suggest that associations between survival and both leaf length and plant circumference in the individual transect/altitude datasets were consistent with those evident from the pooled analysis, at least for the seedling data. This supports the conjecture that at high-altitude sites, leaf length and circumference are being selected in the opposite direction when compared to low-altitude sites.
Common garden experiment
This experiment provided additional evidence for genotypic differences associated with the altitude and transect. The two-way ANOVA indicated a significant effect of altitude (length, F2, 207= 16.35, P < 0.001; circumference, F2, 207= 15.39, P < 0.001) and transect (length, F2, 207= 15.67, P < 0.001; circumference, F2, 207= 6.95, P < 0.01), as well as a significant interaction (length, F4, 207= 6.64, P < 0.001; circumference, F24, 207= 3.99, P < 0.01). The interaction plots (Fig. 9) showed that, for mean leaf length, values tended to be higher for lower altitude sites, and also that values were higher for New Country than the other sites. For circumference, the opposite trend was apparent, with lower values for the low altitude sites and in this case higher overall values for the Cope site. Mid-altitude sites tended to be intermediate (such as for leaf length for Cope) or more similar to one of the parental values (such as for leaf length at New Country). Interaction effects reflected the fact that altitudinal patterns were weaker at some transects.
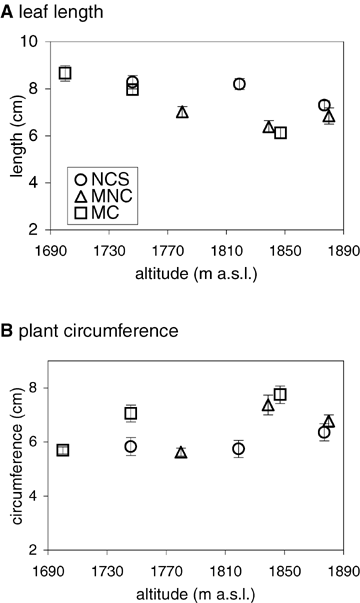
Interaction plot for mean (± SE) leaf length (A) and plant circumference (B) from common garden experiments. The x-axis represents altitude of origin. Key depicts transect of origin: NCS (New Country Spur), MNC (Mount Nelse Central), MC (Mount Cope).
Discussion
GROWTH FORM, FITNESS, AND ALTITUDE
The field measurements indicate that considerable variation in growth form exists in populations of P. hiemata within the Bogong High Plains in Victoria, Australia. This variation contributed to a significant linear association with altitude. The pattern was consistent across all three transects, suggesting adaptive differentiation or environmental effects common to the transects. Transplant experiments indicated that these patterns had a genetic component as well as being due to plastic responses. High altitude genotypes generally have a shorter (leaf length) and wider (circumference) appearance, whereas low altitude genotypes maintain a taller, slimmer stature regardless of planting altitude (high, low). This suggests that the traits have undergone past directional selection. Genotypes for small plant size have been found at high altitudes in other locations, where environmental conditions tend to reduce the size of all genotypes (Clausen et al. 1941; Conover and Schultz 1995; Angert and Schemske 2005). The Poa results complement other studies that suggest evolutionary adaptation to an expanded range is likely to require modifications in vegetative development (size, growth) (Geber and Eckhart 2005).
Our estimates of G and E suggest that on average around half the differences between plants from high and low sites are due to each of these components. The estimates were similar for clonal material and for seedlings even though the former may have included effects exerted early in plant development. There were some differences in patterns detected in clonal and seedling material, particularly focused around the effects of transect origin on growth form, but these differences could also have reflected the years across which measurements were taken, given that seedlings were transplanted at a later date than the clonal material.
Both the vegetative clonal and seedling transplants indicate that local plants tended to have relatively higher survival; this form of crossing interaction indicates a fitness advantage for populations growing in their home site. This result matches many other studies demonstrating local adaptation of plants to different environments (Barber 1965; Grant and Antonovics 1978; Briggs and Walters 1984). Site advantage is partly related to altitude, because populations from the low-altitude sites of other transects tended to perform relatively better than those from high altitudes. However site advantage also relates to local conditions on transect as reflected by differences between transects in the ANOVAs.
We found evidence that both leaf length and circumference relate to relative fitness of plants at the different altitudes and directly or indirectly affect survival of genotypes. As gene flow can act as a potentially large barrier to local adaptation (Linhart and Grant 1996; Sambatti and Rice 2006), in light of apparent local adaptation for both traits, either low levels of gene flow are present along the altitudinal gradients, or else selective pressures are strong enough to maintain differentiation between high- and low-altitude sites despite gene flow. Analyses of selection coefficients on various annual or short-lived herbaceous (small stature) species (Linhart and Grant 1996) suggest that for values of s = 0.1, differentiation usually can occur at 15–25 m, although the effect of gene flow can reduce this to 7 m. When values of s = 0.5 are reached, differentiation can occur in less than 10 m. In our case the selection coefficients are around 0.1 or greater, suggesting that genetic differences could easily have developed over the 1000-m transects considered here. The fitness analysis indicates that selection coefficients change between low- and high-altitude sites, and that they are in opposite directions for the two traits measured. The graphs suggest that selection is toward the extreme values of the range, reflecting strong directional selection on both traits that seem directly related with survival at different altitudes in the alpine zone. Other studies that have incorporated local adaptation experiments and selection analyses have demonstrated that traits in a population can be selected in opposing directions across environmental gradients. Because of this, immigrant genes may be selected against, and high- and low-altitude sites may remain genetically distinct even when migration occurs between them (Nagy and Rice 1997). The direction of altitudinal selection is consistently in the same direction suggesting parallel selection pressures at the different transects.
These selection gradients generate cogradient selection patterns: the reaction norm has been pushed in the same direction as the trait expression. Because both environmental and genetic differences act in the same direction, the plastic responses as well as genetic changes are in the direction of increased adaptation. Classic work (Turesson 1922; Clausen et al. 1941) and many subsequent studies (Bradshaw 1965; Schlichting and Levin 1986; Schlichting and Smith 2002) have shown the potential of phenotypic plasticity in having an adaptive basis and this can change in response to climate (Jump and Penuelas 2005).
The exact reason why shorter leaves and a wider circumference might be favored at high altitudes with the converse phenotype being favored at a low altitude is unclear. There are many potential selective factors associated with changes in altitudinal gradients, such as moisture, temperature, exposure, competition, wind, snowmelt, and soil conditions. The effects of single factors could not be isolated in this study and the variation in growth form may reflect selection due to a combination of these factors (Linhart and Grant 1996). At higher altitude, plants may be exhibiting smaller phenotypes as a selection response to reduce water loss through transpiration (Herms and Mattson 1992; Etterson and Shaw 2001). At lower altitudes biotic pressures may be playing a role, as competition for space causes a shift in selective pressures (Arendt 1997).
RESPONSE TO FUTURE CLIMATE CHANGE
As conditions become warmer and drier in the Australian Alps, selection will favor different phenotypes, and population persistence may depend on evolutionary change in P. hiemata across its range. The high-altitude genotypes of P. hiemata are already at the geographical edge of their range limit. Over time there may be evolution in the direction of local adaptation that favor trait values selected at lower altitudes. There is already evidence that low-altitude species are shifting up mountains to higher altitudes (Parmesan 2006). However, environmental changes could be countered by natural selection if there is genetic variation within alpine species. The findings from this study indicate that P. hiemata has the potential to respond to global warming that includes an adaptive plastic response as well as genetic shifts. We anticipate that cogradient selection may lead to longer leaves and a smaller plant circumference in P. hiemata. However this will depend on the nature of the changes in climatic conditions and biotic factors over time and the types of traits favored by these changes. Revegetation efforts of alpine sites often involve P. hiemata. The genetic background of P. hiemata is likely to influence the long-term success of such efforts, as local adaptation might aid or hinder establishment of the plants. Given the effects of altitude and transect on plant survival, revegetation could best be achieved through material from a local population or a mix of altitudes to maximize the potential for adaptation.
Associate Editor: J. Kohn
ACKNOWLEDGMENTS
The authors thank Parks Victoria for vehicle access to sites and other advice, N. Walsh and H. Wahren for assistance with Poa taxonomy, L. McPhee and A. Campbell for advice on plant propagation, K. Kent and E. Thomas for fieldwork assistance, S. Hadden for flora permits as well as D. Williams and F. Jarrad for comments on the manuscript. The authors acknowledge financial support from Australian Research Council (ARC) through their Linkage and Federation Fellowship programs as well as the Department of Natural Resources and Environment, Parks Victoria, E.S Link, Mount Hotham Alpine Resort Management, Howmans Gap Alpine Centre, and Falls Creek Alpine Resort Management.




