Determination of Trace Elemental Concentrations in Document Papers for Forensic Comparison Using Inductively Coupled Plasma–Mass Spectrometry
Abstract
Abstract: With improvements in manufacturing procedures, comparing physical characteristics of paper samples may not offer sufficient discrimination among different vendors. In this work, the potential to differentiate paper samples based on trace element concentrations was investigated. Paper samples from two different vendors were microwave-digested and trace element concentrations (Mg, Al, Mn, Fe, Sr, Y, Ba, Ce, and Nd) were determined using inductively coupled plasma–mass spectrometry. Differences in concentration were assessed statistically using two-way ANOVA and Tukey’s honestly significant differences test. Elemental concentrations were shown to be consistent across a single sheet as well as within a single ream of paper for each vendor. Reams from vendor A were differentiated based on Al and Ba concentration while reams from vendor B were differentiated based on Mg, Mn, and Sr concentrations. Paper was differentiated according to vendor based on significant differences in Ba, Sr, Ce, and Nd concentrations.
Crimes committed with falsified or counterfeit documents cost our country billions of dollars each year (1). Nearly every major life event now involves some sort of written or typed document, be it a birth certificate, marriage certificate, property deed, or death certificate. Most daily transactions involve the transfer of a document, including checks, business contracts, and passports. Any of the aforementioned documents may be forged or falsified in some manner for personal or financial gain. Documents are also used in other crimes, for example as threatening letters and ransom notes. Questioned documents like these are submitted to the forensic lab for a variety of reasons: to determine their authenticity, to identify the author of the writings, or to determine the printer/typewriter that produced the document.
The analysis of questioned documents therefore involves different types of analyses including comparisons of the handwriting, ink, typescript, or print, as well as physical and chemical characterization of the paper itself. There has been considerable emphasis on comparing the ink (2–9) and toner (10–14) used in a document and even studies to determine age of the ink (15–21). While identifying the ink is certainly important, there are other aspects of document analysis that can be equally valuable, particularly analysis and comparisons of the paper on which the document is printed or written.
Traditional analysis of paper involves a comparison of the physical properties (e.g., dimensions, weight, and color) and the fluorescent properties which are used to determine the presence of optical brighteners in the paper. The fiber content of the paper can be determined microscopically and an experienced examiner can even identify the pulping process used during manufacture (22). Finally, the chemical composition of the paper can be determined using a variety of analytical techniques. Many chemical additives are used during the production of the paper to improve its color, strength, and opacity, to adjust pH, and even to prevent growth of microorganisms (23). Infrared reflectance spectroscopy can be used to determine the coating based on characteristic adsorption bands for the different coating materials (e.g., starch, calcium carbonate, and casein). The fillers and pigments in papers (e.g., kaolin clay, calcium carbonate, talc, mica, and titanium dioxide) are typically crystalline in nature and therefore can be determined by X-ray diffraction analysis (22).
Due to improvements in quality control during the modern manufacturing process, the final paper product is very uniform with few physical or chemical differences among paper samples of the same type. However, even if the main components of the paper are similar, there can still be variation in the trace elemental composition. As paper is produced from natural materials (e.g., wood pulp and clays) and recycled consumer waste, it is highly unlikely that different paper manufacturers will produce a product with identical elemental composition (1). Hence, elemental analysis can associate or discriminate paper samples that are otherwise indistinguishable based on physical and fluorescent properties.
Elemental analysis has been performed on document papers using a variety of analytical techniques (24–30). Blanchard and Harrison (25) determined an elemental “fingerprint” based on the clay content of specific papers using neutron activation analysis (NAA). Five paper samples were prepared from pulp with 15% of different clay filler added to each, then analyzed by NAA. Based on a comparison of the elements present in the paper and clay samples, the five paper samples were correctly associated with the clays that were added as fillers. Also using NAA, Brunelle et al. (26) determined that Ta, As, and Sn were less commonly observed in 600 paper samples from 10 manufacturers and hence, these elements would be most valuable for discrimination.
Although successful for this application, NAA is limited by the lack of availability in forensic labs and the need for a nuclear reactor. Polk et al. (27) used scanning electron microscopy with electron dispersive X-ray spectroscopy (SEM–EDS) to determine elemental composition of several writing papers. The most abundant elements detected in the paper samples were Al, Si, S, Ca, and Ti. Relative standard deviation (RSD) values for a single sheet and within a box of paper for all these elements was low (<5% for sheet and <10% for box), which enabled discrimination of sheets from different but identically labeled boxes. However, SEM–EDS has limited sensitivity for elements with atomic numbers larger than Na and relative, rather than absolute, concentrations are used for discrimination.
Inductively coupled plasma–mass spectrometry (ICP–MS) is a sensitive, multi-element technique that has also been applied for the determination of trace elements in document paper (29,30). Spence et al. (29) considered 17 different paper manufacturers: one ream was selected from each of 16 manufacturers and six reams were obtained from a single manufacturer. Five sheets were selected at random from each ream and a sample (30 × 40 mm) was cut from each sheet. The samples were then microwave-digested and analyzed using ICP–MS. Nine elements (Na, Mg, Al, Mn, Sr, Y, Ba, La, and Ce) were selected as potential discriminating elements; concentrations of these elements did not vary significantly in sheets from the same ream. Samples from all 17 paper manufacturers could be distinguished based only on elemental concentrations of Mn and Sr. In a separate study, papers from the same manufacturer but four different batches (sampled monthly) were statistically distinguished using Al, Zr, and Mn; however, differences in consecutively manufactured rolls could not be determined (29).
While the success of trace elemental composition in differentiating papers from different manufacturers has been demonstrated, differences within and among reams of the same type of paper from the same vendor have not yet been explored in detail. Forensically, it would be useful to not only differentiate paper from different vendors but also to differentiate paper from the same vendor according to ream. The ability to detect such differences would add another level of specificity to the current analysis and comparison of paper.
In this research, the trace elemental composition of 100% recycled document (multipurpose copy) paper was determined, aiming to specifically investigate not only the potential of differentiating reams of paper from the same vendor but also differentiating reams of paper from different vendors. In this work the term “vendor” is used to describe a commercial label where different vendors sell paper that may or may not have been produced by the same manufacturer. Document paper from two U.S. vendors (five reams from each) was purchased and samples were microwave-digested prior to ICP-MS analysis. Several statistical comparisons were made to assess the potential of differentiating the paper samples based on elemental composition. First, the homogeneity of the elemental composition across a single sheet and within a ream of paper was investigated to choose elements suitable for comparison between reams. Next, a statistical comparison was made among reams from the same vendor to investigate differentiation of the reams. Finally, a comparison among reams from the two vendors was made, aiming to distinguish different vendors based on elemental content of the papers.
Materials and Methods
Paper Samples
Five reams (each containing 500 sheets) of white document paper (8½ × 11 inches) made from 100% postconsumer waste were obtained from two different vendors, chosen due to their ready availability in the Lansing, MI area. All reams were 20 lb weight and were labeled acid- and chlorine-free. For vendor A, four reams were obtained from different offices in the Chemistry building at Michigan State University while the fifth ream was obtained from a local branch of a national chain of copy centers. All five reams had the same vendor label and each ream had a different number, which was potentially a lot or production batch number but this could not be confirmed. For vendor B, all five randomly selected reams were purchased from a local branch of a national office supply chain and no unique markers were observed on the packaging. Three sheets were selected from each ream for analysis: the top sheet, a sheet from approximately the middle of the ream, and the bottom sheet.
Five samples (one from each corner and one from the center of the page) were cut from each individual sheet of paper, using plastic scissors. All samples were subsequently handled using plastic tweezers and gloves to prevent any elemental contamination. Each sample was c. 23 × 18 mm, which corresponded to a mass of 0.029 ± 0.001 g.
Microwave Digestion of Paper Samples
Paper samples were digested in a microwave digestion unit (Ethos EX; Milestone, Inc., Shelton, CT) equipped with an internal temperature probe. The paper samples were placed individually into quartz vessels (Milestone, Inc.) that had previously been acid-washed. To the quartz vessel, 1.5 mL of Optima grade nitric acid (69%; Fisher Chemicals, Pittsburgh, PA) and 0.75 mL of hydrogen peroxide (30%; J.T. Baker, Phillipsburg, NJ) were added. The quartz vessel was then capped and placed into an outer Teflon® vessel (Milestone, Inc.) to which 11 mL of ultra high purity water (Barnstead, Dubuque, IA or Millipore, Jaffrey, NH) and 1 mL of 30% hydrogen peroxide (J.T. Baker) were added. The Teflon® vessel was then sealed with a Teflon® cap. Five samples and one procedural blank (prepared in the same way but without paper present) were prepared at once using the following digestion program: heat from room temperature to 210°C over a 15-min period, then hold at 210°C for 10 min. The system automatically adjusted applied microwave power (wattage) to obtain and maintain the desired temperature.
Following digestion, the Teflon® vessels were allowed to cool to below 100°C. The quartz vessels were removed from the Teflon® vessels and allowed to cool to room temperature with the quartz tops in place to prevent evaporative changes in volume. The paper in the quartz vessel was partially digested; the resulting solution was clear with a small amount of white particulate matter. The particulate matter was chemically characterized and is discussed in more detail in the accompanying Technical Note (31). Through characterization and analysis of the particulate matter, it was determined that the concentrations of the elements of interest in the clear digest solution were not affected by the presence of the undigested particulates. Hence the clear digest solution was used for subsequent analysis.
The digests were further diluted to a final concentration of 2% HNO3, and stored in previously acid-washed 15 mL polystyrene conical vials (BD Falcon™, BD Biosciences, Franklin Lakes, NJ) until analysis. Between digestions of paper samples, the quartz vials were cleaned by performing a microwave digestion, using the same procedure described above, without the addition of a paper sample. The digest liquid from the cleaning step was discarded and the vials rinsed in ultra high purity water before being used again.
Analysis of Paper Digests by ICP–MS
Paper digests were analyzed on a Micromass Platform quadrupole ICP–MS (now Thermo Fisher Scientific, Waltham, MA) with hexapole collision cell using a CETAC ASX-500 autosampler (CETAC, Omaha, NE). Instrumental operating and data collection parameters are given in Table 1. Tune conditions were optimized daily using a 10 ppb solution of Be, Co, In, Ce, Bi, and U (plasma grade, Spex Certiprep, Metuchen, NJ) prepared in a 2% HNO3 solution.
| ICP–MS Operating Parameters | |
| Rf power (W) | 1350 |
| Ar flow rates (L/min) | |
| Outer | 13.00 |
| Auxiliary | 0.72 |
| Nebulizer | 0.71–0.73 |
| Sampling cone | Ni with Cu core, 1.14 mm diameter orifice |
| Skimmer cone | Ni, 0.89 mm diameter orifice |
| MS resolution | Unit mass |
| Data Collection Parameters | |
| Mode | Selected ion recording (peak jumping) |
| Dwell time (s) | 0.1 |
| Repeats | 1 |
| Autosampler | |
| Sample read delay (s) | 105 (1.75 m) |
| Rinse time (s) | 90 |
- ICP–MS, inductively coupled plasma–mass spectrometry.
The instrument was calibrated using multi-element external calibration standards (plasma grade; Spex Certiprep) prepared in 2% HNO3. Elements were grouped by concentration range in the calibration standards: 5–1000 ppb for Mg, Al, and Fe; 0.1–100 ppb for Sc, Mn, Zn, Sr, Zr, Sn, Ba, Pb, and U; and 0.05–10 ppb for Y and Nd. Prior to analysis, 1 mL of an internal standard solution containing 20 ppb of Bi and In (plasma grade; Spex Certiprep), also prepared in 2% HNO3, was added to 2 mL of each paper digest. Samples were analyzed using the conditions in Table 1. Calibration standards were run after every 20 samples.
The instrument response for each element was normalized to the internal standard; 115In was used as an internal standard for 24Mg, 27Al, 45Sc, 55Mn, 56Fe, 66Zn, 88Sr, 89Y, 90Zr, 120Sn, 138Ba, 140Ce, and 146Nd while 209Bi was used to normalize instrument response for 208Pb, 232Th, and 238U. The concentrations for the paper digest samples were then calculated using the calibration curve generated from the appropriate calibration standards run immediately before and after the digest to be quantified. Element concentrations in each digest were corrected for dilution factors and normalized to the paper weight measured prior to digestion to yield the concentration in μg element/g paper.
Statistical Treatment of Data
All statistical analyses were performed using OriginPro for windows 7.5 SR 4 (v7.5853; OriginLab Corp., Northampton, MA) or Excel 2000 (Microsoft® Corp., Redmond, WA). The paper samples were initially treated as two separate groups by vendor. The average elemental concentration (μg/g paper) was calculated for each sheet (n = 5) along with the RSD. Q-tests were performed on all data sets that had RSDs higher than 15%, removing the data points that were statistically determined to be outliers (confidence limit of 95%). Elements with an RSD greater than 15% after removing statistical outliers for any of the sheets tested were not used in any further data analysis procedures.
Two-way ANOVA was applied to the average elemental concentrations for each sheet tested to identify those elements that showed significant differences within and between reams. Significant differences were further tested using Tukey’s honestly significant differences (HSD) test.
To determine significant differences in elemental concentrations between the two vendors, Student’s t-test was performed, comparing the average concentration of each element for all paper samples between the two vendors. An F-test was performed for each element to compare the variance of the elemental concentrations for each vendor, at the 95% confidence limit. If the variances were statistically different the degrees of freedom (df) for the t-test comparison were calculated (32). The t-statistic was compared with tables of values at the 95% and 99% confidence limits (two-tail) using 8 degrees of freedom (n−2, n = 10) or the value calculated for the degrees of freedom.
Results
Selection of Elements to Discriminate Among Paper Samples
To identify elements suitable for discriminating the paper samples, a multi-step process was conducted. First, a review of the literature was undertaken to identify elements likely to be present in the paper samples (24–30). Then, a full mass scan of one paper digest (vendor A) was collected and the elements present were compared with the list generated from the literature review. Procedural blanks were then analyzed to identify and hence eliminate elements present due to contamination arising from the sample preparation procedure. Finally, the RSDs of the remaining elements within a single sheet of paper were determined to eliminate those elements with unacceptably high RSDs (>15%). High RSDs indicate nonuniformity across the sheet of paper and hence, those samples are not representative of the full sheet.
From the literature review, the following elements were identified as potentially useful for discrimination: 7Li, 23Na, 24Mg, 27Al, 45Sc, 55Mn, 52Cr, 55Rb, 56Fe, 59Co, 63Cu, 66Zn, 88Sr, 89Y, 90Zr, 107Ag 114Cd, 121Sb, 138Ba, 139La, 140Ce, 146Nd, 208Pb, and 232Th (24–30). However, based on the full mass scan, 7Li, 23Na, 52Cr, 55Rb, 59Co, 63Cu, 107Ag, 114Cd, 121Sb, and 139La were removed from the list as the concentrations of these elements were below background levels. Additional elements (e.g., 120Sn) were observed in the paper digest and were added to the list. At this stage, the list of elements to be considered in further analyses consisted of the following elements: 24Mg, 27Al, 45Sc, 55Mn, 56Fe, 66Zn, 88Sr, 89Y, 90Zr, 120Sn, 138Ba, 140Ce, 146Nd, 208Pb, and 238U. However, after all the paper samples had been analyzed, it was discovered that 238U concentration was significantly less in all samples than in the initial scan and was actually below the detection limit of the instrument, so 238U was eliminated from further analysis.
Following analysis of the procedural blanks, the list of potentially discriminating elements was reduced further. The elements 66Zn, 208Pb, and 90Zr were eliminated based on high variability (high RSDs relative to average concentration present) while 120Sn was eliminated based on high concentration (5 ppb) relative to the other elements in the procedural blanks. Such elements were not considered useful in discriminating the papers due to the high and variable background levels.
The variability in elemental concentration across a single sheet of paper was then assessed. Because only a portion of the sheet would be sampled in a forensic analysis scheme, it is important that a sample is representative of the whole sheet. RSD values were calculated for each sheet of paper based on the five samples analyzed per sheet (one from each corner and one from the center). Statistical Q-tests were used to check for outliers for elements with RSDs greater than 15%. Determined outliers were eliminated and the RSD recalculated. If the RSD was still greater than 15%, the element was eliminated from the suite of discriminatory elements. In general, the RSD for an elemental concentration within a sheet was 5–10%.
The instrument was then calibrated for each element remaining and 45Sc was subsequently eliminated due to a nonlinear calibration. Limits of quantitation for the remaining elements were determined as the lowest point on the linear calibration curve or listed as less than the lowest standard; these are listed in Table 2. A single digest sample was run in triplicate and yielded concentrations that had less than 3% RSD for the elements remaining in the list. Hence, the suite of elements that was used in all subsequent comparisons was as follows: 24Mg, 27Al, 55Mn, 56Fe, 88Sr, 89Y, 138Ba, 140Ce, and 146Nd.
| Element | Limit of Quantitation (ppb) |
|---|---|
| 24Mg | <5 |
| 27Al | 10 |
| 55Mn | <0.1 |
| 56Fe | <5 |
| 88Sr | 0.25 |
| 89Y | <0.05 |
| 138Ba | 0.25 |
| 140Ce | <0.05 |
| 146Nd | <0.05 |
Variation Within and Among Reams from Vendor A
Five samples from three sheets per ream were digested and analyzed by ICP-MS. The average concentrations of each element in each ream (n = 15) from vendor A are displayed in Table 3.
| Element | Within Ream Concentrations (μg element/g paper) | |||
|---|---|---|---|---|
| Vendor A | Vendor B | |||
| Average Concentration (μg element/g paper) | % RSD | Average Concentration (μg element/g paper) | % RSD | |
| Mg | (1) 1074.9 | 6.6 | (1) 852.8 | 8.7 |
| (2) 956.0 | 7.5 | (2) 942.5 | 7.4 | |
| (3) 1058.3 | 7.7 | (3) 616.7 | 4.8 | |
| (4) 969.4 | 5.3 | (4) 1004.4 | 6.9 | |
| (5) 1005.2 | 5.7 | (5) 853.2 | 9.0 | |
| Al | (1) 338.0 | 10.5 | (1) 410.4 | 15.3 |
| (2) 560.9 | 8.6 | (2) 458.7 | 9.0 | |
| (3) 321.6 | 9.9 | (3) 697.8 | 7.7 | |
| (4) 561.6 | 10.1 | (4) 477.2 | 9.1 | |
| (5) 445.5 | 22.9 | (5) 394.3 | 15.3 | |
| Mn | (1) 4.2 | 6.2 | (1) 4.1 | 8.1 |
| (2) 3.8 | 7.2 | (2) 5.0 | 7.3 | |
| (3) 4.1 | 7.2 | (3) 6.0 | 6.9 | |
| (4) 3.8 | 6.4 | (4) 4.7 | 8.7 | |
| (5) 4.1 | 8.2 | (5) 4.1 | 8.3 | |
| Fe | (1) 88.9 | 6.8 | (1) 75.9 | 11.5 |
| (2) 80.1 | 8.1 | (2) 89.6 | 6.1 | |
| (3) 83.0 | 7.0 | (3) 92.7 | 5.5 | |
| (4) 79.7 | 6.7 | (4) 91.5 | 7.1 | |
| (5) 83.2 | 7.8 | (5) 76.5 | 14.7 | |
| Sr | (1) 36.5 | 6.5 | (1) 57.6 | 8.1 |
| (2) 29.3 | 6.7 | (2) 53.4 | 7.4 | |
| (3) 35.5 | 8.2 | (3) 28.6 | 6.1 | |
| (4) 29.6 | 5.0 | (4) 56.4 | 7.4 | |
| (5) 33.8 | 10.8 | (5) 62.8 | 6.3 | |
| Y | (1) 0.10 | 8.6 | (1) 0.11 | 12.3 |
| (2) 0.09 | 10.1 | (2) 0.13 | 8.1 | |
| (3) 0.10 | 9.7 | (3) 0.23 | 8.5 | |
| (4) 0.10 | 7.4 | (4) 0.13 | 8.7 | |
| (5) 0.10 | 13.1 | (5) 0.11 | 12.9 | |
| Ba | (1) 12.0 | 7.4 | (1) 2.24 | 9.8 |
| (2) 7.0 | 8.4 | (2) 2.51 | 7.2 | |
| (3) 11.1 | 8.1 | (3) 2.66 | 7.3 | |
| (4) 6.9 | 10.1 | (4) 2.60 | 6.6 | |
| (5) 9.5 | 21.1 | (5) 2.18 | 8.1 | |
| Ce | (1) 0.34 | 5.8 | (1) 0.29 | 12.3 |
| (2) 0.34 | 7.6 | (2) 0.32 | 6.5 | |
| (3) 0.33 | 9.6 | (3) 0.26 | 5.8 | |
| (4) 0.34 | 5.6 | (4) 0.33 | 18.2 | |
| (5) 0.36 | 11.1 | (5) 0.28 | 13.39 | |
| Nd | (1) 0.16 | 4.8 | (1) 0.14 | 12.9 |
| (2) 0.15 | 6.5 | (2) 0.15 | 7.4 | |
| (3) 0.15 | 8.5 | (3) 0.15 | 7.1 | |
| (4) 0.16 | 5.6 | (4) 0.15 | 5.1 | |
| (5) 0.16 | 10.2 | (5) 0.13 | 15.4 | |
- RSD, relative standard deviation.
Two-way ANOVA was applied to the average elemental concentrations for each sheet tested to identify those elements that showed significant differences within and between reams. The two variables tested were the variance among sheets (five samples from each sheet) and the variance among the reams (15 samples; three sheets with five samples each). The null hypotheses were that there was no difference in the mean of the elemental concentrations of the sheets and that there was no difference in the mean of the elemental concentrations of the reams. The alternate hypotheses were the mean of the elemental concentrations of the sheets and reams were not equal. A p ≤ 0.05 was chosen to define a significant difference between elemental concentrations; at this p-value, the probability that the mean of the elemental concentrations were the same was 5% or less. Shown in Table 4 are the resulting p-values from the two-way ANOVA analysis for paper samples from vendor A.
| Element | p-Values from Two-Way ANOVA | |
|---|---|---|
| Sheets (n = 5) | Reams (n = 15) | |
| Mg | 0.491 | 0.007 |
| Al | 0.647 | 0.001 |
| Mn | 0.676 | 0.064 |
| Fe | 0.660 | 0.121 |
| Sr | 0.408 | 0.004 |
| Y | 0.495 | 0.258 |
| Ba | 0.453 | 0.001 |
| Ce | 0.453 | 0.405 |
| Nd | 0.201 | 0.486 |
- Significant differences are defined as p ≤ 0.05 and are shown in italics.
None of the elements showed significant variation in concentration within the three sheets tested from a single ream; all the p-values were >0.05. Hence, variation in elemental concentration was not significant within a single ream. This is important in forensic cases where only a small sample is available; it is important that the sample analyzed is representative of the whole sheet. Furthermore, a single sheet that has been removed from the ream will have the statistically indistinguishable elemental concentrations compared to other sheets in that ream. Also, there did not appear to be any contamination from contact with the paper wrappers on the reams as the top and bottom sheets were sampled and did not show significant elemental differences from a sheet sampled in the middle.
The average elemental concentrations among the five reams from vendor A were then compared and the resulting p-values are also displayed in Table 4. Four elements (Mg, Al, Sr, and Ba) were found to vary significantly among the reams and Tukey’s HSD test was then applied to investigate these differences further. The null hypothesis was there is no difference in the elemental concentration of the reams and the alternate hypothesis was there is a difference in the mean elemental concentration of the reams. The results of the Tukey’s HSD test are displayed in Fig. 1.
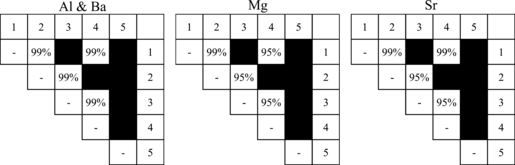
—Tukey’s honestly significant differences test results for vendor A. Significant differences between values with p ≤ 0.05 are represented by 95% and p ≤ 0.01 are represented with 99% to indicate the confidence that these two concentrations are different between the two reams. Black squares indicate the comparison between the two reams did not yield a statistically significant concentration difference. The numbers in the top row and in the right column indicate ream number.
Reams 1 and 3 were not distinguished from each other and reams 2 and 4 were not distinguished from each other based on the elements used for discrimination (Mg, Al, Sr, and Ba). However, reams 1 and 3 were distinguished from reams 2 and 4 (and vice versa) at least at the 95% confidence level based on concentrations of Mg, Al, Sr, and Ba. Ream 5 was not distinguished from any of the reams. Upon further inspection, elemental concentrations for ream 5 are within the range of concentrations in the other four reams. For example, ream 5 has a concentration of 445.5 μg Al/g paper, which falls within the range of 321.6 μg Al/g paper to 561.6 μg Al/g paper observed for reams 1 through 4. Interestingly reams 1, 3, and 5 had the same marking on the ream packaging while reams 2 and 4 had very similar markings on the packaging, suggesting that the ream markings had some association with a batch or manufacturing time frame. However, as the exact meaning of the marking could not be determined, this hypothesis cannot be substantiated.
Based on the number of pairs distinguished, Al and Ba were considered the most discriminatory elements to differentiate reams of paper from vendor A.
Variation Within and Among Reams from Vendor B
The average concentrations of each element in each ream (n = 15) from vendor B are displayed in Table 3. Elemental concentrations in sheets and reams of paper from vendor B were then statistically compared using two-way ANOVA, with results shown in Table 5. Similar to vendor A, there was no significant variation in elemental concentration within a ream; that is, elemental concentrations for a single sheet were consistent with corresponding concentrations in the other two sheets sampled from the same ream. Again no contamination was observed from the ream packaging as the top and bottom sheets did not show any significant differences in elemental concentrations.
| Element | p-Values from Two-Way ANOVA | |
|---|---|---|
| Sheets (n = 3) | Reams (n = 5) | |
| Mg | 0.139 | 6.79 × 10 −5 |
| Al | 0.512 | 2.07 × 10 −4 |
| Mn | 0.539 | 1.95 × 10 −4 |
| Fe | 0.193 | 3.84 × 10 −2 |
| Sr | 0.384 | 2.08 × 10 −6 |
| Y | 0.407 | 1.27 × 10 −6 |
| Ba | 0.544 | 2.58 × 10 −2 |
| Ce | 0.480 | 3.02 × 10 −2 |
| Nd | 0.391 | 0.498 |
- Significant differences are defined as p ≤ 0.05 and are shown in italics.
When differences among reams were considered, there were significant differences in elemental concentrations for all elements with the exception of Nd. Further investigation of these differences among the five reams was conducted using Tukey’s HSD test to obtain pairwise comparisons to determine which reams were distinguishable. Results are shown in Fig. 2. It should be noted that Fe is not included in Fig. 2: no statistically different pairs were determined using Tukey’s HSD test, despite a p-value of 0.04 from the previous ANOVA. Hence, discrimination of the reams from vendor B was based on differences in the concentrations of Mg, Al, Mn, Sr, Y, Ba, and Ce.
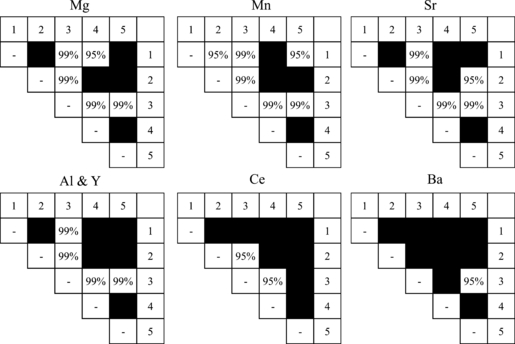
—Tukey’s honestly significant differences test results for vendor B. Significant differences between values with p ≤ 0.05 are represented by 95% and p ≤ 0.01 are represented with 99% to indicate the confidence that these two concentrations are different between the two reams. Black squares indicate the comparison between the two reams did not yield a statistically significant concentration difference. The numbers in the top row and in the right column indicate ream number.
Two of the reams of paper (1 and 3) were discriminated from all other reams based on these elemental concentrations. For ream 1, the Mn concentration was significantly different from reams 2 and 5 at the 95% confidence level, the Mg concentration was different from ream 4 at the 95% confidence limit, and Mg, Al, Mn, Sr, and Y concentrations were different from ream 3 at the 99% confidence limit. Ream 3 had significantly different concentrations of Mg, Al, Mn, Sr, and Y at a 99% confidence interval. In addition, ream 2 was found to have a different Sr concentration from ream 5 at a 95% confidence limit. Reams 2 and 4 could not be differentiated from each other nor could reams 4 and 5.
Upon further inspection, ream 3 had significant differences in elemental compositions compared with all other reams. For example, ream 3 had an average concentration of 697.8 μg Al/g paper, compared with between 390 and 478 μg Al/g paper for the remaining four reams. Similarly, the average concentration of Mg in ream 3 was 616.7 μg Mg/g paper compared with between 852.8 and 1004.4 μg Mg/g paper for the other four reams. Due to such differences in average concentrations, it is possible that ream 3 came from an entirely different production batch. However, this hypothesis cannot be confirmed as there were no markings on the packages of paper from vendor B.
Ream 1 could also be differentiated from all the others but every elemental concentration did not differ from all other reams. This is promising because only a very small population (n = 5) of reams was tested, all purchased at the same time, yet significant differences were still observed. However, it should also be noted that there were pairs of reams in this sample set (e.g., reams 2 and 4, reams 4 and 5) that could not be differentiated.
Based on these observations, Mg, Mn, and Sr were the most useful elements for distinguishing paper from vendor B as these elements discriminated the largest number of pairs. While Al and Y were not useful discriminating elements in this small sample population (n = 5 reams), there was one ream in this population (ream 3) that was statistically distinguishable from the other reams based on the concentration of these two elements. Additionally, the suite of discriminatory elements was optimized using paper digests from vendor A so it is possible that elements only present in paper from vendor B were not selected and such elements could offer further discrimination among these samples.
Variation in Elemental Concentration Between Vendors
The average elemental concentrations for all paper samples analyzed for each vendor are shown in Table 6. Student’s t-test was used to investigate significant differences in the mean of the average elemental concentrations between paper samples from vendors A and B. The average concentrations of each of the samples and the results of the t-test are shown in Fig. 3. In this figure, the bars represent the average concentration of the element throughout all the samples analyzed from the vendor (n = 75, five reams, three sheets from each and five samples per sheet) and the error bars represent the standard deviation of these measurements. Pairs of samples with significant differences are connected by a line, with *p ≤ 0.05 and **p ≤ 0.01 indicating the significance level. From the t-test results there were significant differences between the two vendors in the Ba and Nd concentrations at the 99% confidence level and in the Sr and Ce concentrations at the 95% confidence limit.
| Element | Average Elemental Concentration (μg/g paper) | |
|---|---|---|
| Vendor A (n = 75) | Vendor B (n = 75) | |
| Mg | 1010 ± 80 | 900 ± 100 |
| Al | 400 ± 100 | 500 ± 100 |
| Mn | 4.0 ± 0.3 | 4.8 ± 0.8 |
| Fe | 83 ± 7 | 90 ± 10 |
| Sr | 33 ± 4 | 50 ± 10 |
| Y | 0.10 ± 0.01 | 0.14 ± 0.05 |
| Ba | 9 ± 2 | 2.4 ± 0.3 |
| Ce | 0.34 ± 0.03 | 0.30 ± 0.04 |
| Nd | 0.16 ± 0.01 | 0.14 ± 0.01 |
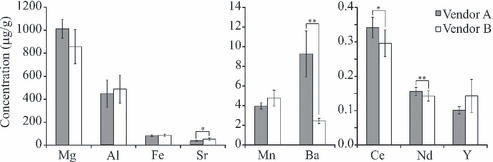
—Concentration of trace metals found in the paper samples from vendors A and B. The error bars represent one standard deviation of the values n = 75. Significant difference between values with *p ≤ 0.05 or **p ≤ 0.01.
Two ternary plots were used to graphically illustrate the differences in ratios of concentrations of Sr, Ba, Ce, and Nd between the two vendors (Fig. 4). In each plot, reams from vendor A are grouped, reams from vendor B are grouped, and the two vendors are clearly distinguished. In Fig. 4a, samples from vendor A have greater spread across the Ba axis than those from vendor B while in Fig. 4b, vendor B samples are more spread across the Sr axis. This is in accordance with previous statistical analyses in which Ba was deemed one of the most discriminatory elements for vendor A while Sr was one of the four most discriminatory elements for vendor B.
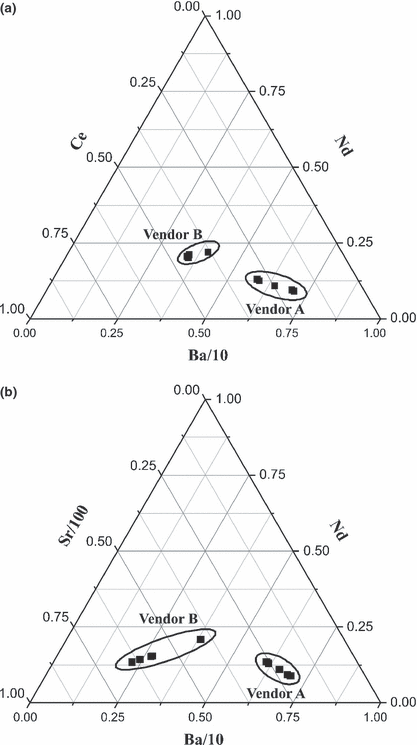
—Ternary plots of (a) the ratio of Ce, Nd, and Ba and (b) the ratio of Sr, Nd, and Ba for vendor A and vendor B. The Ba and Sr concentrations were significantly higher than the Nd concentration and therefore, were divided by 10 and 100, respectively, to make the plot easier to read. Each point represents an average ream value and five reams from each vendor are plotted.
Elemental concentrations of Ba and Nd were also significantly different between the two vendors. The Ba concentration in the papers from vendor A is significantly greater than the concentration in the vendor B paper (9.3 ± 2.3 cf. 2.5 ± 0.3 μg Ba/g paper). The Nd concentration is c. 10% greater in the samples from vendor A (0.16 ± 0.1 cf. 0.14 ± 0.1 Nd/g paper). This is also evident in the ternary plot (Fig. 4b) as the greatest separation between the two vendors is along the Ba axis. Although the differences in Ba and Nd concentrations are statistically significant at the 99% confidence limit, Ba may be a better choice for discrimination in a universal method as the detection limit of the instrument does not need to be as low.
Discussion
Elemental analysis of paper samples from two different vendors has been demonstrated to be a promising addition to traditional document analysis procedures. Trace elemental concentrations were shown to be consistent across a single sheet (five samples per sheet) as well as within a single ream of paper (three sheets per ream) for each vendor. Hence, forensically, each ream can be treated as a single source as it can be assumed that a single sheet from the ream will have the statistically indistinguishable elemental concentrations as any other sheet from the same ream.
There were statistically significant differences in element concentrations among reams from the same vendor. In addition, different elements offered the best discrimination of the reams for each vendor. For vendor A, Al and Ba were the most discriminatory while Mg, Mn, and Sr were the most discriminatory elements for vendor B. In fact, vendor B had more elements that varied between the reams, but one ream was different from all others and accounted for a large number of the variations. It is useful to note that a paper from the same vendor can vary so much in elemental concentration even when purchased from the same store and at the same time as the other reams tested.
Papers from different vendors were easily discriminated from one another, with Ba being the most discriminatory element; the Ba concentration in paper from vendor A was significantly higher than the corresponding concentration in paper from vendor B. Other elements (Sr, Ce, and Nd) also had significant differences, allowing discrimination between the two vendors. Due to the relatively small sample size used in this study, multivariate statistical procedures were not investigated. However, such procedures could be used to compare levels of all common elements between the two vendors, potentially offering greater discrimination between vendors and even among reams from the same vendor.
This study was completed on a small number of samples collected over a relatively short time frame. A ream is an arbitrary packaging of 500 sheets of paper and it may not be known to a consumer, or forensic scientist, if two reams are from the same production batch which may produce thousands of reams of paper. It is possible that the reams from each vendor sampled in this study were from the same batch; however, differences were still observed among the reams. Overall ICP–MS is a promising technique for forensic analysis of paper, allowing discrimination of paper of the same type from the same vendor.




