Isolation, Partial Purification, and Immunogenicity of Flagella from Tritrichomonas foetus
Abstract
Abstract. Tritrichomonas foetus, the agent of bovine trichomoniasis, is a flagellate protozoan responsible for substantial economic losses to the dairy and calf industries worldwide. As yet, there is no approved treatment nor is there a sensitive diagnostic method. All these problems suggest that immunization is the best control strategy. In view of this, we isolated and partially purified flagella of the parasite by vortex homogenization followed by low-speed differential centrifugation. The resulting enriched flagellar preparation termed “crude flagellar prep” was purified further by sucrose and percoll gradients. Microscopic analysis showed that the flagellar membrane was intact. Analysis by sodium dodecyl-sulfate polyacrylamide gel electrophoresis revealed three prominent protein bands of 42, 49, and >250 kDa, and several minor bands. Immunoblotting of flagellar and whole-cell extracts revealed many flagellar antigens.
BOVINE trichomoniasis, caused by the protozoan parasite Tritrichomonas foetus, is characterized by permanent infertility, pyometra, abortion, and fetal death (Felleisen 1999; Tachezy et al. 2002; Yule, Skirrow, and BonDurant 1989). The prevalence of bovine trichomoniasis is high and has a world-wide distribution: 3.3–35% infection rate in Australia; (Clark, Dufty, and Parsonson 1984) and 5.8–7.8% of bulls infected in the USA (Hall et al. 1986). The economic losses due to reduced milk and calf production in a California dairy were calculated as $665.00 per infected cow (Goodger and Skirrow 1986; Yule et al. 1989) and $2,000 to $2,500 per infected bull (Herrick 1989). The true prevalence of bovine trichomoniasis in the United States is impossible to estimate because there is no mandatory reporting of the infection except in the state of Idaho (Fox, Hobbs, and Rogers 1995). Despite the presumed high prevalence of bovine trichomoniasis and the substantial economic losses, there are no effective treatment and control strategies. Moreover, the current diagnostic InPonch methods are insensitive, and take a long time to complete diagnosis (Yule et al. 1989). Vaccination has been considered the best control strategy (Clark, Dufty, and Parsonson 1983).
In addition to the presently available whole-cell vaccine (Kvasnicka et al. 1992), there are on-going studies on the surface antigen (TF1.17) (BonDurant, Corbeil, and Corbeil 1993; Corbeil et al. 2001). Nevertheless, more studies are required to identify novel cellular or subcellular molecules that may have vaccinogenic potential, have diagnostic potential or serve as targets for chemotherapy. The development of protective immunoprophylaxis, rapid, and sensitive immunodiagnostic methods depend on a better understanding of the immunobiology of the T. foetus cell and its subcellular components such as flagella.
Flagella were chosen as the focus of this study because flagella of pathogenic microorganisms are known to be highly immunogenic (Drake and Montie 1987; Feldman et al. 1998; Piras, De Rodriguez, and Piras 1981). Consequently, they were found to be of vaccinogenic and diagnostic values (Drake and Montie 1987; Piras et al. 1981; Ruiz et al. 1986).
In this report, we describe the isolation and partial purification of flagella from T. foetus. We also present experimental data from Western blots with rabbit, bovine antisera, and monoclonal antibody to support the hypothesis that the flagella of T. foetus are immunogenic.
MATERIALS AND METHODS
Parasite cell culture and isolation of flagella. Tritrichomonas foetus, strain 330.13, was cultured axenically at 37°C in Diamond's trypticase-yeast extract-maltose (TYM) medium, pH 7.2, supplemented with 10% heat-inactivated horse serum (Diamond 1957). Gentamicin and fungizone were added at 20 and 0.25 μg/ml to control bacterial and fungal contamination, respectively. Two-liter cultures were harvested at mid-logarithmic phase and used for each flagellar preparation experiment. Flagella of T. foetus were isolated essentially as previously described (Jemilohun 1998) with some modifications, using calcium or mastoparan deflagellation buffer protocols (Quarmby and Hartzell 1994; Sanders and Salisbury 1989). The cells were washed with PBS buffer and suspended in 20 ml of deflagellation buffer (DB) containing 0.25 M sucrose, 20 mM HEPES, (pH 7.2), 2 mM MgCl2, 15 mM CaCl2, and 1 mM 2-mercaptoethanol. Phenylmethylsulfonyll fluoride (PMSF) and Na-p-tosyl-l-lysine chloro-methyl ketone (TLCK) were added to final concentrations of 3 and 5 mM, respectively. The cell suspension was deflagellated by vortex homogenization for 3 min. Cell lysis was minimal and about 95% deflagellation was achieved (data not shown). Deflagellation was monitored by phase contrast microscopy.
The homogenate was diluted 10 times with DB and centrifuged at 153 g for 10 min to remove the deflagellated cells. The supernatant containing the flagella was collected and the pellet was resuspended in fresh buffer, diluted, and centrifuged as before. The supernatant was collected and added to the first. The combined supernatant was centrifuged at 153 g for 10 min and the resulting supernatant was centrifuged at 9,820 g for 20 min to pellet the flagella. The flagellar pellet was suspended in 40-ml DB and centrifuged at 153 g for 10 min to remove sub-cellular contaminants. The supernatant was carefully aspirated into another centrifuge tube and centrifuged at 9,820 g for 20 min. The resulting pellet was suspended in 2-ml buffer and termed the “crude flagellar prep.” Observation by phase-contrast microscopy showed that the crude flagellar prep was enriched with flagella and without intact cell contaminants. The crude flagellar preparation was further purified by a 1–1.8 M linear sucrose gradient centrifugation (Opperdoes et al. 1984) and percoll discontinuous centrifugation (Morgado Diaz and De Souza 1997). Both sucrose and percoll gradients were centrifuged at 22,600 g for 2 h in a Beckman J2-HS centrifuge using JA 13.1 rotor (Beckman Instruments Inc., Palo Alto, CA). The bands containing the flagella were carefully removed, diluted with DB, and centrifuged at 9,820 g for 20 min. The flagellar pellet was carefully suspended in DB with PMSF and TLCK added to 3 mM final concentration and stored at −20°C until use.
Sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The flagellar preparations and the whole-cell sample were solubilized in buffer consisting of 50 mM Tris-HCl (pH 8.2), 5 mM EDTA, 1% (v/v) NP-40, 0.1% (w/v) SDS, 3 mM TLCK, and 1 mM PMSF. Protein concentration was determined by the bicinchoninic acid (BCA) method using a commercial kit (Pierce, Rockford, IL) (Smith et al. 1985). Bovine serum albumin was used as the protein standard. Flagellar preparations yield an average of 17 mg of flagellar proteins. SDS-PAGE was performed in a mini-gel apparatus (Bio-Rad Laboratories, Hercules, CA) with 10% separating polyacrylamide gel and 5% stacking system. The samples were solubilized under denaturing conditions in 5 × sample buffer (20% (v/v) glycerol, 10% (v/v) 2-mercaptoethanol, 4% (w/v) SDS, 125 mM Tris-HCl, pH 6.8). The samples were heated at boiling for 5 min prior to loading equal amounts of proteins (15 μg) to the wells of the gel. Broad-range molecular mass standards of 250, 150, 100, 75, 50, 37, 25, 20, 15, and 10 kDa (Bio-Rad Laboratories, Hercules, CA) or molecular mass ladders, 220, 160, 120, 100, 90, 80, 70, 60, 50, 40, 30, 25, 20, 15, and 10 kDa (Invitrogen, Carlsbad, CA) were used. Protein separation was accomplished at 200 constant volts (Laemmli 1970). To visualize protein bands, gels were stained with 0.1% (w/v) Coomassie Blue R-250 in solution containing 40% (v/v) methanol and 10% (v/v) acetic acid. The gels were destained in solution containing 40% methanol and 10% acetic acid (Bio-Rad Laboratories).
Antibodies and immunoblot analyses. To identify flagellar immunogens, flagellar prep and whole-cell samples were analyzed by immunoblots. The blots were probed with bovine antisera, rabbit antisera, and a monoclonal antibody kindly provided by Drs. Donald E. Burgess (ATTC, Manassas, VA) and Lynette B. Corbeil (UC, San Diego, CA). The bovine antibodies were prepared by immunizing cattle intramuscularly twice at biweekly intervals with 107T. foetus cells in 1% formalinized saline mixed with incomplete Freund's adjuvant. Serum was collected 10 d after the last immunization (Corbeil et al. 1989). The monoclonal antibodies were raised by intraperitoneal injection of mice with approximately 107T. foetus cells mixed with complete Freund's adjuvant. Mice were boosted by intravenous injection with T. foetus 3 d prior to fusion. Positive clones were selected and hybridoma clones were prepared by limiting dilution (Burgess 1986). Rabbit antibodies were prepared by immunizing rabbits with 106T. foetus cells mixed with an equal vol. of Freund's complete adjuvant. The immunizations were repeated with Freund's incomplete adjuvant (Corbeil, Hodson, and Widders 1991; Shaia et al. 1998).
After fractionation of the samples by SDS-PAGE, the proteins were electroblotted to nitrocellulose membrane for 1.5 h at 100 V (Towbin, Staeehelin, and Gordon 1979). The membranes were washed briefly in PBS buffer and the non-specific binding sites of the nitrocellulose membranes was blocked at room temp. for 2 h in a blocking buffer (5% (w/v) blotto, 0.2% (v/v) Tween 20 in PBS). The membranes were briefly rinsed with 0.2% Tween 20 in PBS (PBST buffer) and probed overnight with 1:250 dilution bovine antisera (Corbiel et al. 1989), 1:250 dilution of rabbit antisera (Corbiel et al. 1991), 1:100 dilution monoclonal antibody (MAb) (Burgess 1986), and 1:200 dilution of rabbit antibodies (Shaia et al. 1998) prepared in PBST containing 1% blotto at 4°C with gentle shaking. The membranes were washed three times for 10 min each with PBST and bound antisera were detected by incubation for 30 min at room temp. in anti-bovine or anti-rabbit or anti-mouse IgG-alkaline phosphatase conjugate diluted with 1% blotto in PBST according to the supplier specifications (Kirkegaard and Perry Laboratories Inc., Gaiththersburg, MD). The blots were washed three times with 1% blotto in PBST and once with PBS and developed with 5-bromo-4-chloro-3-indolyl-phosphate (BCIP) as the substrate and nitroblue tetrazolium (NBT) as the chromogenic indicator (Kirkegaard and Perry Laboratories Inc.). The reaction was stopped by washing the blots in distilled water.
RESULTS
Microscopic analyses. Although some of the parasite cells were broken in the process of deflagellation, which led to the presence of subcellular particles, these unwanted contaminants were removed by low speed differential centrifugation and linear sucrose gradient centrifugation. The flagellar preparation was about 85–95% pure based on analysis by phase contrast microscopy (Fig. 1). Further purification of the flagellar fraction was achieved by linear sucrose gradient or percoll gradient centrifugation. The flagellar sheath (membrane) remained intact and was not affected by the method of deflagellation nor the purification scheme employed (Fig. 2). Flagellar complexes (C), flagellar fragmentation (F), and flagellar spherical formation (S) were apparent (Fig. 2).
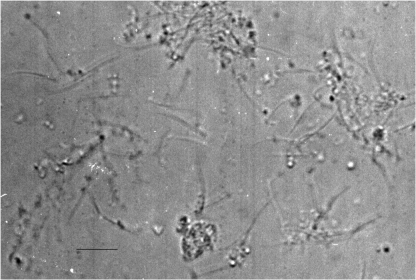
Light micrograph of crude flagellar preparation from Tritrichomonas foetus. Flagellar fragments and presence of sub-cellular particles are apparent. Bar=10 μm.
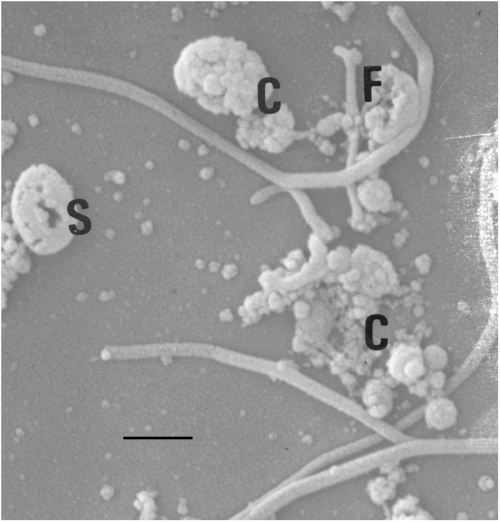
Scanning electron micrograph of partially purified flagella from Tritrichomonas foetus. Flagellar membrane is intact. Flagellar complex formations (C), flagellar fragmentation (F), and circularization of flagella (S) were consistently observed in the flagellar preps. Bar=5 μm.
Sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Highly reproducible gel patterns were obtained from analyses of several different flagellar preparations. The whole flagella showed a complex pattern of polypeptides ranging from about 10 to 300 kDa (Fig. 3). Three prominent proteins 42, 49, and >250 kDa, and several minor proteins were consistently observed in the partially purified flagellar preps. These three prominent proteins were enriched in the flagellar preparation compared to the whole-cell extract, suggesting that they are important flagellar proteins (Fig. 3).
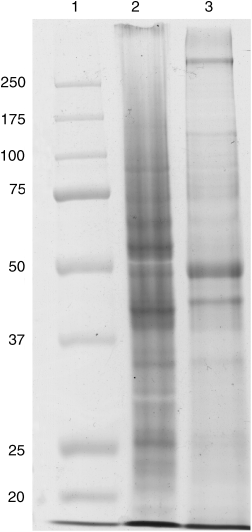
SDS-PAGE analysis of whole-cell and flagellar extracts from Tritrichomonas foetus. Proteins were resolved in a 10% gel and stained with Coomassie brilliant blue. Molecular mass standards in kDa (lane 1), whole-cell extract (lane 2), and flagellar extract (lane 3).
Immunoblot analyses. The bovine antisera demonstrated immunoreactivity mainly with high molecular mass antigens of about 100, 116, and 180 kDa from both whole-cell and flagellar extracts. The 116-kDa antigen was uniquely located on the flagella as its band-intensity on the whole cell extract was reduced (Fig. 4A, lane 2). The MAb showed strong immunoreactivity with an 84-kDa protein and other higher molecular mass antigens, suggesting that these antigens share some epitopes with the 84-kDa antigen. Along with several other antigens, the 84-kDa antigen was strongly recognized in both whole-cell and flagellar samples (Fig. 4B). The two heterologous rabbit antisera showed broad-spectrum immunoreactivity with flagellar extract (Fig. 5), while the other rabbit antisera reacted essentially with high molecular mass antigens (Fig. 5B).
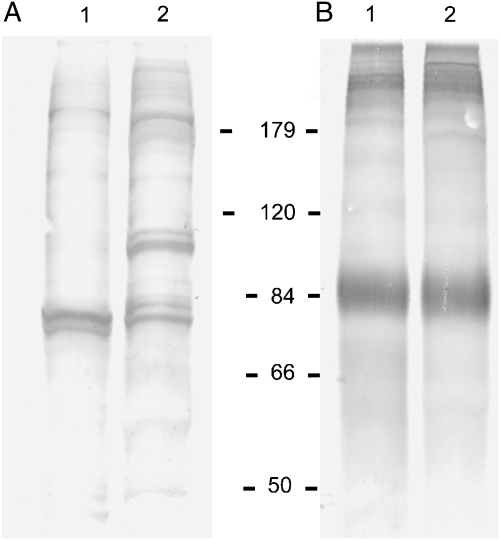
Western blots of proteins from whole-cells (lane 1) and flagellar extracts (lane 2) from Tritrichomonas foetus. Protein samples were fractionated on a 10% SDS-PAGE, electroblotted, and probed with bovine anti-T. foetus sera (panel A) and mice-derived monoclonal antibody (panel B). The molecular mass standards are in kilodaltons.
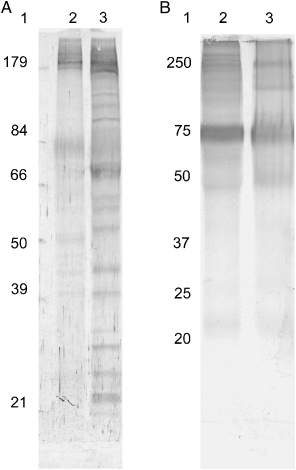
Western blots of proteins from whole-cell and flagellar extracts of Tritrichomonas foetus probed with two anti-T. foetus sera from rabbits. Extracts were separated on a 10% SDS-PAGE, electroblotted, and probed with rabbit antisera. Lane 1 represents molecular mass markers in kilodaltons. (A) Lanes 2 and 3 represent whole-cell and flagellar extracts, respectively. (B) Lane 2 represents flagellar extract and lane 3 is the whole-cell extract.
DISCUSSION
We employed mechanical homogenization coupled with centrifugation techniques to isolate and partially purify the flagella of T. foetus. The isolation and purification scheme did not compromise the integrity of the flagella as the flagellar sheath (membrane) remained intact. Despite precautions taken to avoid flagellar breakage, some flagellar fragmentation was apparent in our flagellar preparations, although this likely has no effect on the molecular composition of the flagella.
Flagella of many protozoans enable adhesion to the host: in trichomonads (Corbeil et al. 1989; Silva Filho, De Souza, and Lopes 1988), in trypanosomes (Cooper, Ribeiro de Jesus, and Cross 1993; Nozak, Haynes, and Coss 1996; Warburg, Tesh, and McMahan-Pratt 1989), and in Chlamydomonas (Bloodgood and Workman 1984). The migration of T. foetus from vagina to the uterus is by means of flagella (Singh et al. 1999; Skirrow and BonDurant 1990). In some other protozoan parasites, the antigenicity of flagella has been used in immunoprophylaxis (Cooper et al. 1993; Piras, De Rodriguez, and Piras 1981; Ruiz et al. 1986).
Our SDS-PAGE analysis revealed a complex array of flagellar proteins ranging from about 10 to 300 kDa in molecular mass. Some of these proteins were prominent in the flagella as their presence in the whole-cell sample was not apparent or greatly reduced. Immunoblot of the whole-cell and flagellar samples showed that some antigens were uniquely located on the flagella. Several polypeptides from both whole-cell and flagellar samples were immunoreactive with bovine and rabbit antisera. An antigen of about 84-kDa from both whole-cell and flagellar samples reacted strongly with a MAb while a flagellar antigen >250 kDa reacted with heterologous rabbit antisera. This 84-kDa antigen was perhaps the protein reported by Corbeil et al. (1989) as an 83-kDa antigen with strong reactivity with bovine antisera. Additionally, the bovine antisera recognized several high molecular mass flagellar immunogens of about 180, 116 and a doublet 84 kDa as in the previous report (Corbeil et al. 1989). The heterologous rabbit antisera showed immunoreactivity with several flagellar proteins including 84, diffuse 180, >250 kDa and many lower molecular mass antigens.
Krieger et al. (1990) implicated motility and adherence in T. vaginalis, the agent of human trichomoniasis. In the case of T. foetus, motility to the uterus is apparently not responsible for pyometra and abortion caused by the parasite. However, flagellar proteins may contribute to the lytic factors that may act in concerted form with motility and result in damage to the uterine wall, ultimately leading to abortion and/or pyometra (Lubick and Burgess 2003). It is interesting that our results showed that T. foetus flagella are immunogenic in bovines and rabbits. This demonstrates the importance of flagellar polypeptides in the immunobiology of T. foetus. In addition to the ultimate goal of preventing bovine trichomoniasis, studies of this parasite will provide insight into the immunobiology of eukaryotic flagella, a subject for which there is little immunological knowledge compared with our knowledge of bacterial flagella (Feldman et al. 1998; Grant et al. 1993; Sbrogio-Almeida et al. 2004). The present study, to the best of our knowledge, is the first isolation and immunological study of flagella from T. foetus. The information presented here should provide a sound basis for more flagellar studies of T. foetus and other trichomonads that could ultimately lead to identification of effective molecules for immunoprophylaxis, for the design of drugs, and for rapid immunodiagnosis of bovine trichomoniasis. We are presently working on biochemical and functional analyses of the flagellar antigens identified during the course of these studies.
Acknowledgments
P. J. is thankful to Drs. William M. Willingham and Clifton Orr for their firm commitment and support that made these studies possible. P. J. would also like to acknowledge the support from Drs. Lawrence Cornett and Helen Benes at UAMS. The provision of some reagents by Dr. Patricia Marks and the assistance by Dr. Raji Abayomi (VCWU, Richmond) with literature acquisition are acknowledged. The professional technical support by Ziba Barber is appreciated. We thank Dr. Lynette B. Corbeil (UC, San Diego) for providing us bovine and rabbit antisera. This study was supported in part by NIH grant # P20 RR-16460 from the BRIN Program of the National Center for Research Resources and NIH-NIAID grant # R15 AI555444.




