Ultrastructure, Eneystment and Cyst Wall Composition of the Resting Cyst of the Peritrich Ciliate Opisthonecta henneguyi
Abstract
ABSTRACT. The cyst wall of Opisthonecta henneguyi has been studied ultrastructurally and cytochemically by light and electron microscopy, as well as by chemical and electrophoretic analyses, to examine the structure of the cyst wall and its composition. The cyst wall consists of four morphologically distinct layers. The ectocyst is a thin dense layer. The mesocyst is the thickest layer and is composed of a compact material. The endocyst is a thin layer like the ectocyst, but less dense. The granular layer varies in thickness and is composed of a granular material. In the resting cyst, kinetosomes of both oral apparatus and trochal band as well as the myoneme system are maintained, and only cilia are resorbed. The sugars present in the cyst wall are predominantly N-acetylglucosamine (90%) and glucose (10%). The niesocyst is composed of chitin, and the endocyst includes glycoproteins and acid mucopolysaccharides. During secretion of the cyst wall, the endocyst and granular layer are secreted from precursors synthesized “de novo”. No cytoplasmic precursors of ectocyst and mesocyst have been detected.
UNDER adverse conditions, such as starvation, many ciliates undergo a process of cell differentiation called en-cystment, in which a vegetative cell is transformed into a resting cyst. The opposite process is termed excystment (Corliss and Esser 1974; Gutierrez, Martin-Gonzalez, and Matsusaka 1990). Eneystment involves various morphological and physiological changes, such as formation of a cyst wall derived from different precursors, cytoplasmic dehydration, high autophagic activity, nuclear condensation, and decrease of cell volume (Calvo, Torres, and Pérez-Silva 1986; Delgado, Calvo, and Torres 1987; Gutierrez, Torres, and Pérez-Silva 1983a; Martín-Gonzalez, Benitez, and Gutierrez 1991; Martín-Gonzalez, Pa-lacios, and Gutierrez 1994, Walker and Maugel 1980; Walker, Maugel, and Goode 1980).
During encystment, there may be total or partial resorption of oral and/or somatic ciliature and infraciliature. The oxytrich ciliates and Blepharisma stoltei (Repak 1968) do not maintain cilia or kinetosomes. Kahiella simplex (Foissner and Foissner 1987), Urostyla grandis (Rios et al. 1985), Gonostomum (Walker and Hoffman 1985) and Paraurostyla weissei (Delgado, Calvo, and Torres 1987) lose cilia but retain a few subpellicular microtubules. Other ciliates, such as Dileptus visscheri (Kink 1973), maintain some microtubular structures and kinetosomes, and resorb cilia. Colpoda inflata (Chessa et al. 1994; Martin-Gonzalez, Benitez, and Gutierrez 1991) resorbs the oral infraciliature, but retains traces of the argyrome and the somatic kineties. Diophrys (Walker and Maugel 1980) and Euplotes (Rawlinson and Gates 1985) maintain somatic and oral cilia as well as subpellicular microtubules.
The presence of different precursors that are synthesized “de novo”, giving rise to the different cyst wall layers during the encystment of various ciliate protozoa, has been reported (Calvo, Torres, and Pérez-Silva 1986; Delgado, Calvo, and Torres 1987; Grimes 1973, Martín-Gonzalez, Palacios, and Gutierrez 1994; Walker, Maugel, and Goode 1980). These precursors originate from the endoplasmic reticulum in Colpoda steinii (Ruthmann and Kuck 1985), or from Golgi vesicles in Histri-culus similis (Calvo, Torres, and Pérez-Silva 1986) and Telo-trochidium henneguyi (synonymous with Opisthonecta dubia) (Walker, Edward, and Suchard 1989).
Ultrastructural studies of resting cysts have demonstrated that the cyst wall is composed of several layers. The stichotrich ciliates Histriculus similis (Calvo, Torres, and Pérez-Silva 1986), Paraurostyla weissei (Delgado, Calvo, and Torres 1987), Oxytricha fallax (Grimes 1973), Laurentiella acuminata (Gutierrez, Torres, and Pérez-Silva 1983b), Onychodromus acu-minatus (Jareňo 1985), Pleurotricha sp. (Matsusaka 1976), Gastrostyla steinii (Rosati, Verni, and Nieri 1983; Walker, Maugel, and Goode 1980), Oxytricha bifaria (Verni, Rosati, and Ricci 1984), and Stylonychia mytilus (Walker, Maugel, and Goode 1975) have four layers: ectocyst, mesocyst, endocyst, and granular layer. The resting cyst of Blepharisma stoltei has three layers (ectocyst, interwall space, and endocyst) (Repak and Pfister 1967; Repak 1968). The cyst wall of Diophrys scutum (Walker and Maugel 1980), Urostyla grandis (Rios et al. 1985) and Colpoda cucullus (Janisch 1980) consists of three morphologically distinct layer, the morphology of these layers is basically similar to that of the three layers of oxytrichds cysts. Euplotes muscicola (Bussers et al. 1986) and Cyrtolophosis elongata (Diaz et al. 2000) have two layers (ectocyst and endocyst).
Studies on the chemical composition of the cyst wall show that proteins and polysaccharides are the main components (Bussers et al. 1986; Calvo et al. 1983; Delgado, Calvo, and Torres 1987; Gutierrez, Torres, and Pérez-Silva 1984; Martín-Gonzalez et al. 1991; Matsusaka and Hongo 1984; Rios, Pérez-Silva and Fedriani 1988; Rios et al. 1989; Rosati, Verni, and Ricci 1984). The nature of glycoprotein carbohydrate moieties has been investigated using different lectins in Histriculus cav-icola (Calvo and De Miguel 1996). The presence of chitin in the resting cysts of ciliates has been investigated using treatment with chitinase (Bussers 1976; Bussers and Jeuniaux 1974) and WGA lectin (Greco et al. 1990; Mulisch and Haustnann 1989). Chitin appears in the walls of the resting cysts, often associated with organic or inorganic substances, such as proteins, polysaccharides, and silica (Bussers 1976; Mulisch 1993). The distribution of chitin in protistan organisms and its phy-logenetic implications have been discussed by Mulisch (1993).
Little is known about the ultrastructure and composition of peritrich resting cysts (Bussers and Jeuniaux 1974; Dodd 1962; Finley and Lewis 1960; Rosenberg 1938; Walker, Edward, and Suchard 1989), and the presence of chitin is not clear. The present work is an ultrastructural study of the resting cyst and the encystment process in the peritrich ciliate Opisthonecta henneguyi. The presence of chitin and the composition of the cyst wall have been investigated by chemical and cytochemical methods.
MATERIALS AND METHODS
Opisthonecta henneguyi was kindly supplied by Dr. A. Guinea (University Complutense of Madrid, Spain). The cultures were kept at 20°C in 0.05% (w/v) cereal leaves (Sigma) with Escherichia coli as food. Mass cultures of vegetative cells in late stationary phase were gently filtered, collected by centri-fugation at 300 g for 1 min, washed several times with mineral water (Font-Vella), and transferred to 10× concentrated Oster-hout solution (not bacterized) to induce encystment. The cells were kept in this medium at 20°C until cysts were completely formed. The duration of the encystment process varied between 18–40 h depending on experiment. Throughout the encystment process, the cell shape changes: the cells become shorter and their volume decreases. Samples were taken at different times during the encystment process for ultrastructural analysis. Cysts and cyst walls were purified and collected as previously described by Rios et al. (1989).
The silver impregnation method (Fernández-Galiano 1994) and electron microscopy were used to distinguish what infra-ciliature was retained in the mature resting cysts.
Encysting cells (for transmission electron microscopy) and mature cysts (scanning and transmission electron microscopy) were fixed for 1 h in a 1:1 mixture of 4% (v/v) glutaraldehyde and 2% (w/v) osmium tetroxide in 0.1 M cacodylate buffer (pH 7.3), and processed as described by Delgado, Calvo, and Torres (1987).
For cytochemical studies with light and electron microscopy, cysts were fixed in 2% (v/v) glutaraldehyde in 0.1 M cacodylate buffer (pH 7.3) and embedded in Spurr's resin. Thin sections of these samples were stained with periodic acid-Schiff (PAS), Alcian blue 8GX (pH 2.5 and pH 5.8), and periodic acid-thio-carbohydrazide-silver proteinate (PA-TCH-SP) (see Delgado, Calvo, and Torres 1987). Cysts fixed in cacodylate-buffered 2% (w/v) glutaraldehyde were stained with dialyzed iron (DI) or high iron diamine (HID) (Calvo et al. 1983), and then embedded in Spurr's resin.
The presence of proteins was determined using 1 % pronase. Resting cysts treated with 1% pronase in distilled water (pH 7.4) for 2 h at 37°C were fixed for 1 h in a 1:1 mixture of 4% glutaraldehyde and 2% osmium tetroxide in 0.1 M cacodylate buffer (pH 7.4), dehydrated, and embedded in Spurr's resin. Ultrathin sections of untreated cysts, fixed as above, were incubated with 1 % pronase.
A battery of seven lectins was used for lectin labeling: five horseradish peroxidase (HRP)-conjugated lectins Canavalina ensiformis (Con A); Tritium vulgaris (WGA); Arachis hypo-gaea (PNA); Dolichos biflorus (DBA) and Ulex europaeus I (UEA I) and two biotin-conjugated lectins Lens culinaris (LCA) and Maackia amurensis (MAA). For light and electron microscopy, the methods described by Calvo and De Miguel (1996) were followed.
For the detection of chitin, thick sections of cysts treated with 1% pronase as described above and untreated cysts were incubated for 24 h at 37°C in 0.0002 u/μl chitinase (SIGMA) and HRP-WGA lectin. The WGA lectin was incubated with 0.4 M N-acetyl-D-glucosamine for the controls. For electron microscopy, cysts were fixed for 1 h in a mixture of 0.5% (V/V) glutaraldehyde and 2% (W/V) paraformaldehyde in 0.1 M cacodylate buffer (pH 7.4), dehydrated, and embedded at 35°C in Lowicryl K.4M. Ultrathin sections were mounted on nickel grids, floated for 24 h at 37°C in 1% pronase, and incubated on the WGA lectin. At the same time, ultrathin sections were sequentially incubated in 1% Pronase, 0.0002 u/μl chitinase, and WGA lectin.
Gel electrophoresis and lectin blotting.
Purified cyst walls were solubilized by 6 M urea in SDS sample buffer pH 9.5 and analyzed on linear gradient 6–18% SDS polyacrylamide slab gels according to the method of Laemmli (1970). Protein quantification was carried out by the Bradford method (Bradford 1976). Gels were stained with 0.1% Coomassie brilliant blue. Lectin blotting with Con A and WGA was carried out according to Calvo and De Miguel (1996).
GLC-MS analysis of sugars.
Isolated cyst walls (30–80 μg) or an insoluble pellet of cyst walls treated with 6 M urea in SDS sample buffer pH 9.5 (30–80 μg) was mixed with meso-inositol, lyophilized, and treated with 35% (w/v) HC1 (100 μl) at 100°C for 2.5 h. Methanol (3 × 500 μl) was added to the mixture, which was then dried under a stream of nitrogen. The samples were reacetylated with 10:1:1 methanol-pyridine-acetic anhydride (900 μ1) at room temperature for 15 min and then dried under a stream of nitrogen. The reacetylated methyl gly-cosides obtained were silylated with 1:1 pyridine-BSTFA (W,s(trimethylsilyl)trifluoracetamide) for 2 h at room temperature. The TMS (trimethylsilyl) derivatives were analyzed by GLC-MS performed with a Micromass AutoSpec-Q instrument fitted with an OV-1 WCOT column (25 m × 0.25 mm i.d.). The temperature program was isothermal at 150°C for 2 min followed by a 10°C/min gradient up to 250°C.
RESULTS
The resting cyst of Opisthonecta henneguyi is spherical as revealed by scanning electron micrography. The outer layer is not smooth, but exhibits many protuberances—the biggest one being plug-like (Fig. 1). During excystment, the vegetative cell emerges through this plug (data not shown).
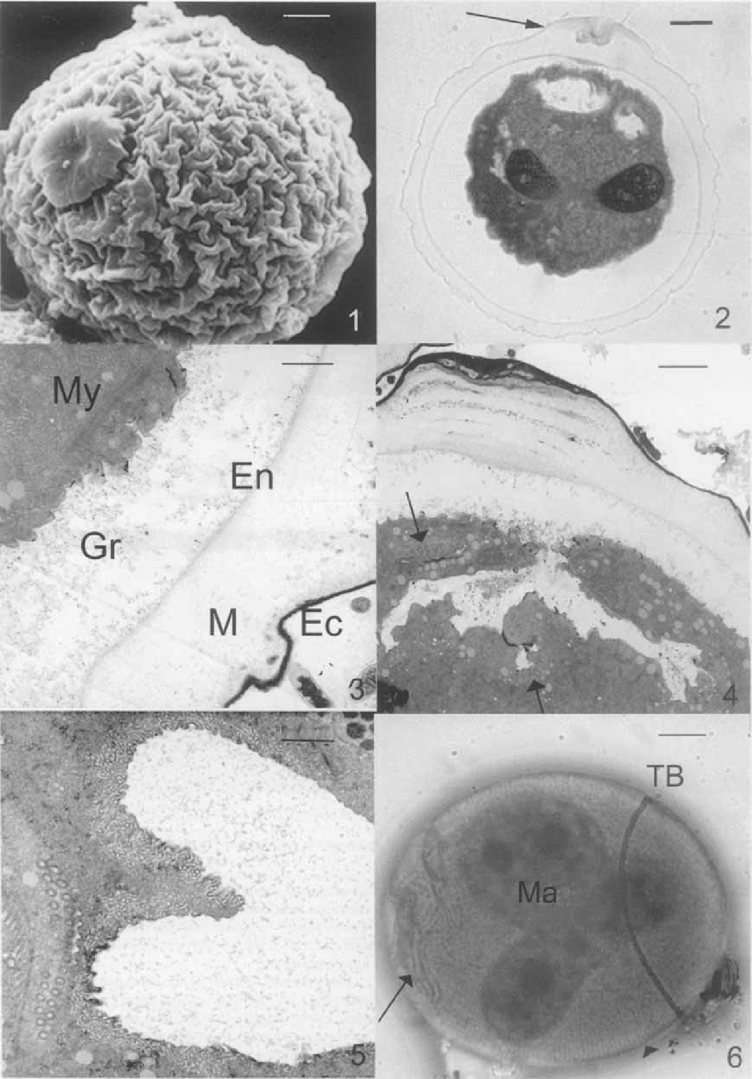
Mature resting cyst of Opisthonecta henneguyi. 1. Scanning electron micrograph (SEM) showing the outer layer (ectocyst) and the plug. Bar = 5 u,m. 2. Light micrograph of a thick section of a resting cyst stained with toluidine blue. The arrow indicates the plug. Bar = 5 μm. 3, 4. Transmission electron micrographs of cyst wall. EC, ectocyst; M, mesocyst; En, endocyst, and Gr, granular layer. Note the myoneme system (My) under the pellicle (Fig. 3, Bar = 1 μm), theoral kinetosomes (arrows), and the thickness of the mesocyst in the plug area (Fig. 4, Bar = 2 μm). 5. Detail of oral kinetosomes. Bar = 1 μm. 6. Cyst impregnated by silver carbonate, showing the trochal band (TB), the oral ciliature (arrow), the argyrome (arrowhead), and the macronucleus (Ma). Bar = 5 μm.
The cyst wall comprises four layers: ectocyst, mesocyst, en-docyst, and the granular layer consisting of granular material located between the endocyst and the pellicle (Fig. 2-4). The ectocyst is a thin, dense layer, which becomes thicker in the plug area (Fig. 2, 4) due to aggregates of detritus-like material trapped in this zone. The mesocyst is the thickest layer; in the region close to the ectocyst, it is composed of a compact material, and a less compact region (i.e. less dense) near the endocyst. Like the ectocyst, the mesocyst increases in size in the plug area (Fig. 2, 4). The mesocyst material in this zone appears denser and more stratified (Fig. 4). The endocyst is a thin layer like the ectocyst, but less dense. The granular layer varies in thickness because it is delimited by the pellicle.
Near the plug, the cytoplasm exhibits a clear area (Fig. 4) like an invaginated area in the cytoplasm, probably originated when the peristome is retracted. This chamber contains material that comes from degenerating cilia. Transmission electron micrographs (1–6, 7–10) and Fernandez-Galiano's silver impregnation of mature resting cysts (Fig. 6) show that the oral and aboral infraciliature, argyrome, and myoneme system are retained, although cilia are resorbed.
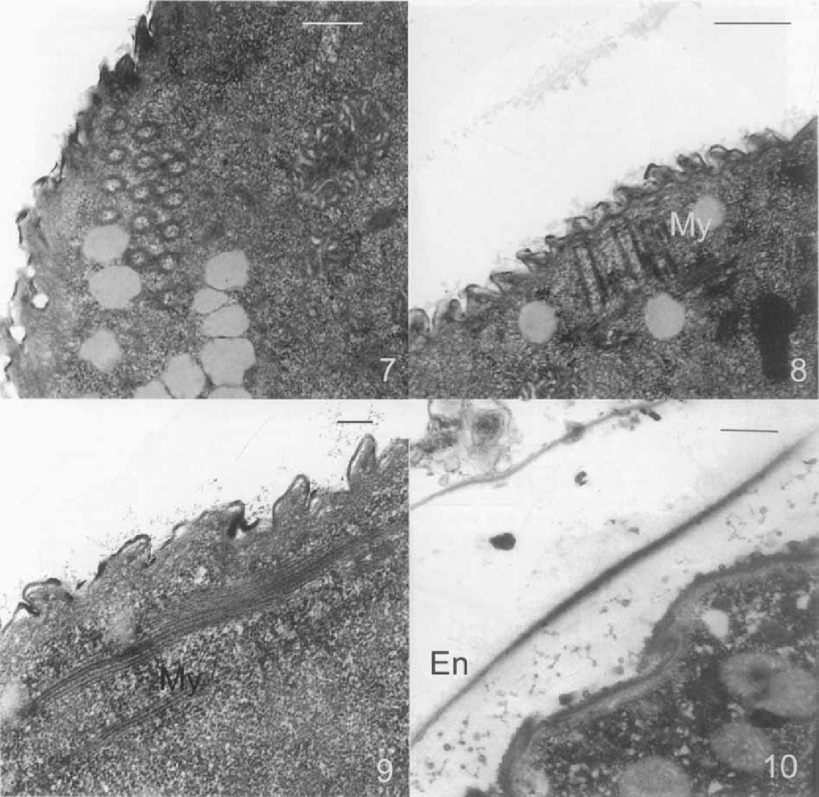
Transmission electron micrographs of the mature resting cyst of Opisthonecta henneguyi. 7. Transverse section of the trochal band. Bar = 0.5 μm. 8. Longitudinal section of the trochal band. My, myoneme. Bar = 0.2 μm. 9. Myoneme system under the pellicle. Bar = 0.2 μm. 10. DI (dialyzed iron) staining. The endocyst (En) shows a strong staining. Bar = 0.5 μm.
The techniques used to demonstrate neutral polysaccharides by light (PAS) and electron microscopy (PA-TCH-SP) showed no staining in the cyst wall layers (data not shown). Acidic mucosubstances were detected by light (Alcian blue pH 2.5, pH 5.8) and electron microscopy (DI and HID) in the endocyst (Fig. 10).
None of the cyst wall layers was stained with PNA, DBA, UEA I, LCA, or MAA lectins. Con A, a lectin that binds to a-D-glucosyl and/or a-D-mannosyl residues, stained the endocyst (Fig. 11). WGA, a specific lectin for the detection of (N-ace-tylglucosamine)n residues, was strongly bound to the endocyst and the mesocyst (Fig. 12).
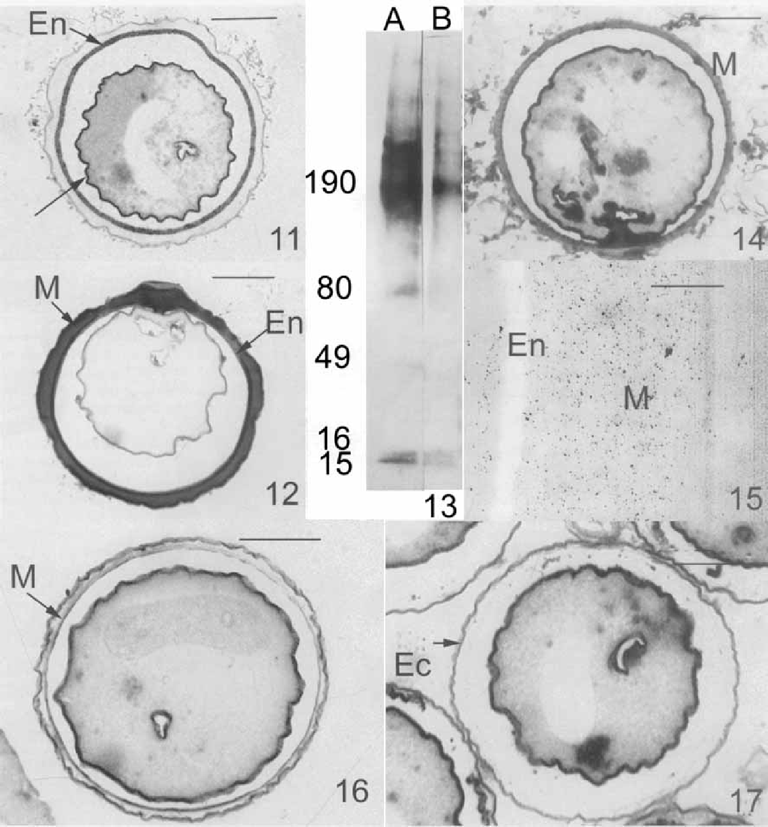
Lectin staining of the cyst wall of Opisthonecta henneguyi. 11. Horseradish peroxidase (HRP)-Con A. The endocyst (En) and the pellicle (arrow) exhibit a strong binding with this lectin. Bar = 10 μm. 12. HRP-Triticum vulgaris (WGA) bind strongly to the mesocyst (M) and endocyst (En). Bar = 10 μm. 13. Lectin blotting of isolated cyst walls solubilized by 6 M urea in Laemmli sample buffer. Lane A-WGA lectin; Lane B-Con A lectin. At the left, molecular weight standard (kDa). 14. Pronase treatment and HRP-WGA labeling. The mesocyst (M) is strongly stained. Bar = 10 μm. 15. Pronase treatment and WGA gold labeling. After pronase treatment, the endocyst (En) is electron-lucent. Gold particles are present in the mesocyst layer (M). Bar = 0.5 μm. 16. Chitinase treatment and HRP-WGA staining. Mesocyst (M) staining is eliminated. Bar = 10 μm. 17. Pronase and chitinase treatments and HRP-WGA labeling. Mesocyst and endocyst are digested, only the ectocyst (Ec) is maintained. Bar = 10 μm.
GLC-MS analysis confirmed the lectin results, and showed the presence of glucose (10%) and (mainly) N-acetylglucosa-mine (90%) in isolated cyst walls. These sugars were identified by comparison with standard samples. To investigate the presence of glycoproteins, isolated cyst wall resolved by SDS-PAGE and was transferred onto nitrocellulose filters that were then incubated with WGA and Con A lectins (Fig. 13). Most of the cyst wall proteins that bind to lectins have a high molecular weight (i.e. > 150 kDa) as is common for glycoproteins. A group of polypeptides with molecular weights above 190 kDa reacted strongly with both Con A and WGA. Some polypeptides of low molecular weight bound to the lectins tested: two polypeptides of 15 and 16 kDa reacted with both Con A and WGA; and two polypeptides of 49 and 80 kDa bound only to WGA.
Since WGA binds to glycoproteins with N-acetylglucos-amine residues, but also has a high affinity for chitin, we investigated the presence of chitin in the cyst wall of O. henneguyi. The deproteinization with pronase before WGA binding revealed by the light (Fig. 14) and electron microscopy (Fig. 15) that there was a strong staining of the mesocyst, while the endocyst became electron-lucent (Fig. 15). Thick sections of cyst incubated in 0.0002 u/μl chitinase before WGA lectin labeling showed an elimination of mesocyst staining (Fig. 16). Thick sections of cyst treated with 1% pronase and 0.0002 u/μ1 chitinase before lectin labeling showed the elimination of endocyst and mesocyst (Fig. 17). Only the ectocyst remained. These results suggest that the mesocyst is composed of chitin, and the endocyst by glycoproteins containing N-acetylglucos-amine residues.
Encystment.
Detail of the complete pellicle of the vegetative cell (Fig. 18, 19). No cytoplasmic precursors of ectocyst surrounded by a unit of membrane has been detected. The ectocyst seems to originate from the outer layer of the pellicle with the appearance of a membrane and its associated glycocalyx. During early encystment, this layer becomes more dense and begins to separate from the rest of cell surface, giving rise to the ectocyst (Fig. 20). We have not detected mesocyst precursors. When the mesocyst is formed, ciliary resorption begins, whereas kinetosomes are maintained (Fig. 21, 22). Probable endocyst precursors appear as spherical vesicles with a fibrillar material surrounded by a unit membrane (Fig. 21, 22). Precursors of the granular layer have variable morphology and are surrounded by a unit membrane (Fig. 23). Their content is similar to the layer from which they originate. When all the cyst wall layers are formed, there appears to be movement of detritus material—originating from ciliary degeneration—to the plug area (Fig. 24).
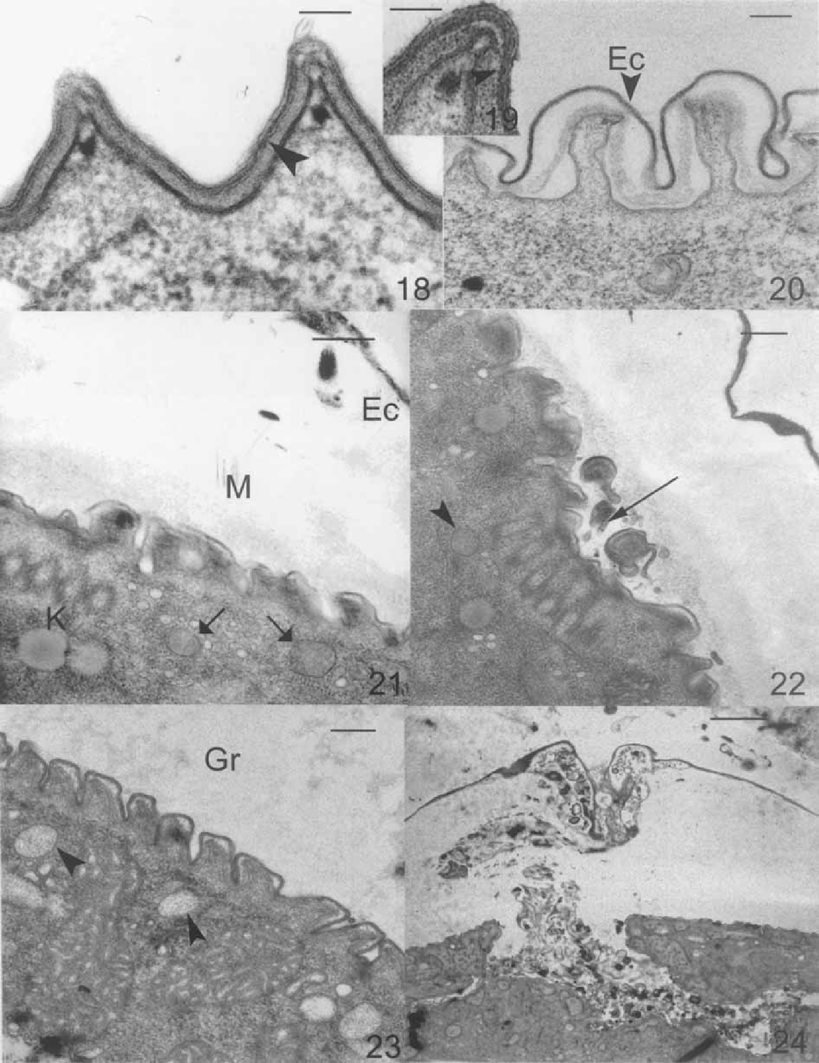
he encystment process of Opisthonecta henneguyi. 18–19. Detail of the pellicle of a vegetative cell. Outer pellicular layer (arrowhead). Bar = 0.1 μm. 20. Beginning of encystment. Separation of the outer pellicular layer, which becomes more dense, giving rise to the ectocyst (Ec). Bar = 0.2 μm. 21. Probable endocyst precursors (arrows). EC, ectocyst; M, mesocyst; K, kinetosomes. Bar = 0.5 μm. 22. Resorption of trochal band cilia (arrow). Probable endocyst precursors (arrowhead). Bar = 0.2 μm. 23. Granular layer precursor (arrowhead). Gr, granular layer. Bar = 0.20 μm. 24. Detritus material (such as the remains of cilia) in the plug area. Bar = 2 μm.
DISCUSSION
The resting cyst of Opisthonecta henneguyi has four cyst wall layers, resembling those of oxytrich ciliates (Calvo, Torres, and Pérez-Silva 1986; Grimes 1973, Gutierrez, Torres, and Pérez-Silva 1983b; Matsusaka 1976; Verni, Rosati, and Ricci 1984; Walker, Maugel, and Goode 1975, Walker, Maugel, and Goode 1980), but with some differences. The ectocyst is composed of dense material, not of plates or discs as in oxytrichs. This layer shows some protuberances, as has been described in Parauros-tyla weissei (Delgado, Calvo, and Torres 1987). However, a plug is never found in oxytrichs. The presence of a protrusion at one end, marking the site of the excystment aperture closed by a hyaline plug, has been described in some heterotrichs, tintinnids, oligotrichs, colpodeans, and papulifers (Reid and John 1983).
The mature resting cyst of Opisthonecta henneguyi maintains the myoneme system and kinetosomes of both oral apparatus and trochal band, as has been described in Telotrochidium (Walker, Edward, and Suchard 1989).
Our results with HID and DI indicate that acid mucopoly-saccharides are present in the endocyst of Opisthonecta henneguyi. GLC-MS analysis and studies with Con A and WGA lectins show the presence of glucose and N-acetylglucosamine. Pronase and chitinase treatments show the presence of proteins and chitin in the endocyst and mesocyst, respectively. The endocyst is digested by pronase treatment, suggesting that this layer is composed of protein. However, the mesocyst is resistant to pronase and is labeled by WGA lectin—we suggest that this layer is composed of N-acetylglucosamine in polymeric form (i.e. chitin). Chitinase treatment eliminates the WGA labeling of mesocyst, supporting the idea that this layer is made of chitin. Double digestion with pronase and chitinase eliminates both endocyst and mesocyst layers—only the ectocyst is left. The glycoproteins detected by WGA and Con A lectin blotting must be located in the endocyst, since this layer is sensitive to pronase treatment and is stained by both lectins. It is well known that Con A and WGA bind to complex N-linked sequences, so we conclude that the proteins present in the endocyst have gly-cans of this kind.
Chitin distribution in the ciliates is heterogeneous. The presence of chitin has been reported in the cyst wall of Hyalophysa chattoni (Landers 199la, b), Cyclogramma blochmanni, Cli-macostomun virens, Phacodinium metschnikoff, Euplotes inky-stans, and Telotrochidium henneguyi (Bussers and Jeuniaux 1974). Chitin has been identified in the mesocyst and endocyst of Nassula picta, Fabrea salina, Bursaria truncatella, Pseu-domicrothorax agilis, and Pseudomicrothorax dubius (Bussers 1976), and in the endocyst and ectocyst of Blepharisma undu-lans (Mulisch and Hausmann 1989) and Euplotes muscicola (Greco et al. 1990). However, chitin appears to be absent in the resting cyst of Bresslaua, Woodruffia, Didinium, tintinnids, col-podid ciliates (except Bursaria), Dileptus, and stichotrichs (Bussers 1976; Bussers and Jeuniaux 1974).
The process of cyst wall production by exocytosis of precursor vesicles synthesized “de novo” is extremely common in ciliate protozoa, although there are some exceptions. In Col-poda steinii, the cyst wall is formed from the membranes or derived from vesicles of the endoplasmic reticulum, which are progressively stacked against the inside of the growing cyst wall, and not by the exocytosis of vesicles containing precursor material (Ruthmann and Kuck 1985). In Pseudomicrothorax agilis, the mesocyst derives directly from the epiplasm of the vegetative cell, and the ectocyst probably arises from an increasingly dense cell membrane (Bussers 1976). In the apos-tome ciliate Hyalophysa chattoni, the ectocyst probably originates from the inner layer of the vegetative cell pellicle and from the endoplasmic reticulum that underlies the pellicle (Landers 199la, b). In Colpoda cucullus, the pellicle thickens and then develops into the first layer of the resting cyst (Ka-wakami and Yagiu 1963a, b). In Euplotes muscicola, the chi-tino-proteic ectocyst seems to proceed from the hypolemme (Bussers et al. 1986).
Opisthonecta henneguyi presents all the ways of cyst wall formation described in ciliate protozoa. The ectocyst is not secreted by exocytosis of cytoplasmic precursors as occurs in Pseudomicrothorax agilis, Hyalophysa chattoni, and Colpoda cucullus. The endocyst and the granular layer of Opisthonecta henneguyi are secreted by exocytosis of different precursors synthesized “de novo”. The precursors of these layers are different from those described in the peritrich ciliate Telotrochidium henneguyi (Walker, Edwards, and Suchard 1989). In the latter species, only one precursor type has been described: sub-pellicular flattened vesicles that fuse with parasomal sacs, releasing their contents. No membrane-limited precursors of the mesocyst have been detected. The mesocyst of Opisthonecta henneguyi is a chitinous layer. Chitin polymerization and secretion probably takes place on the outer cell membrane, as has been described in most chitin-secreting systems, such as fungi, yeasts, arthropods, flagellates, and some ciliated protozoa with chitinous cyst wall, such as Euplotes muscicola (Greco et al. 1990) and Hyalophysa chattoni (Landers 1991a). Chitin secretion also occurs by chitosomes (vesicles that transport chitin synthetase to the cell surface in fungi). This method of chitin secretion is unusual in ciliates, and has been described only during the lorica formation of Eufolliculina uhligi (Mulisch and Hausmann 1983; Mulisch et al. 1983).
ACKNOWLEDGMENTS
Financial support was provided by the Dirección General de Investigaciones Científica y Técnica (DGICYT), project: PB97-0710-C02-01. We are grateful to Dr Antonio Gil and M E. Soria both of the Departamento de Química Orgánica, Facultad de Química, for their help in the GLC-MS analysis of sugars.




