Expression of Toxoplasmic 65 kDa Cystic mRNA by RT-PCR in Patients with Toxoplasma gondii Infection Relapses
The conversion of Toxoplasma gondii bradyzoites to tachyzoites may be responsible of toxoplasmic encephalitis (TE) in immunocompromised patients. To date there are no definitive systems available to detect bradyzoites in clinical samples [4]. Among identified bradyzoite-specific antigens, the 65 kDa MAGI is abundantly located in the cyst's matrix, and at lower levels in tachyzoites [6]. However, the antigenic fragment encoded by the 1.2 kb exon of the MAGI gene was recently demonstrated to be specific for the bradyzoite stage [5]. Moreover, unlike other bradyzoite-specific genes, MAGI transcription is regulated during the development of the bradyzoite stage. This was demonstrated by the detection of the MAGI transcript in preparations containing parasites in the process of conversion from the tachyzoite stage to the bradyzoite stage, indicating a potential role of the MAGI transcript as a bradyzoite marker [2,5]. “In house” oligonucleotides deduced from the 1.2 kb portion of the MAGI gene were employed in reverse transcriptase (RT)-PCR to investigate the mRNA expressed in cerebrospinal fluid (CSF) specimens obtained from AIDS patients with relapse of TE. Amplified fragments were then analyzed by Southern blot employing internal oligonucleotides as specific probes. The Bl gene [1] was also investigated by RT-PCR and Southern blot for comparison.
MATERIALS AND METHODS
CSF specimens were collected from nine AIDS patients with TE relapse. These patients were taking long-term anli-Toxoplasma prophylaxis or treatment at time of CSF collection. Samples were centrifuged at 1,000 g for 5 min at 4°C. Total RNA was extracted from fresh or stored (at −80°C) CSF (200 μl), by a RNeasy Mini Kit according to manufacturer's (QIAGEN) instructions. The RNA preparations were resuspended in water and stored at −80°C.
cDNA synthesis and amplification were performed in single tubes using an Access RT-PCR System (Promega). Briefly, 10 μl of total RNA were added in 50 μl of reaction mixture containing 50 mM Tris HC1 (PH 8.3 at 25°C), 50 mM KC1, 1.5 mM MgSO4, 2.5 mM spermidine, 10 mM dithiothreitol, 0.5% Tween 20®, 0.2 mM each of dNTP, 5 U of avian myeloblastosis virus (AMV) reverse transcriptase, 5 U of Thermus flavus (Tfl) DNA polymerase, 0.4 μM and 0.8 μM downstream and upstream outer primers for MAGI (Fig. 1) and Bl genes, respectively. 20 μ1 of DNA- and RNA-free water was used instead of RNA as negative control. Reverse transcription was performed in a thermal cycler at 48°C for 45 min for the reverse transcription, and at 94°C for 2 min for the inactivation of AMV-RT and the denaturation of RNA/cDNA/primer. This step led directly into the second strand cDNA synthesis by PCR amplification, using outer primers which amplify the 35-fold repetitive Bl gene and the MAGI genes. PCR products underwent a second round of amplification by inner primers (Fig. 1), in a final volume of 50 μ1. RT-PCR conditions for the MAGI gene were 95°C for 10 min; 35 cycles at 95°C for 30 s, 48°C for 30 s, and 68°C (required by Tfl DNA polymerase) for 60 s. RT-PCR conditions for the Bl gene were 95°C for 15 min; 40 cycles at 95°C for 30 s, 58°C for 90 s, and 68°C for 90 s. Amplicons were analyzed by agarose gel electrophoresis and visualized by ethidium bromide staining. Following the RT-PCR procedure, 15 μl of the PCR product fragments were separated on 1% agarose gel, denatured in neutral buffer, and then transferred to a nylon membrane (Nytran N 0.2 μm; TurboBlotter System, Schleicher & Schuell) in 20X SSC transfer buffer. Nylon membranes were pre-hybridized at 45°C for 60 min. and then hybridized by labeled oligonucleotide probes for Bl and MAGI genes (tailed at the 3′-end with fluorescein-11-dUTP (Fl-dUTP) (Amersham International plc, England). The final concentration was 10 ng ml−1; incubation was for 2 h at 45°C in a Shake ‘n’ Stack hybridization oven (Hybaid). A clinical sample was considered to be T. gondii DNA-positive if the RT-PCR amplicons hybridized with the probe in Southern blotting experiments.
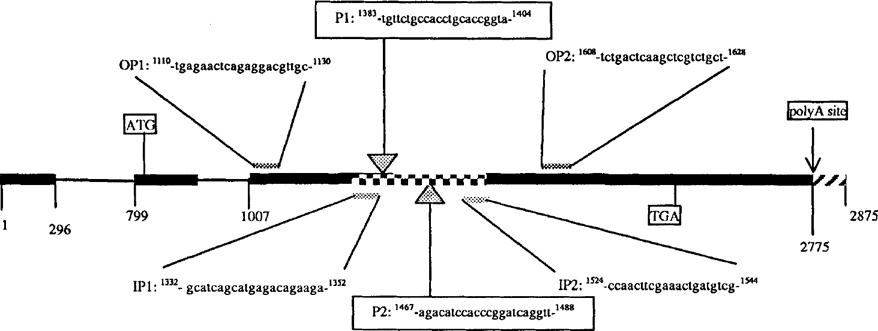
Representative structure of the MAGI gene containing two introns (thin line) and three exons (bold line) coding for the 65 kDa cyst antigen. Oligonucleotides employed as outer- and inner-primers in RT-PCR assay, and as probes PI and P2 in Southern blot hybridization are shown.  212 bp amplified fragment;
212 bp amplified fragment;  not-transcribed fragment
not-transcribed fragment
RESULTS AND CONCLUSIONS
MAGI RT-PCR was more sensitive and specific than Bl in detecting T. gondii during TE relapse, indicating that there was a major degree of expression of the MAGI gene during bradyzoite differentiation. The production of MAGI mRNA was detected in all 9 CSF samples (100%) compared to Bl mRNA (6 out of 9 samples, 66.6%). Southern blotting by the PI and P2 probes of the MAGI gene confirmed results obtained by RT-PCR. Both probes showed a specific hybridization signal (Fig. 2), confirming that the 212 bp RT-PCR-amplified fragment was within the 1.2 kb exon coding for the antigen expressed in cysts only. In contrast, the Bl gene was detected with the MAGI gene in most of the patients receiving either long-term maintenance prophylaxis or specific anti-Toxoplasma treatment. This indicates that the Bl gene has limited value in monitoring the effects of therapy or prophylaxis [3].
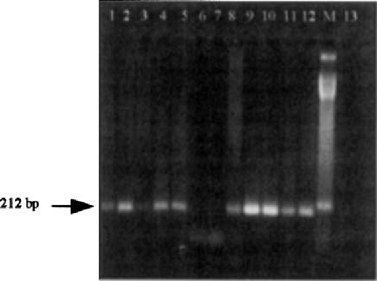
Representative RT-PCR with MAGI oligonucleotide primers. Lanes 1, 2, 4, 5, 8, 9, 10: CSF positive samples; lanes 11–12: total RNA from T gondii RH strain control; M: molecular mass standard of 50 bp; lane 13: negative control. The arrow indicates MAGI-specific amplification signal demonstrated in bradyzoites (left).
The bradyzoite markers have not previously been investigated as potential diagnostic tools in human studies. Thus, the employment of bradyzoite-specific genes such as MAGI may represent a new approach for detecting latent phases of T. gondii infection. Since MAGI transcription has been experimentally determined to occur during conversion to the bradyzoite stage, our system might be useful in processing clinical specimens especially from patients with TE reactivation.
ACKNOWLEDGMENTS
This work was made by grants of the Ministry of University and Scientific and Technologic Research (ex 60% and ex 40% 1999–2000) and of the “Cassa di Risparmio di Ferrara” Foundation.




