Production and Role of Nitric Oxide in the Alveolar Immune Response to Pneumocystis carinii
The importance of nitric oxide in the immune response to Pneumocystis carinii (P. carinii) is unclear. We examined the role of nitric oxide (NO) in the pulmonary immune response against Pneumocystis carinii in an in vitro culture system and in rats. It has been shown that P. carinii can stimulate normal alveolar macrophages to produce NO [3,5,6], and that previous P. carinii exposure increases NO production in unactivated macrophages exposed to P. carinii in vitro, but not in activated macrophages [4].
MATERIALS AND METHODS
The in vitro culture system used for the growth of P. carinii has been described previously [2]. Human embryonic lung fibroblast cells (HEL-299) were cultured in 24-well tissue culture plates, and the monolayers inoculated with 7 × 105P. carinii trophozoites. The nitric oxide generators spermine NONOate or diethylamine NONOate (0.1 mM in 0.01 mM NaOH) were incubated with some HEL cells and P. carinii growth compared to the following controls: cultures treated with 0.01 mM NaOH or cultures treated with TMP/SMX (50/250g/ml) harvested on days 1, 3, 5 and 7.
To determine whether nitric oxide plays a role in the clearance of P. carinii in normal rats, female Sprague-Dawley rats were infected by transtracheal inoculation with approximately 7.8 × 108 organisms as previously described [1]. In some rats, nitric oxide production was inhibited by IP injection of L-N5-(l-iminoethyl)ornithine (L-NIO) which was given on days 4, 5, and 6 for a one week experiment, days 9, 11, 12, and 13 for a two week experiment. At time of harvest, the rats were anesthetized and lavaged with 100 ml phosphate-buffered saline. The BAL fluid was centrifuged at 300 ×g for 5 min, and the alveolar macrophages were counted. Some alveolar macrophages were incubated with PBS, IFN-γ 100 U/ml) and LPS (2 g/ml), IFN-γ and 1 × 108P. carinii trophozoites, or IFN-γ and 1 × 108 Group B streptococci bacteria for 18 hr. NO production was calculated, and the P. carinii burden was assessed in the rats treated with L-NIO.
One-way ANOVA or Student's t-test was performed where appropriate to determine significant differences among or between samples using the SigmaStat software package (Jandel Scientific).
RESULTS AND DISCUSSION
Nitric oxide was toxic to P. carinii in in vitro experiments using the nitric oxide generators diethylamine NONOate and spermine NONOate, reducing P. carinii trophozoite numbers by 76.5% and 95%, respectively, after seven days in culture (Table 1).
| P. carinii trophozoites/oil immersion field | ||||
|---|---|---|---|---|
| Days after Incolucation | ||||
| 1 | 3 | 5 | 7 | |
| C | 2.89 0.35 | 6.03 1.27 | 8.8 2.07 | 10.99 4.54 |
| D | 2.21 0.21 | 2.14 0.53 | 3.83 0.48 | 2.59 0.05a |
| C | 2.98 0.09 | 8.94 0.81 | 13.83 2.25 | 19.94 0.97 |
| S | 2.08 0.81 | 2.11 0.34 | 1.75 0.71 | 1.00 0.37* |
In a dexamethasone-immunosuppressed, transtracheally inoculated rat model of P. carinii infection, production of nitric oxide by alveolar macrophages was reduced by 95% during P. carinii pneumonia even though iNOS mRNA levels were comparable to normal alveolar macrophages activated with IFN-γ and LPS or IFN-γ and P. carinii (data not shown). These results suggest that inhibition of nitric oxide production in P. corinii-infected rats takes place downstream of transcription of iNOS. In contrast, normal rats transtracheally inoculated with P. carinii had a 100% increase in nitric oxide production (Figure 1).
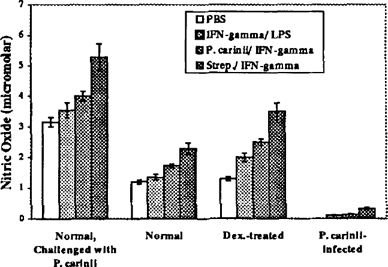
Nitric oxide production by lavaged alveolar macrophages. Alveolar macrophages lavaged from normal, P. carinii-challenged normal, dexamethasone-treated, and dexamethasone-treated and P. carinii-infected rats were incubated for 18 hr in media with 20 1 PBS, 10 U IFN-γ and 2 g/ml LPS, 100 U IFN-γ and 1 × 102P. carinii trophozoites, or 100 U IFN-γ and 1 × 102 Gp. B streptococci bacteria. Nitrites were measured using the Griess reagent. In all samples, nitrites were secreted in increasing amounts with stimulation (PBS < IFN-γ/LPS < IFN-γIP. carinii IFN-γ/Strep.) In all conditions, alveolar macrophages from P. carinii-infected rats produced significantly reduced amounts of nitrites, p<0.05. Likewise, in all conditions, alveolar macrophages from normal, P. carinii challenged rats produced significantly more nitrites, p<0.05.
In all samples, nitrites were secreted in increasing amounts with stimulation (PBS < IFN-γ/ IFN-γIP. carinii < IFN-γ/ Strep). In all conditions, alveolar macrophages from P. carinii-infected rats produced significantly reduced amounts of nitrites (p<0.05). Likewise, in all conditions, alveolar macrophages from normal, P. carinii-challenged rats produced significantly more nitrites (p<0.05).
To test whether nitric oxide plays a role in the clearance of P. carinii in normal rats, nitric oxide production was inhibited by IP injection of L-NIO in rats challenged with P. carinii. After five days of treatment, initiated nine days after inoculation of organisms, nitric oxide production by alveolar macrophages from these animals was reduced by 85% as compared to alveolar macrophages from untreated rats challenged with the same number of organisms.
Also, rats that received the NO inhibitor had 38% fewer alveolar macrophages as compared to infected rats not treated with L-NIO. The P. carinii burden in L-NIO treated animals, as measured on impression smear of lung tissue, was 1.5–3 times greater than in control rats, indicating that nitric oxide may play a role in the directing the alveolar immune response to and the clearance of P. carinii in normal rats (Figure 2).
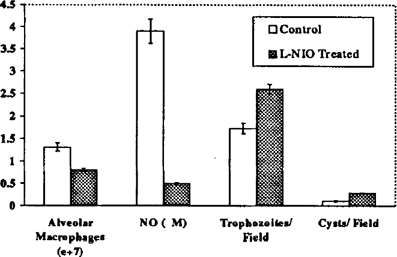
L-NIO mediated inhibition of nitric oxide production impairs clearance of P. carinii from normal rats. Normal rats were challenged with P. carinii transtracheally and then some rats were treated with L-N3-(l-iminoethyl)omithine (L-NIO, 2 mg/kg/treatment day) on days 9, 11, 12, and 13 after inoculation. Treated animals and control animals were sacrificed on day 14. The increase in alveolar macrophage number seen in the control rats was inhibited in L-NIO treated rats by 38.1%. Also, nitric oxide secreted by lavaged alveolar macrophages from L-NIO treated rats was only 13.3% of that from control animals. The P. carinii burden in these animals was increased 150% for trophozoites and 263% for cysts in L-NIO treated rats as compared to control rats.
These results indicate that nitric oxide is toxic to P. carinii in culture, and that alveolar macrophages from P. carinii-infected rats secrete little nitric oxide despite high iNOS levels. Nitric oxide may play a role in the clearance of P. carinii from healthy rats, because inhibition of nitric oxide production increases the P. carinii burden in healthy, P. carinii-challenged rats.




