Effect of the Transcription Factor GATA-2 on Phagocytic Activity of Alveolar Macrophages from Pneumocystis carinii-Infected Hosts
Alveolar macrophages from normal hosts are able to bind, phagocytose, and degrade P. carinii [6,10], but those from P. carinii-infected hosts are defective in phagocytosis of P. carinii organisms [4,5]. Recently, the expression of the transcription factor GATA-2 gene was found to be down-regulated during P. carinii infection in alveolar macrophages [8]. GATA-2 has been shown to play a crucial role in the development of hematopoietic cells [7,9] and regulation of a variety of genes [2,3]. la this study, we performed experiments to determine whether down-regulation of GATA-2 correlates with the defect in phagocytosis of alveolar macrophages from P. carinii-infected rats.
MATERIALS AND METHODS
Female Sprague Dawley rats, each weighing 124-138 g, were used. Some rats were given regular water and used as controls, some rats were immunosuppressed with dexamethasone as previously described [1], and some were immunosuppressed and transtracheally inoculated with P. carinii as previously described [1], At specific time points, rat lungs were lavaged with approximately 100 ml of PBS. The lavage fluid was centrifuged at 300 xg for 5 minutes at 25°C to pellet cells. The cells were then resuspended to 1.5 × 106/ml in complete media containing RPMI 1640 (Sigma, St. Louis, MO) supplemented with 10% FBS, 1 mM pyruvate, 1% non-essential amino acids, 14 mM glucose, 17.9 mM NaHCO3, 10 mM HEPES, 100 units/ml penicillin, and 0.1 mg/ml streptomycin.
Alveolar macrophages were incubated in complete media at 37°C in a 5% CO2 atmosphere for 18 hr and then treated with IFN-γ (100 units/ml) for 2 hr and LPS (2 g/ml) for 30 min before phagocytosis assay. Fifty million fluorescein isothiocyanate (FITC)-conjugated, 1 m diameter, carboxylated latex beads (Sigma Chemical Co., St. Louis, MO) were added to 1 ml aliquots of macrophages (1 × 106/ml). The cells were incubated at 37°C/5% CO2 for 2 hr with gentle agitation every 10 min and then centrifuged through 3 ml FBS at 300 xg for 5 min at 25°C to remove beads that were not phagocytosed. Cells (30,000–50,000) were placed on a Superfrost+ slide (Fisher, Pittsburgh, PA) by cytospin (Cytospin n, Shandon, Pittsburgh, PA) at 750 rpm for 5 min at 25°C, stained with Giemsa stain, and then examined on a fluorescent microscope (Olympus BH-2, Tokyo, Japan) at 400x. The number of FTTC-labeled beads in at least 300 random macrophages from at least 50 random fields was determined, and an average number of FITC-labeled beads per macrophage was calculated.
RESULTS AND DISCUSSION
Alveolar macrophages from the normal rats phagocytosed an average of 1.89 beads per macrophage, and those from the dex-suppressed rats showed an average of 1.27 beads per macrophage. Surprisingly, alveolar macrophages from the P. carinii-infected rats had an average of only 0.075 (40% reduction as compared to dex-suppressed) beads per macrophage (Figure 1). This result indicates that alveolar macrophages from P. carinii-infected rats have a reduced ability to phagocytose and that the previously discovered inability of macrophages from P. carinii- infected hosts to phagocytose is not specific for P. carinii but is a general defect in phagocytosis.
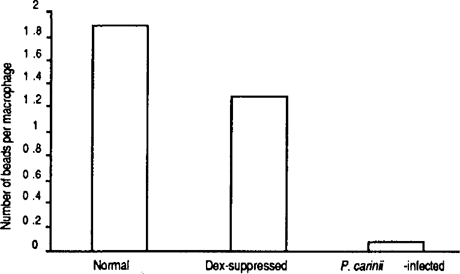
Phagocytic activity of alveolar macrophages from normal, daxsuppressed, and P. carinii-infected rats. Alveolar macrophages were obtained from three different rats of each group and assayed for phagocytosis using FITC- labeled latex beads. Total number of beads in 300 macrophages from each rat were counted, and an average number of beads per macrophage from each group of rats is graphed.
To determine whether down regulation of the GATA-2 gene correlates with the reduced phagocytic activity of alveolar macrophages in P. carinii-infected hosts, normal alveolar macrophages were treated with GATA-2-specific antisense oligonucleotides and then assayed for phagocytic activity. The sequence 5′-GCTGCAGTGGGGGTGAGG-3′located from nucleotides 628 to 645 (473 bp downstream from the initiation codon) of the mouse GATA-2 cDNA (GenBank accession no. NM 008090) was chosen.
Both sense (5′-GCTGCAGTGGGGGTGAGG-3′) and antisense (5′- CCTCACCCCCACTGCAGC-3′) oligonucleotides were synthesized (Synthetic Genetics, San Diego, CA) using phosphorothioated dNTP. The sense oligonucleotide was used as a control in each transfection experiment. Fifteen micrograms of oligonucleotide was complexed with 30 g of cationic lipid (Superfect, Qiagen) in a tube for 10 min at roan temperature before incubation with the cells for 8 hr. The cells were then treated with IFN-γ 2 hr and LPS 30 min prior to phagocytosis assay. An aliquot of macrophages were incubated with cationic lipid alone to control for any effects this reagent may have on phagocytic ability.
The phagocytosis assay was repeated three times using alveolar macrophages from three different animals of each condition. Alveolar macrophages that were treated with sense oligonucleotide phagocytosed an average of 1.38 beads per cell. This number is comparable to that of untreated alveolar macrophages (1.97 beads/cell) (Figure 2). Cells from normal rats treated with GATA-2 antisense oligonucleotides phagocytosed only 28% (0.38 beads/cell) as many beads as those treated with sense GATA-2 oligonucleotides. These results indicate that the sense GATA-2 oligonucleotide did not affect the production of GATA-2 whereas the antisense GATA-2 oligonucleotide caused a significant reduction in the production of GATA-2 and resulted in a 72% inhibition in phagocytic activity of alveolar macrophages. Similar results were observed in dex-suppressed rats. Alveolar macrophages from dex-suppressed rats phagocytosed only 35% (0.41 beads/cell) as many beads when incubated with antisense GATA-2 oligonucleotides as compared to those treated with sense oligonucleotides (1.18 beads/cell).
Effect of GATA-2 on phagocytic activity of alveolar macrophages. Antisense or sense GATA-2 oligonucleotides were introduced into alveolar macrophages from normal or dex-suppressed rats. Phagocytosis assay was then performed, and the results are graphed as described in the legend of Fig. 1.
To determine whether the defect in phagocytosis can be corrected, a GATA-2 expression vector was introduced into alveolar macrophagesfrom P. carinii-infected rats. In order to construct the GATA-2 expression vector, the complete coding region of the rat GATA-2 gene was first obtained by RT-PCR with GATA-2 specific primers (forward: 5′-ATGGAGGTGGCGCCTG AGCAG-3′, nucleotide number 155 – 175 of mouse GATA-2 cDNA, GenBank accession no. NM 008090; reverse: 5′-CTAGCCCA TGGCAGTCACCATG-3′, nucleotide number 1576 – 1597 of the same sequence). The PCR products were cloned into the TOPO TA vector (Invitrogen, Carlsbad, CA). The sequence and the orientation of the cloned fragment were determined by DNA sequencing. The rat GATA-2 coding region was found to be 1440 bp encoding 480 amino acids.
Both sense and antisense GATA-2 clones were subcloned into pCEP4 (Invitrogen, Carlsbad, CA) so that the expression of the GATA-2 gene is driven by the CMV promoter. The pCEP4 clones, pGATA2sense and pGATA2antisense were then introduced into alveolar macrophages from P. carinii-infected rats. The one with the GATA-2 gene in the antisense orientation served as the control. The GATA-2 expression constructs (15 g) were incubated with 25 g Superfect cationic liposome (Qiagen, Valencia, CA) for 10 min at room temperature. Cells were incubated with the sense construct, antisense construct, or Superfect alone for 36 hr and then assayed for phagocytosis.
Alveolar macrophages from P. carinii-infected rats treated with antisense construct or Superfect alone phagocytosed similar numbers of FITC-latex beads (0.053 and 0.068 beads/cell, respectively, Figure 3). The same cells treated with the sense GATA-2 expression vector regained some of their phagocytic function, as noted by their increased uptake of FITC-latex beads (0.208 beads/cell, Fig. 3). This 392% increase indicates that GATA-2 plays an important role in phagocytosis.
Restoration of phagocytic activity by GATA-2 over expression. A GATA-2 expression vector (sense) was mixed with Superfect reagent and then introduced into alveolar macrophages from P. carinii-infected rats. The macrophages were then assayed for phagocytosis. Alveolar macrophages treated with Superfect reagent alone or with a control vector which contains the GATA-2 gene in die antisense orientation were used as controls.
We conclude from the results of this study that the defect in phagocytosis of alveolar macrophages from P. carinii-infected hosts is a general defect in phagocytosis, not specific for P. carinii. This defect correlates with down regulation of GATA-2 expression during P. carinii infection since suppression of GATA-2 production in alveolar macrophages from normal rats renders them defective in phagocytosis. The possibility to restore the phagocytic activity of alveolar macrophages from P. carinii infected hosts by GATA-2 overexpression further confirms that GATA-2 plays an important role in the regulation of phagocytosis in alveolar macrophages.




