Recombinant CD40 Ligand Administration Does Not Decrease Intensity of Pneumocystis carinii Infection in Scid Mice
Abstract
SUMMARY: X-linked Hyper IgM Syndrome (HIM) is a rare congenital immunodeficiency recently demonstrated to be caused by a mutation in the gene encoding CO40 ligand. These patients are susceptible to Pneumocystis carinii pneumonia, which implies an important role for CD40L in host defense against P. carinii. In this study we undertook to investigate whether treatment of P. carinii infected scid mice with murine recombinant CD40 ligand trimer (muCD40L) for 21 days would facilitate clearance of the organisms. We found no significant difference in organism burden in treated compared to control animals. Therefore in this model treatment with muCD40L alone is ineffective in clearing P. carinii infection.
Pneumocystis carinii is an opportunistic pathogen causing pneumonia in patients with AIDS, cancer and other immunocompromised patients. X-linked Hyper-IgM Syndrome (HIM) is a rare congenital immunodeficiency recently demonstrated to be caused by a mutation in the gene encoding CD40 ligand [3]. The syndrome is characterized by normal to elevated serum IgM levels, but decreased or absent levels of IgA, IgE and IgG. Patients with HIM are susceptible to opportunistic infections such as P. carinii pneumonia (PCP), suggesting a critical role for CD40L in host defense against P. carinii.
CD40L is present on activated T-lymphocytes. Its receptor CD40 is expressed by B cells, macrophages, dendritic cells and endothelial cells as well as a variety of other cell types. The interaction between CD40-CD40L is known to stimulate expression of accessory molecules such as MHC class II on antigen presenting cells. Additionally, CD40/CD40L interaction induces production of inflammatory cytokines such as TNF-α and IL-12 by macrophages. In B cells the binding of CD40L to CD40 is essential for antibody class switching, and thereby is critical to the production of antigen specific IgG. The purpose of this study was to investigate whether treatment with murine recombinant CD40 ligand would facilitate clearance of P. carinii organisms from the lungs of infected severe combined immunodeficiency (scid) mice.
MATERIALS AND METHODS
Twelve balb/c scid mice were infected with P. carinii by co-housing with infected animals. Trimeric murine CD40 ligand/leucine-zipper fusion protein (muCD40L) was administrated intraperitoneally (i.p; 30 μg/dose) to 6 mice every other day for 20 days. The remaining 6 mice were injected i.p. with buffer alone at the same time points.
On day 21 animals were sacrificed and lungs were removed. Impression smears of the lungs were stained with Diff-Quik. One lobe of the lung was fixed in Histochoice with 20% ethanol and embedded in paraffin. Slides from fixed tissue were stained with Hematoxylin and Eosin (H&E) or by an immunoperoxidase technique using monoclonal antibody 4D7, which is specific for P. carinii [1]. Impression smears were rated for intensity of P. carinii infection on a scale from 0 to 5.
RESULTS AND DISCUSSION
On day 11 two mice from the muCD40L treated group were euthanized due to respiratory distress, and on day 13 one mouse from the control group was found dead. Remaining mice survived for the 21 days of the protocol. All animals were infected with P. carinii. The mean impression smear ratings in the muCD40L trimer treated group was 2.25 compared to 2.0 in the control group (p=NS). The intensity of infection based on histology using H&E as well as immunostaining with 4D7 was consistent with these findings. Fig. 1A and 1B shows lung tissue from a muCD40L trimer treated and a control respectively stained with mAb 4D7. The difference in organism burden between the 2 groups was not significant, and therefore in this model treatment with muCD40L alone was inadequate to clear P. carinii.
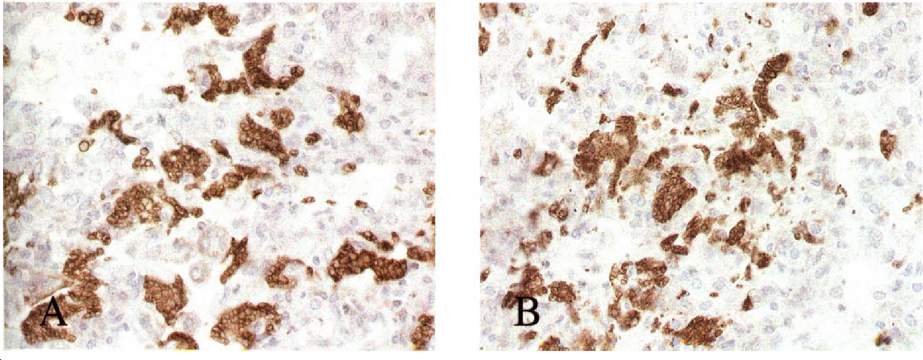
Staining of lung tissue with 4D7 1° mAb, which is specific for P. carinii. 2° mAb: Horse radish peroxidase-conjugated rat anti-mouse. Clusters of P. carinii (brown) seen in lungs from both muCD40L treated (A) and control mouse (B).
Other studies have looked at the role of CD40L in host defenses against P. carinii. In one study scid mice with PCP were reconstituted with purified CD4 cells from immunocompetent donors and administered anti-CD40L mAb. These mice were impaired in the ability to clear P. carinii infection compared to controls administered an irrelevant control antibody [4]. These data suggests an important role for CD40L interacting with a cell type other than B cells. In another study dexamethasone treated Sprague-Dawley rats were partially protected from P. carinii infection by prophylactic administration of muCD40L at the onset of immunosuppression [2].
In our study we also used scid mice, which do not have mature B or T cells. A potential effect of muCD40L in this model would therefore require the interaction of CD40L with its receptor CD40 on a cell type other than B cells. Macrophages are known to be the main effector cells against P. carinii. We hypothesized that an effect of CD40L-CD40 interaction would result in activation of macrophages and potentially improve effector functions for clearing P. carinii. However, in this study we did not see any effect of treatment on the intensity of P. carinii infection. This could be due to a lack of cytokine production from CD4+ cells that may be required as costimulatory signals for macrophage activation. Lack of opsonization of P. carinii organisms by antibodies could further diminish the efficiency of macrophage effector functions. In addition, animals in this study were heavily infected, a factor that might require higher doses or longer duration of treatment with muCD40L.
ACKNOWLEDGMENTS
We would like to thank Elaine K. Thomas, Ph. D, Immunex Corporation, Seattle WA for kindly providing the Trimeric murine CD401igand/leucine-zipper fusion protein.




