Development of Theluhuniu solenupsue in Red Imported Fire Ants Solenupsis invicta from Polygynous Colonies Results in Formation of Three Spore Types
Microsporidia that are widespread in the environment should be monitored for their potential danger to humans with impaired immune systems. The latest findings of the close relationship between Vavraiu culicis and Truchipleistophora hominis, as well as of Brachioh (Nosm) algeri development in mammalian cell lines in spite of nonpermissive temperatures, strongly support this idea. Like V. culicis or B. algeri, infecting mosquitoes, Thelohaniu solenopsae (T.s), parasitizes a widespread insect species, red imported fire ant (RIFA), that intensively contacts humans. Additionally. T.s is a promising biocontrol agent, and its introductions in RIFA populations are being attempted in certain areas of the United States. According to previous research [1,2], T.s development results in the formation of two types of spores: “Nosemu”- and “Thelohunia-like”. the latter maturing inside sporophe IWS vesicles and also called octospores or meiospores. Although the ultrastructure of this microsporidium was described more than 20 years ago [l], its caste and tissue specificity and mode of transmission remain obscure. This research has been undertaken to fill these gaps.
MATERIAL AND METHODS
Infected and uninfected S.invictu colonies were cultivated for 14 months in the laboratory [3]. Brood infected with T.s was kindly provided by David Oi (USDA-ARS). It was introduced into laboratory colonies of ants, eventually resulting in miaosporidian infection throughout the colony. Sixty two female alates, as well as individuals of other castes and stages. were examined 48-60 weeks post colony infection. Fresh preparations, Giemsa and Dapi stained smears of cuticles, of abdominal tissues, and of ovaries, were examined in light (WON equipped with PhC. DIC and Epifluorescence) (ISUI) and electron (JEMlOOCX) microscopes (EM) using conventional protocols.
RESULTS AND DISCUSSION
In addition to two spore types previously recorded for T.s– diplokaryotic Nosemu-like free spores and octospores with single nuclei, maturing inside sporophorous vesicles in sets of 8 – a new spore type has been discovered. “megaspores” with two nuclei (Table). The latter possess a thick endospore and a polar filament of 20-22 coils (Table, Fig.).
| Spore type | Lengh, μm ±SD | Length range | Width, μm ±SD | Width range | nn |
|---|---|---|---|---|---|
| Mega spores | 7.2±0.51 | 6.2–8.0 | 3.8±0.26 | 3.3–4.4 | 23 |
| Nosema like spores | 4.5±0.29 | 3.5–5.2 | 2.6±0.23 | 2.2–3.7 | 28 |
| Octaspores | 3.5±0.24 | 2.6–4.0 | 2.4+0.20 | 1.8–3.3 | 28 |
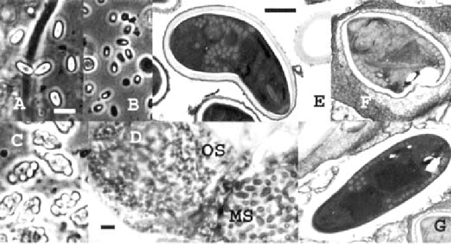
A-C – phase contrast, E-G – elearon microscopy. A - megaspores (MS); B,G - Nosema-like spores; C,F - octospores (0s). D - hemithii d o n through ovaries with two neighbor oocytes infected with 0s and MS. A-D – bar =10 m, E-G–bar=l m
The infected laboratory population of RIFA exhibited uneven distribution of spore types among the castes and the brood. In imagoes of all castes, three spore types were found, but in brood only Nosema-like spores and megaspores were detected. The microsporidium displayed also certain tissue specificity. In workers, the infection was restricted to the fat body. Mature spores were concentrated between the subcuticular epithelium and the muscle layer. In female imagoes, only ovaries were infected. Both LM and EM analysis of the infected females revealed heavy infection of wytes. Adjacent oocytes in one female occasionally were infected simultaneously, one with octospores, octospores, the neighboring oocyte with megaspores (Fig. d). In oocytes infected with octospores, few free binuclear Nosema-like spores were detected. EM studies revealed that the latter were lacking internal structure, looking like discharged spores. No “empty” spores were observed among octospores or megaspores. “Nosema like” spores were the only type of spores that easily discharged their polar filaments in fresh preparations.
Concluding remarks. (1) Transovarial transmission might represent the principle way for T.s to spread inside RJFA populations, in which only one or a few females (queens) are responsible for the reproduction of the whole colony. (2) The consistent occurrence of megaspares in field and laboratory RIFA populations suggests that these spores constitute a regular part of the parasite life cycle. Their occurrence in larvae rather than in imagoes, and their development in ovaries alongside octospores. indicate that in the T.s life cycle megaspores might be formed alternatively to octospores, presumably when spore morphogenesis starts before the mother sporont cell undergoes meiosis. (3) The frequent occurrence of “empty”“Nosema-like” spores in the infected tissue, as well as their thin endospores, suggest that this spore type is responsible for autoinfection. (4) Further studies of T.s will elucidate the exact role of each spore type in the T.s life cycle and might lead to refining the systematic status of this microsporidium.
ACKNOWLEDGMENTS
The authors thank Arthur R. Richter for technical assistance. Supportec by a Louisiana State Board of Regents grant and by USDA South. ern Regional IPM grant ## 99-34103-8067.




