Encephalitozoon cuniculi Infection in Mice with the Chronic Granulomatous Disease (CGD) Disorder
Encephalitozoon cuniculi, a single-celled parasite in the phylum, Microsporidia, causes chronic infections in immune competent mammals and can cause lethal infections in T lymphocyte-deficient hosts[3]. As an obligate intracellular parasite, E. cuniculi can replicate within resting or resident macrophages, while macrophages activated by cytokine signals (eg. interferon-gamma; IFN-O kill intracellular E. cuniculi organisms [1,4]. Nitrogen intermediates contribute to the macrophage-mediated killing of E. cuniculi in vitro, although additional factors must participate since inducible nitric oxide synthase deficient (knockout) mice did not die after experimental E. cuniculi infections[2,7]. B6.129S6Cybbtml mice have the recessive disorder for chronic granulomatous disease (CGD) which is characterized by a defective phagocytic oxidative respiratory burst[9]. The purpose of this study was to determine if the CGD mice are susceptible to infection with E. cuniculi which would implicate oxygen intermediates in also playing a role in the macrophage-mediated control of these organisms.
MATERIALS AND METHODS
Five female B6.l29S6Cybbtml CGD mice and five B6.129SF2/J control wild-type (WT) mice were purchased from Jackson Laboratory (Bar Harbor, ME) at 5 weeks of age. Mice were housed in sterile filter-topped cages on HEPA filtered ventilator rack shelves and were fed sterile food and water ad libitum. After one week's acclimation, the mice were inoculated intraperitoneally (ip) with 1 X 107 tissue culture-harvested and Percoll-purified E. cuniculi spores, as previously described [5]. The mice were monitored for clinical signs of infection. One and three weeks after inoculation, peritoneal exudate cells (PEC) were obtained after ip injection and recovery of 3 ml sterile Hank's buffered salt solution per mouse. Cytospin preparations of the PECs were fixed with methanol for 10 min, stained for 5 min with Calcofluor White M2R (0.1% w/v in water adjusted to pH 8.0 with potassium hydroxide; Sigma, St. Louis, MO; catalogue number F3543), rinsed with water, counterstained with Evan's Blue (0.5% w/v in phosphate buffered saline; Sigma, St. Louis, MO; catalogue number E0133), rinsed again in water, dried, and viewed under fluorescence microscopy at an excitation wavelength of 395 run and at 600X magnification. IgG antibody responses were measured against solubilized E. cuniculi proteins by ELISA as previously described [6].
RESULTS AND DISCUSSION
One week after inoculation with E. cuniculi, organisms could be identified in the Calcofluor-stained peritoneal macrophages of each WT and CGD mouse. An average of approximately 60% of the peritoneal macrophages from the CGD mice were infected with microsporidia compared with approximately 30% of the peritoneal macrophages from the WT mice (Fig. 1). Three weeks later, however, no infected peritoneal macrophages from the WT mice could be found after viewing more than 100 fields per cytospin while infected peritoneal macrophages could be identified in four of the five CGD mice inoculated with E. cuniculi (Fig. 1, inset).
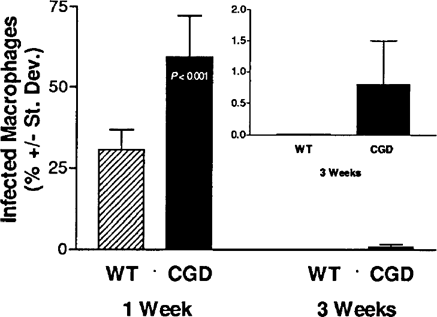
Comparison of the percent of infected peritoneal macrophages recovered from WT and CGD mice after ip inoculation of E. cuniculi.
IgG antibody titers against E. cuniculi were measured in sera collected three weeks after inoculation. The mean ELJSA titers of sera from the CGD and WT mice were identical at 1:12,800 (Fig. 2).
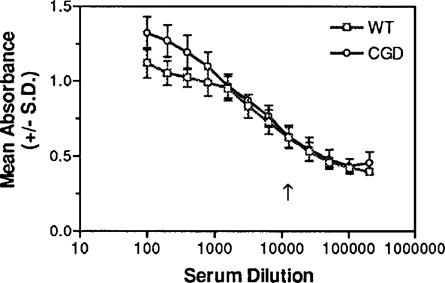
IgG ELISA titration against solubilized E. cuniculi organisms of sera from WT and CGD mice obtained three weeks after ip inoculation with E. cuniculi. The endpoint titers were determined as the highest dilutions that were statistically significantly higher (P < 0.05) than the baseline absorbance values of control sera.
In addition, no differences could be discerned in the Western blot immunodeteclion profiles of sera from mice of both groups (not shown) indicating that the CGD disorder did not prevent these mice from generating humoral immune responses against E. cuniculi similar to that of the WT mice.
All mice survived at least five weeks after inoculation with E. cuniculi. At necropsy, organisms could be identified in liver sections stained with Calcofluor White from the CGD mice but not in liver sections from the WT mice (not shown). These results suggest that the phagocytic respiratory oxidative burst (via reactive oxygen intermediates) contributed to resistance against E. cuniculi infections. The CGD mice carried a higher parasite burden one week after inoculation while nearly twice as many peritoneal macrophages contained organisms compared with those of the WT mice. Even three weeks after inoculation, infected peritoneal macrophages could still be identified in four of five CGD mice while no infected peritoneal macrophages from WT mice could be found.
Since the CGD mice did not succumb to infection with E. cuniculi, other factors, in addition to the oxidative respiratory burst, probably contributed to resistance. Nitrogen intermediates were found to contribute to IFN-(- and LPS-activated macrophage killing of E. cuniculi in vitro, but inducible nitric oxide synthase (iNOS) deficient mice did not succumb to infection [2,7]. Additional studies are needed to determine if the iNOS deficient mice, similar to the CGD mice, carried a higher parasite burden and required more time to resolve acute E. cuniculi infections compared with the WT mice. Furthermore, Khan and colleagues have presented evidence that resistance is dependent upon CD8+ T cells [8]. It is unclear yet if these CD8+ T cells function primarily by expressing cytotoxicity for the microsporidian spores, cytotoxicity for infected host cells, or if they secrete specific cytokine signals needed to activate macrophage killing of microsporidia. Taken together, the study presented here and the previously-published studies suggest that multiple factors contribute to resistance against lethal E. cuniculi infections and include CD8+ T cells, IFN-(, and macrophages which release reactive oxygen intermediates and reactive nitrogen intermediates.
ACKNOWLEDGMENTS
The authors gratefully acknowledge funding from the National Institutes of Health (RR00164).




