Genotyping Encephalitozoon Parasites Using Multilocus Analyses of Genes with Repetitive Sequences
Encephalitozoon cuniculi, Encephalitozoon hellem, and Encephalitozoon intestinalis are three of the four most common human microsporidian parasites. In addition to humans, E. cuniculi and E. intestinalis have been found in various mammals such as rabbits, rats, mice, horses, foxes, cats, dogs, muskrats, leopards, and baboon, and E. hellem infection is common in some birds [3,7]. Thus zoonotic infection can be a significant source of human infection under certain circumstances. As a result, the development of intra-species genotyping tools that can differentiate Encephalitozoon parasites from various hosts is important to studies of human microsporidiosis.
Thus far, three genotypes of E. cuniculi have been identified based on the number of GTTT repeats in the internal transcribed spacer (ITS) of the rRNA gene: a genotype or strain I first isolated from rabbits containing 3 repeats, a genotype or strain II first isolated from mice containing 2 repeats, and a genotype or strain III first isolated from dogs containing 4 repeats [4], Thus far, both genotypes I and III of E. cuniculi have been found in humans [11]. A recent ITS sequence characterization of 5 human E. hellem isolates from Europe and Africa has identified 3 genotypes of E. hellem, even though the significance of the genetic diversity is not clear [9]. ITS sequence analysis, however, failed to reveal any heterogeneity in 13 human E. intestinalis isolates [6].
Currently, genotyping of Encephalitozoon parasites mainly involves DNA sequencing of ITS, which is not practical in most diagnostic laboratories because of the technical demands and high cost. Thus, there is a need for the development of simpler genotyping tools that are more cost-effective and can be performed in a greater number of diagnostic laboratories. In this study, we examined the genetic diversity within E. cuniculi, E. hellem, and E. intestinalis, using sequence analyses of genes with repetitive sequences, such as the ITS, polar tube protein (PTP) and spore wall protein I (SWP-1). Results of this study support a recent observation in the length polymorphism of the PTP gene of E. cuniculi and E. hellem [10].
MATERIALS AND METHODS
Parasite isolates
Twelve E. cuniculi isolates, 24 E. hellem isolates and 11 E. intestinalis isolates were used in this study. E. cuniculi isolates used included 7 isolates from humans (CDC:V282 from Colorado. CDCV449A from Michigan, CDC:V385 from California, USP A-1 and USP A-2 from Spain, and CDC:V446 and 3275 from Italy), 1 isolate from a dog (Ca-d from Texas), and 1 isolate from a rabbit (CDC:V428A from Ohio). Three previously characterized reference strains of E. cuniculi, strain I from a rabbit (Ja-r from Texas), strain II from a mouse (Va-m from New York), and strain III from a dog (Ja-d from Texas), were also used as controls. The E. hellem isolates used in this study included 24 human isolates from 20 patients in the United States (11 isolates), Puerto Rico (1 isolate), Italy (10 isolate), Switzerland (1 isolate), and Spain (1 isolate). The E. intestinalis specimens used were isolated from AIDS patients in the United States (10 isolates) and Peru (1 isolate).
Molecular analysis
DNA was extracted from infected tissues or cultured spores by conventional phenol-chloroform method. Nucleic acid from each sample was used in PCR amplification of the ITS, SSU rRNA, PTP, or SWP-1 gene, using primers listed in Table 1. PCR products were sequenced in both directions on an AB1377 autosequencer (Applied Biosystems, Foster City, Calif.). The sequences obtained were aligned with each other and the published sequence using the Wisconsin Package Version 9.0 (Genetics Computer Group, Madison, Wis.).
| Species | E. cuniculi | E. hellem | E. intestinalis |
|---|---|---|---|
| SSU rRNA | CACCAGGTTGATTCTGCCTGA | CACCAGGTTGATTCTGCCTGA | CACCAGGTTGATTCTGCCTGA |
| CCAACTGAAACCTTGTTACGACTT | CCAACTGAAACCTTGTTACGACTT | CCAACTGAAACCTTGTTACGACTT | |
| ITS | GTGCCAGC(C/A)GCTGGCAC | GGTGGTGCATGGCCG | GTGCCAGC(C/A)GCTGGCAC |
| GTT(G/A)GTTTCTTTTCCTC | GTT(G/A)GTTTCTTTTCCTC | GTT(G/A)GTTTCTTTTCCTC | |
| FTP- I | GCAGTTCCAGGCTACTAC | CATGCTTGCCAACACAGG | AAGATGAAAGGTATTTCT/GAAG |
| AGGAACTCCGGATGTTCC | TGGAGGCATTGCAATAGG | AGCAAGC/TGTTGCATGGAG | |
| SWP-i | ACTGACAAGTACCACATC | ||
| TTGGACTCACACATTAGG |
Nucleotide sequence accession number
The SSU rRNA, ITS and PTP nucleotide sequences were deposited in the GenBank database under accession no. AF310677-AF310679, AF33836 to AF338368, AF340007-AF340012, and AY024342.
RESULTS AND DISCUSSION
E. cuniculi
Nucleotide sequences of the ITS obtained from 12 E. cuniculi isolates identified 3 genotypes, with the number of GTTT repeats identical to each genotype previously reported. Genotype I had 3 GTTT repeats and was seen in 4 isolates. Genotype II had 2 GTTT repeats and was seen in 1 isolate. Genotype III had 4 GTTT and were seen in 7 isolates (Table 2). One nucleotide difference was present among the 3 genotypes in the 3′ end of the small subunit rRNA gene: genotype I had A (5′-CGGGACAGTG-3′) whereas genotypes II and III had T (5′-CGGGACTGTG-3′) at nucleotide position 716 of L07255 in the GenBank database.
| Species | No. of isolates | ssu rRNA | ITS | PTP-1 | SWP-1* |
|---|---|---|---|---|---|
| E. cuniculi | 12 | 2 | 3 | 3 | 5 |
| E. hellem | 24 | 3 | 2 | 4 | — |
| E. intestinalis | 11 | 1 | 1 | 1 | — |
- *SWP-I primers failed to amplify DNA from E. hellem and E. intestinalis.
Three types of PTP sequences were obtained from the 12 E. cuniculi isolates: 1) genotype I sequence was identical to the previously published sequence (AJ005666 from Ref. 2), and was obtained from the reference isolate strain I, a rabbit isolate (CDC:V428A), and 2 human isolates (CDC:V385 and CDGV446); 2) genotype II sequence had 4 bp differences from genotype I, and was obtained from the reference strain II: and genotype III had a deletion of one of the 78-bp repeats and 2 bp differences from genotype I (Table 3), and was obtained from the reference isolate strain III, a dog isolate (Ca-d) and 5 human isolates (CDC:V282, USP A-1, USP A-2, CDC:V449A, and 3275). The 3 PTP genotypes were identical to those previously described [10]. There was a complete concordance in genotyping results of samples between sequence analyses of ITS and PTP (Table 2).
| E. cuniculi genotype | E. hellem genotype** | ||||||
|---|---|---|---|---|---|---|---|
| I | II | III | IA | IB | 1C | IIB | |
| ITS* | 37 | 33 | 41 | 46 | 46 | 46 | 50 |
| PTP | 312 | 312 | 234 | 360 | 420 | 480 | 510 |
| SWP-1 | 255,306 | 219,270 | 348,429 | — | — | — | — |
- *Full length of the gene.
- **SWP-I primers failed to amplify DNA from E. hellem
Analysis of the repeat region of the SWP-1 gene revealed that only 3 isolates (CDC:V385, CDC:V428A, and the reference strain I) produced a PCR fragment of the expected 399 bp in size based on the published sequence (AJ133745 from Ref. 1). Other isolates yielded either a single band of different sixes or double bands. Sequence analyses of the 399-bp PCR products revealed a nucleotide sequence identical to the published sequence AJ 133745. As expected, the repeat region contained 5 36-bp repeats and 5 15-bp repeats (Fig. 1). DNA sequencing of other PCR products, including those of the double bands produced nucleotide sequences similar but not identical to AJ 133745. All together, 6 types of SWP-1 sequences were obtained, and they differed from each other in the number of 36- and 15-bp repeats in the repeat region, which was responsible for the size differences in the electrophoresis of PCR products (Table 3).
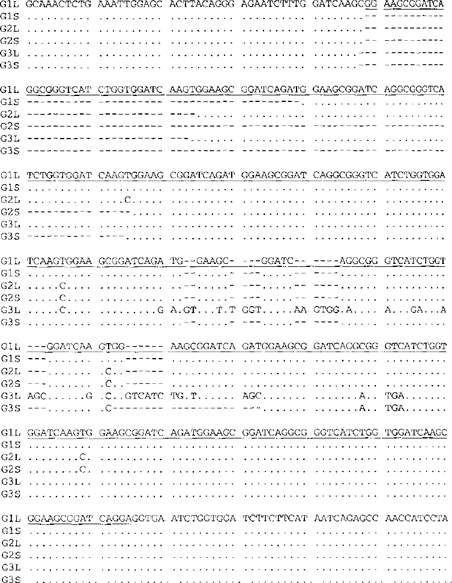
Sequence differences in the SWP-1 gene among 3 E. cuniculi ITS genotypes. Dots denote sequence identity to sequence type G1L, and dashes depict nucleotide deletions. The repeat region is underlined. Gl, G2, and G3: ITS genotype I, II, and III, respectively. L: long PCR product. S: short PCR product.
The 11 E. cuniculi isolates sequenced were divided to 5 genotypes based on SWP-1 banding patterns and sequences: 1) genotype la had one PCR band (299 bp) and 10 repeats (255 bp in total repeat length) and was seen in reference strain I and isolates CDC:V428A and CDC:V385; 2) genotype Ib had one PCR band (450 bp) and 12 repeats (306 bp in total repeat length) and was seen in isolate CDC:V446; 3) genotype II had two PCR bands (363 and 414 bp), one with 9 repeats (219 bp in total repeat length) and one with 11 repeats (270 bp in total repeat length, and was seen in reference strain II; 4) genotype IIIa had two PCR bands (348 and 429 bp), one with 8 repeats (204 bp in total repeat length) and one with 12 repeats (285 bp in total repeat length), and was seen in isolates CDC:V282, USP A-l and USP-A2; and 5) genotype IIIb had one PCR band with a sequence identical to the longer PCR product with 12 repeats in genotype IIIa and was seen in reference strain III and isolates CDC:V449A and Ca-d. Beyond the differences in the number of repeats, the long and short SWP-1 PCR products were related to each other for each genotype.
E. hellem
All E. hellem isolates used in this study were sequenced for the ITS gene. Nucleotide sequences obtained from 23 of the 24 isolates were identical to the genotype I sequence previously reported [9]. One isolate (CDC:V261), however, had an ITS sequence similar to those of genotypes 2 and 3 reported before [9], which differed from the rest in the number of CTTT repeats, length of the T-repeat, and by point mutation. Three types of SSU rRNA sequences were obtained from the 24 isolates studied (Table 2). Fifteen isolates had SSU rRNA sequences identical to the genotype I sequence reported before: CDC:V278, CDC:V242, CDC:V274, Le, CDC:V257, CDC:V258, CDC:V281, Wo, Ye, Ly, He, CDC:V213, CDC:SP-1, LEPV-2–93, and PV-3–93. Eight isolates had a similar sequence, except for an insertion of G at position 162: PV-8–95, VRPV-1–93, PV-5–95, PV-11–98, PV-10–97, PV-10–97EI, MIPV-6–95, and PV-9–96. One isolate (CDC:V261), had a SSU rRNA sequence identical to genotype 2 sequence previously described [8], which had 7 nucleotide base differences from genotype 1.
All E. hellem isolates were also analyzed at the PTP locus (Table 3). Four different sizes of PTP PCR products were detected, as reflected in the different migration rates in agarose gel electrophoresis. Genotype 1A was generated from the smallest PCR products from the 10 E. hellem isolates (CDC:V278, CDC:V242, CDC:V274, Le, CDC:V257, CDC:V258, CDC:V281, Wo, Ye, and Ly), of 461-bp in length, and each was identical to the published sequence (AF044915 from Ref. 5). In contrast, genotypes 1B, 1C and 2B were 521, 581 and 611-bp long, and found in 5 isolates (He, CDC:V213, CDC:SP-1, LEPV-2–93, and PV-3–93), 8 isolates (PV-8–95, VRPV-1–93, PV-5–95, PV-11–98, PV- 10–97, PV-1O-97EI, MIPV-6–95, and PV-9–96.) and 1 isolate (CDC:V261), respectively. Differences in PTP sequence length among genotypes 1 A, IB, and 1C were due to variations in the number of a 60-bp tandem repeat: each of them had 6, 7 and 8 copies of the 60-bp repeat, respectively. Genotype 2B had 3 copies of the 60-bp repeat and 5 copies of a 66-bp repeat. In the 66-bp repeat, a 6-bp sequence (GGAAGC or GGAAGT) was repeated once at the beginning of the 60-bp repeat. Sequence variations among genotypes were also seen in the repeat and non-repeat regions.
E. intestinalis
All 11 E. intestinalis isolates were characterized at ITS, SSU rRNA and PTP-1 loci. All sequences obtained from these isolates were identical to those previously published (data not shown).
Results of this study suggest that genes with repetitive sequences such as the PTP and SWP-1 can be good targets for genotype analysis. Sequence comparison of the PTP gene divided E. cuniculi into three genotypes in congruence with ITS analysis. The advantage with PTP gene is the length polymorphism, which allows the development of simple PCR-based genotyping tools. Genotype III has a deletion of one copy of the 78-bp central repeats, enabling it to be differentiated from genotypes I and II by electrophoresis of PCR products. In addition, genotypes I and II can be differentiated from each other by RFLP analysis, which provides a mechanism for confirmation and circumvents the need for DNA sequencing. Similar results were observed at the SWP-1 gene. Length polymorphism originated from variations of repeat numbers also existed among the three E. cuniculi genotypes. This length variation was further seen within some genotypes and between different copies of the SWP-1 gene, which divided the genotypes I and III into several subgenotypes.
PCR and sequence analysis of the PTP gene in E. hellem also support the use of repetitive sequences as genotyping targets. Four genotypes of E. hellem were found in the 24 isolates analyzed at this genetic locus. This typing resolution is much higher than the one produced by sequence analysis of the ITS, which yielded 2 genotypes. In fact, the typing resolution at the ITS locus was even lower than the sequence analysis of SSU rRNA, which divided the 24 isolates into 3 genotypes. Like in E. cuniculi, the PTP gene had an additional advantage of having length polymorphism, which enabled the differentiation of the E. hellem genotypes by electrophoresis of PCR products without restriction digestion or sequence analysis. No genetic heterogeneity was detected in E. intestinalis in this study. The SSU rRNA, ITS, and PTP-1 genes analyzed, however, had no repetitive sequences in E. intestinalis.
In summary, results of this study indicate the existence of extensive genetic diversity in E. cuniculi and E. hellem isolates from humans. This genetic diversity was previously underestimated by the analysis of ITS sequence, but now can be assessed easily by the analysis of the repetitive region of the SWP-1 or PTP gene. Genetic targets with repetitive sequences are needed for genotype analysis of E. intestinalis. With the development of simple PCR based genotyping tools, more extensive epidemiologic studies and characterizations of large number of isolates from humans and animals will enable the evaluation of the significance of the genetic diversity, the role of animals in human infection, and the transmission dynamics of microsporidiosis.
ACKNOWLEDGMENTS
We thank C. del Aguila, J. Yee, E. Pozzio, A. Tosoni, M. Scaglia, L. Felchle F. Bornay-Llinares, and R. Weber for providing some of the microsporidia isolate, and Mary E. Bartlett and Daniel G. Colley for suggestions on the improvement of the manuscript.




