The Identification of rRNA Maturation Sites in the Microsporidian Encephalitozoon cuniculi Argues Against the Full Excision of Presumed ITS1 Sequence
Abstract
ABSTRACT. In Encephalitozoon cuniculi like in other microsporidia, the primary transcript for SSU and LSU rRNAs includes only one internal transcribed spacer (ITS1) which separates SSU rRNA from the 5.8S region associated with LSU rRNA. The extraction of total RNA from E. cuniculi-infected MRC5 cells using a hot phenol/chloroform procedure enabled us to perform primer extension and S1 nuclease protection experiments in the aim of identifying rRNA maturation sites. Our data support a simple processing (four cleavage sites) with elimination of only nine nucleotides between SSU and LSU rRNA regions. Most of the presumed ITS1 sequence characterized by strain-dependent polymorphism therefore remains linked to SSU rRNA 3′ end. A new secondary structure for the sixth domain of E. cuniculi LSU rRNA is proposed following the identification of its 3′terminus.
In most eukaryotes, the ribosomal DNA (rDNA) unit transcribed by RNA polymerase I comprises genes encoding small subunit (SSU), 5.8S, and large subunit (LSU) rRNAs, separated by two internal transcribed spacers (ITS1 and 2) and flanked by two external transcribed spacers (5′ and 3′ ETSs). Processing of the primary transcript into mature rRNAs therefore involves the removal of these spacers. In microsporidia, there is no evidence for free 5.8S rRNA and a prokaryote-like fusion of 5.8S and LSU rRNA sequences occurred, as first shown in Vairimorpha necatrix [15]. Thus, the microsporidian SSU and LSU rRNA genes appear to be separated by only ITS1. The sizes of the two corresponding mature rRNAs were estimated through gel electrophoresis [2], alignments of rDNA sequences and prediction of rRNA secondary structures [3,7,11,14]. Taken together, the data indicate that the two major microsporidian rRNAs are highly reduced in length, relative to other eukaryotes and even most prokaryotes, but the so-called 16S and 23S rRNAs harbor typical eukaryotic domains. However, the precise sites of maturation of these rRNAs are not known. Here, we report a first study of microsporidian rRNA processing through primer extension and S1 protection experiments in the mammal-infecting species Encephalitozoon cuniculi.
MATERIALS AND METHODS
The mouse isolate GB-M1 [1] of E. cuniculi strain I was routinely cultivated on MDCK (Madin-Darby Canine Kidney) cells. Spores collected from culture supernatants were then used for infecting confluent MRC5 cells (24 flasks each containing 15 ml of culture inoculated with ∼106 spores). One-day infected cells were harvested, washed twice in PBS then gently homogenized with a Potter apparatus (10-15 strokes). After removal of large debris (200 g, 5 min), parasites and remaining host cells were pelleted at 2,000 g for 10 min then suspended in 5 ml of lysis buffer (180 mM Tris, 90 mM LiCl, 4.5 mM EDTA, 1% SDS, pH 8.2). Total RNA was extracted following addition of one volume of hot phenol/chloroform solution and incubation at 55°C for 30 min. After four successive phenol extraction steps and ethanol precipitation, RNA was suspended in 200 1 of sterile water and stocked at -80°C. Contaminating DNA was removed by treatment of 10- 1 samples for 10 min with 10 U of DNase (Promega) and 40 U of RNasin (Promega) in 0.1 M NaCl. For negative controls, the same procedure was applied to uninfected MRC5 cells.
After electrophoresis in 1.2% formaldehyde-agarose gel, RNAs were blotted onto a nylon membrane (Hybond+, Amersham) according to the manufacturer's instruction. 32P-labeled riboprobes were produced as described [12]. Hybridizations were performed in 50% formamide, 0.75 M NaCl, 1% SDS, 5% dextran sulfate. 50 g. ml−1 heparin and 50 g. ml−1 salmon sperm DNA for 12 h at 42°C. Before autoradiography, blots were washed twice in 2X SSPE and 1% SDS for 15 min at room temperature then twice with the same solution at 42°C.
All nucleotide (nt) positions given for extension primer and S1 protection analyses are based on AJ005581 (EMBL) reference sequence. Oligonucleotides 5′ GCTATCACTGAGCCGTCCGCT AATCCCCCAC 3′ and 5′ CTCTCCGCATCCGATTTCAATCG CACTCGCCGCTGCTGC 3′, complementary to nt between 2492 to 2960 and 3319 to 3354, were used for primer extension studies of SSU rRNA and LSU rRNA, respectively. Primers were 32P end-labeled using T4 polynuclotide kinase and [γ-32P]-ATP. Purified labeled oligonucleotides were incubated with total RNA in 80% formamide, 40 mM PIPES (pH 7.4), 400 mM NaCl and 1 mM EDTA (pH 8.0) for 10 min at 95°C then 12 h at 30°C. The extension reaction was done with ∼2 g of total RNA, in the presence of AMV reverse transcriptase (40 U), RNasin (40 U) and the four different dNTPs (0.5 M). Samples were incubated for 1 h at 42°C. Primer extension fragments were analyzed in 6% polyacrylamide-7 M urea gel, in parallel with known sequences as size markers.
For S1 mapping, two amplified fragments (2942–3471) and (5427–5444) were cloned in PGEM-T Easy Vector System I (Promega). After PstI digestion, DNA vectors were incubated for 1 h at 37°C with 5 U of T7 RNA polymerase, 10 mM DTT, all four rNTPs (0.5 M), 40 U of RNasin and 10 Ci of [α-32P]-UTP. Riboprobes were allowed to hybridize with total RNA in the same conditions as for primer extension. 300 U of S1 nuclease were added to each reaction mixture. Samples were incubated for 1 h at 37°C and reactions were stopped by 2 M ammonium acetate, 10 mM EDTA and 5 g ml−1 glycogen. After precipitation, S1-resistant fragments were analyzed as mentioned above.
RESULTS AND DISCUSSION
Transcriptionally active intracellular stages of microsporidia are still difficult to isolate, hindering studies on gene expression. In addition, sporogony involves the formation of a highly resistant cell wall and no experimental protocol ensuring specific degradation of this wall currently exists. Thus, when working with the whole cell host - parasite system and applying most RNA extraction procedures adapted to animal cells, the yield of the extraction of microsporidian rRNA precursor and intermediate forms is strongly limited. In this study, we applied to E. cuniculi-infected MRC5 cells a hot phenol/chloroform method which is generally efficient for extracting RNA from plant cells. A relatively complex pattern was observed after agarose gel electrophoresis under denaturing conditions (Fig. 1a). The most intense ethidium bromide-stained RNA bands can be seen at the positions expected for host cell rRNAs. However, the presence of bands representative of parasite rRNAs was demonstrated through high stringency hybridization with either E. cuniculi SSU rRNA (Fig. 1b) and E. cuniculi LSU rRNA (Fig. 1c) riboprobes. The corresponding sizes are estimated to be ∼1.3 kb and ∼2.6 kb, close to those deduced from rDNA sequence analyses: 1299 nt [13] and 2487 nt [11]. The large size difference between host cell and E. cuniculi LSU rRNAs is evident.
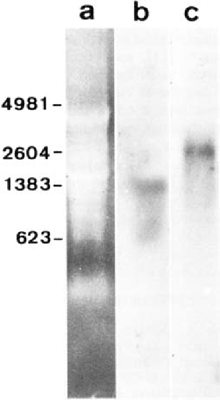
Ethidium bromide-stained electrophoretic pattern of RNAs from MRC5 cells infected with E. cuniculi (lane a) and Northern blots obtained with E. cuniculi-specific SSU rRNA (Une b) and LSU rRNA (lane c) probes. The sizes of four RNA markers are indicated in nucleotides.
Primer extension and S1 mapping experiments were carried out to try to define the exact position of E. cuniculi rRNA extremities. Given that extracted RNAs were of dual origin, the end-labeled primers were chosen to be specific of E. cuniculi sequences. As expected, control experiments using RNA from non-infected MRCS cells did not generate any cDNA extension product. For SSU rRNA, two extension products differing in size by only one nucleotide were obtained (Fig. 2a). As demonstrated through a study of SSU rRNA maturation in Entamoeba histolytica leading to a similar result [9], the lower band may result from a migration artifact. The exact maturation site is therefore deduced from the upper band. The corresponding fragment of 100 bp indicates the occurrence of a cleavage just before an A residue at the position 1750. No additional signal suggestive of 5′ ETS maturation steps was observed, even after longer exposure times. Further experiments using larger RNA amounts and/or primers designed to different parts of the 5′ SSU rRNA region also failed to provide evidence for successive steps. This suggests either a very rapid or a very simplified maturation. Specific secondary structures are known to be directly or indirectly required for the first cleavage step (A0) of 5′ ETS in mouse [10] or budding yeast [6]. The 5′ flanking region of E. cuniculi SSU rRNA gene does not contain such structures. Thus, it seems reasonable to conclude that no intermediary step of 5′ ETS processing exists in E. cuniculi.
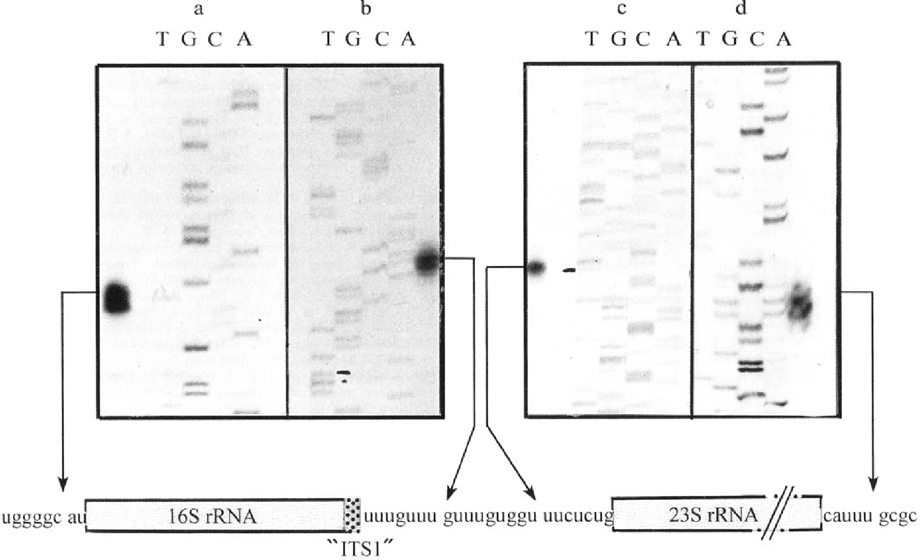
Results of primer extension (a, c) and S1 nuclease protection (b, d) analyses for rRNA processing in E. cuniculi. DNA fragments were separated on a standard sequencing gel. Dideoxy sequencing reaction products from unrelated sequences were used as size markers. Below autoradiographs, are represented the positions of the four deduced cleavage sites (arrows) producing mature SSU (16S) and LSU (23S) rRNAs, and the association of 16S rRNA with a major part of previously considered ITS1. The sequence centered on the short eliminated region is also shown.
As regards to the determination of SSU rRNA-associated 3′ termini, S1 nuclease mapping showed two protected fragments which also differ by a single nucleotide (Fig. 2b). Considering the slow-migrating fragment, we deduce that the cleavage site is located within the presumed ITS1 sequence, just between two successive GUUU repeats.
Surprisingly, no fragment resulting from a cleavage closer to the 3'SSU rRNA extremity inferred from multiple rDNA sequence alignments was detected. The unique extension product (269 bp) provided by the analysis of 5′ termini associated with LSU rRNA reveals a cleavage occurring nine nucleotides downstream of the 3′ SSU rRNA cleavage site (Fig. 2c). Thus, the ITS1 maturation would be restricted to the elimination of the nonanucleotide GUUUGUGGU. Correlatively, the largest part of the presumed ITS1 sequence represents in fact the extreme 3′region of SSU rRNA. This sequence has been considered as a useful strain marker because of intra-species variations in the number of the repeated tetranucleotide GUUU motif [4]. The isolate here used belongs to strain I which is characterized by three repeats. Taking into consideration the fusion of SSU rRNA with most of “ITS1” sequence, the true length of SSU rRNA in E. cuniculi strain I should be 1326 nt. The strain-dependent fluctuations between two and four GUUU repeats can be thought to have no significant incidence on SSU rRNA functions. The predominant nucleotide (U) in such a sequence has been suggested to play an important role in nuclease recognition [8].
In a previous study, the 3′ end of E. cuniculi LSU rRNA was predicted on the basis of some secondary structure elements and of the relative position of repeated motifs within the 3′flanking region [11].
S1 mapping of this extremity is quite suggestive of a simple maturation (Fig. 2d) and indicates a more internal cleavage site. Thus, in contrast to SSU rRNA, the length of mature LSU rRNA was overestimated. The new estimate is 2449 nt. This implies that the sixth domain of LSU rRNA secondary structure must be redrawn, as shown in Fig. 3. As for the other five domains [11], most conserved structural features of domain VI are present in E. cuniculi. The lack of other protected fragments suggests that the E. cuniculi 3′ ETS is either very short or absent. In some protozoan species, this spacer has been shown to include only a few nucleotides, e.g. 14 nt in the ciliate Tetrahymena [5].
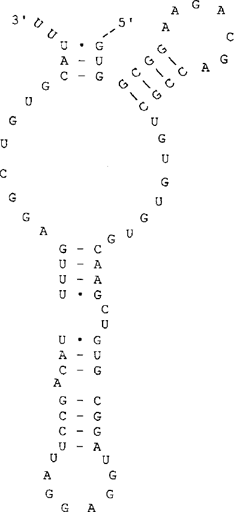
Predicted secondary structure of the domain VI characteristic of the 3′ region of E. cuniculi LSU rRNA.
Clearly, the present data support a rather extreme simplification of rRNA maturation processes in E. cuniculi (four cleavage sites). Like ITS2 [15], 5′ and 3′ ETSs are apparently lacking. The cleavages occurring between the two rRNA coding regions would lead to the removal of only 9 nucleotides. Further studies are obviously required to determine the relationship with specific features of the E. cuniculi rRNA processing machinery and to test the occurrence of possible variations in this processing among other microsporidian species.




