Inter-Strain Variability of Insertion/Deletion Events in the Encephalitozoon cuniculi Genome: A Comparative KARD-PFGE Analysis
Abstract
ABSTRACT. We applied a two-dimensional pulsed-field gel electrophoresis procedure to the genomes of two karyotype variants assigned to two different strains of the microsporidian Encephalitozoon cuniculi, termed D (strain III) and F (strain II). Data obtained for BssHII and Mlul restriction fragment length polymorphisms in each chromosome are compiled and compared to the reference strain I variant A. Six Insertion/Deletion (InDels) are found in subterminal position, some of these being characteristic of either D or F. Like in strain I, the terminal fragments extending between each telomere and rDNA locus are conserved in length for each chromosome. They are however smaller than in reference variant. This size reduction is estimated to be 2.5 kbp for the strain III isolate and 3.5 kbp for the strain II isolate. We hypothesize that for the three E. cuniculi strains, all chromosome extremities are prone to a constant process of sequence homogenization through mitolic recombination between conserved regions.
Although most microsporidian species can be considered as parasites with a highly restricted host specificity, those belonging to the genus Encephalitozoon, especially E. cuniculi, are capable of infecting a wide range of mammalian host species [16]. Three different E. cuniculi strains (I, II and III) have been clearly differentiated on the basis of molecular criteria such as sequence variations of the unique rDNA internal transcribed spacer TTS1 [13] and of the polar tube protein PTP1 [22]. Each strain seems to be preferentially, but not exclusively, associated with an animal host (strain I: rabbit, strain II: fox, strain III: dog), and both immunological and molecular analyses of various E. cuniculi isolates lent credit to the hypothesis that human infections with E. cuniculi are a zoonosis [5,12,14,19,21].
It should be recalled that E. cuniculi has a highly reduced genome (∼2.9 Mb) divided into eleven chromosomes [6]. Chromosome size polymorphism has been demonstrated among isolates assigned to different strains, leading to the discrimination of six karyotype variants (A to F) [5]. The development of a new protocol of genome fingerprinting based on two-dimensional pulsed-field gel electrophoresis (2-D PFGE), we termed “Karyotype And Restriction Display 2-D PFGE (KARD-PFGE)” [8,9], enabled us to show that this polymorphism can result of subtelomeric insertion-deletion events (InDels) in two karyotype variants (A and C) of strain I [10]. Isolates belonging to strains II and III were previously found to have significantly reduced chromosome size ranges, compared to strain I isolates [5]. This prompted us to extend the application of KARDPFGE to two other E. cuniculi isolates of different origins and respectively assigned to strains II and III. To facilitate a comparison with the reference isolate (karyotype A, strain I), the same restriction enzymes (Mlul and BssHH) were used.
MATERIALS AND METHODS
Cell culture
The E. cuniculi isolate USA-D1 (strain n) was from a domestic dog in USA and kindly provided by Dr E. Didier. The isolate N-F220 (strain in) was from a fanned blue fox in Norway and kindly provided by Drs P. Deplazes and R. Weber. The isolate GB-M1 from a mouse (strain I), routinely used for various studies in our laboratory, served as a reference. Parasites were grown at 37°C on MDCK (Madin Darby Canine Kidney) cells [3].
KARD-PFGE
Purification of E. cuniculi spores and preparation of agarose blocks for PFGE analyses were done as described in [9]. Contour-clamped homogeneous electric field PFGE in a Gene Navigator(tm) (Pharmacia) system was performed at 12°C, using 1.3% chromosomal grade agarose (SeaKEM(tm) GTG, FMC Bioproducts) in 0.5 × TBE (45 mM Tris base, 45 mM boric acid, 1 mM EDTA) and 16.5-s pulses for 38 h at 200 V. KARD-PFGE separations and DNA labeling were performed as previously described [9]. For visualising radioactive DNA, gels were vacuum-dried for 1.5 h with a gel slab dryer (Model 224, BioRad), and placed against a Biomax(tm) MS film (Kodak) between two intensifying screens at - 80°C. Data for restriction fragment lengths have been generated semi-automatically with the Melanie n software (ISB, Geneva). Autoradiographs were scanned on a GS700 imaging densitometer (BioRad).
Hybridization
The 23S rDNA probe was designed downstream of the Mlul restriction site associated with 23S rDNA gene, as previously described [8] and was synthesised using a PCR procedure with Universal and Reverse primers (GTTTTCCCAGTCACGAC and AACAGCTATGACCATG, respectively) and a pUC 18-based vector. The corresponding insert was amplified then labeled with 10 Ci [α32P]dCTP, 25 M d[A,G,T]TP and 2 mM MgCl2 after 30 cycles of amplification comprising 30 s at 94°C, 30 s at 53°C and 2 min at 72°C. The synthesis of the 16S rDNA probe was done with primers described in [11]. Radiolabeled probes were hybridised to PFGE-separated restriction fragments directly in dried gels. All hybridizations were done at 65°C for 18 h, in 5X SSPE, 1% SDS and 5X Denhardt solution. Membranes or gels were washed three times in 0.1X SSPE, 0.1% SDS, at 65°C for 30 min each, before exposition to Biomax™ MS film (Kodak).
PCR amplification
To amplify DNA from subtelomeric regions, an oligonucleotide was designed on the basis of the consensus sequence of E. cuniculi chromosome I [23]. The corresponding sequence was: 5′CCGCGAAAGCTTCTGTCGACGAAAG 3′. The other primer was derived from the 23S rDNA gene, downstream of the Mlul restriction site: 5′AGTACGAGAGGAACTCTTGATTGCG 3′. Long-range PCR experiments were performed using a Long Expand PCR kit (Roche Molecular Biochemicals). The expected size for the PCR product of reference isolate was 5 kbp.
RESULTS
Restriction profiles of chromosomal DNAs from two E. cuniculi isolates representative of strains II and III were obtained by KARDPFGE, a procedure which involved successively: (i) PFGE-separation of chromosomal bands (molecular karyotype), (ii) cutting of an agarose band which includes the full karyotype then in-gel digestion of DNA with a restriction enzyme, (iii) in-gel 32P labeling of digested DNA using Klenow fragment, (iv) deposition of the agarose band at the top of a second PFGE gel, electrophoresis of radiolabeled restriction fragments in a direction orthogonal to that of molecular karyotype, and (v) visualization of separated fragments by autoradiography. Each autoradiograph therefore reveals specific restriction patterns of all the chromosomes in the order imposed by the first electrophoretic step. The designations for the karyotype forms of the strain III isolate (dog) and strain II isolate (blue fox) were D and F, respectively [5]. Tables 1 and 2 show the results of spot analysis after either Mlul or BssHII digestion, for these two variants and the reference strain I variant A. Figure 1 summarizes schematically our inferences about the positions of polymorphic elements in the eleven chromosomes of the E. cuniculi genome The three different “genomic fingerprints” are illustrated by the upper photographs of Fig. 2.
| E. cuniculi chromosomes MluI restriction pattern | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||||||||||
| Karyotype | A | D | F | A | D | F | A | D | F | A | D | F | A | D | F |
| 12 | 9,5 | 8,5 | 3 | 3 | 3 | 5 | 5 | 5 | 12 | 9,5 | 8,5 | 8 | 8 | 8 | |
| 12 | 9,5 | 8,5 | 3 | 9,5 | 8,5 | 12 | 9,5 | 8,5 | 12 | 9,5 | 8,5 | 12 | 9,5 | 8,5 | |
| 53 | 53 | 53 | 12 | 9,5 | 8,5 | 12 | 9,5 | 8,5 | 26 | 26 | 26 | 12 | 9,5 | 8,5 | |
| 133 | 133 | 133 | 12 | 21 | 21 | 18 | 18 | 18 | 38 | 38 | 38 | 17 | 17 | 17 | |
| 21 | 27 | 27 | 23 | 30 | 30 | 157 | 65 | 58 | 17 | 17 | 17 | ||||
| 27 | 27 | 27 | 30 | 44 | 44 | 93 | 93 | 42 | 42 | 42 | |||||
| 27 | 41 | 41 | 40 | 49 | 49 | 44 | 44 | 44 | |||||||
| 41 | 87 | 87 | 44 | 69 | 64 | 96 | 89 | 89 | |||||||
| 82 | 46/49 | ||||||||||||||
| TOTAL | 210 | 205 | 203 | 230 | 225 | 223 | 230/233 | 234 | 227 | 245 | 241 | 232 | 248 | 236 | 234 |
| E. cuniculi chromosomes MluI restriction pattern | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 | 7 | 8 | 9 | 10 | 11 | |||||||||||||
| Karyotype | A | D | F | A | D | F | A | D | F | A | D | F | A | D | F | A | D | F |
| 12 | 9,5 | 8,5 | 12 | 9,5 | 8,5 | 8 | 8 | 8 | 12 | 9,5 | 8,5 | 7 | 7 | 7 | 4 | 4 | 4 | |
| 12 | 9,5 | 8,5 | 12 | 9,5 | 8,5 | 12 | 9,5 | 8,5 | 12 | 9,5 | 8,5 | 9 | 9 | 8,5 | 12 | 9,5 | 8,5 | |
| 47 | 47 | 47 | 21 | 21 | 21 | 12 | 9,5 | 8,5 | 15 | 15 | 15 | 12 | 9,5 | 8,5 | 12 | 9,5 | 8,5 | |
| 52 | 52 | 52 | 28 | 28 | 28 | 20 | 20 | 20 | 21 | 21 | 21 | 12 | 9,5 | 9 | 19 | 19 | 19 | |
| 59 | 52 | 58 | 53 | 53 | 53 | 21 | 21 | 21 | 28 | 28 | 28 | 21 | 21 | 21 | 20 | 20 | 20 | |
| 72 | 68 | 63 | 134 | 134 | 134 | 53 | 53 | 53 | 83 | 83 | 83 | 23 | 26 | 26 | 21 | 21 | 21 | |
| 53 | 53 | 53 | 109 | 109 | 107/109 | 23 | 48 | 48 | 27 | 27 | 27 | |||||||
| 87 | 87 | 89 | 80 | 80 | 80 | 32 | 32 | 32 | ||||||||||
| 105 | 84 | 84 | 54 | 54 | 54 | |||||||||||||
| 103 | 100 | 100 | ||||||||||||||||
| TOTAL | 254 | 238 | 237 | 260 | 255 | 253 | 266 | 261 | 261 | 280 | 275 | 271/273 | 292 | 294 | 292 | 304 | 296 | 294 |
| E. cuniculi chromosomes BssHII restriction pattern | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||||||||||
| Karyotype | A | D | F | A | D | F | A | D | F | A | D | F | A | D | F |
| 9,5 | 7 | 6 | 9,5 | 7 | 6 | 9,5 | 7 | 6 | 9,5 | 7 | 6 | 4 | 4 | 4 | |
| 9,5 | 7 | 6 | 9,5 | 7 | 6 | 9,5 | 7 | 6 | 9,5 | 7 | 6 | 9,5 | 7 | 6 | |
| 19 | 19 | 19 | 25 | 25 | 25 | 104/107 | 107 | 107 | 18 | 19 | 18 | 9,5 | 7 | 6 | |
| 20 | 30 | 30 | 38 | 38 | 38 | 107 | 113 | 109 | 50 | 50 | 44 | 20 | 20 | 20 | |
| 30 | 30 | 30 | 45 | 45 | 45 | 50 | 50 | 50 | 27 | 29 | 29 | ||||
| 30 | 30 | 30 | 50 | 50 | 50 | 54 | 54 | 54 | 27 | 29 | 29 | ||||
| 30 | 30 | 30 | 53 | 53 | 53 | 54 | 54 | 54 | 29 | 47 | 47 | ||||
| 30 | 52 | 52 | 29 | 93 | 93 | ||||||||||
| 32 | 93 | ||||||||||||||
| TOTAL | 210 | 205 | 203 | 230 | 225 | 223 | 230/233 | 234 | 228 | 245 | 241 | 232 | 248 | 236 | 234 |
| E. cuniculi chromosomes BssHII restriction pattern | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 | 7 | 8 | 9 | 10 | 11 | |||||||||||||
| Karyotype | A | D | F | A | D | F | A | D | F | A | D | F | A | D | F | A | D | F |
| 8 | 8 | 8 | 9,5 | 7 | 6 | 4 | 4 | 4 | 9,5 | 7 | 6 | 9,5 | 7 | 6 | 8 | 5 | 6 | |
| 9,5 | 7 | 6 | 9,5 | 7 | 6 | 7 | 7 | 6 | 9,5 | 7 | 6 | 9,5 | 7 | 6 | 9,5 | 7 | 6 | |
| 9,5 | 7 | 6 | 11 | 11 | 11 | 9,5 | 7 | 6 | 126 | 89 | 80 | 52 | 52 | 52 | 9,5 | 7 | 6 | |
| 25 | 25 | 25 | 11 | 11 | 11 | 9,5 | 7 | 7 | 135 | 37 | 44/46 | 221 | 228 | 228 | 18 | 18 | 18 | |
| 79 | 79 | 79 | 28 | 28 | 28 | 13 | 13 | 13 | 135 | 135 | 22 | 22 | 22 | |||||
| 123 | 112 | 112 | 44 | 44 | 44 | 29 | 29 | 29 | 22 | 22 | 22 | |||||||
| 55 | 55 | 55 | 29 | 29 | 29 | 25 | 25 | 25 | ||||||||||
| 92 | 92 | 92 | 38 | 38 | 38 | 35 | 35 | 35 | ||||||||||
| 58 | 58 | 60 | 37 | 37 | 37 | |||||||||||||
| 69 | 69 | 69 | 39 | 39 | 39 | |||||||||||||
| 39 | 39 | 39 | ||||||||||||||||
| 40 | 40 | 40 | ||||||||||||||||
| TOTAL | 254 | 238 | 236 | 260 | 255 | 253 | 266 | 261 | 261 | 280 | 275 | 271/273 | 292 | 294 | 291 | 304 | 296 | 295 |
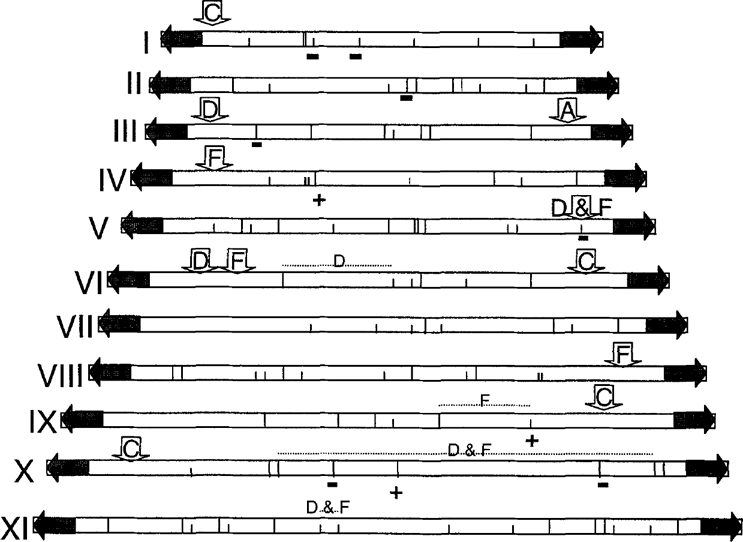
Representation of the different elements of polymorphism deduced from the comparison of KARD patterns of different E. cuniculi isolates, positioned on the reference Mlul and firaHII restriction maps. Mlul and BssHII restriction sites are represented by large and small vertical lines within chromosome schemes. +/− indicate additional site/lacking site, relative to variant A. Inlrachromosomal rearrangements are marked by horizontal dashed lines. Subterminal InDels are shown by vertical arrows with letters (A, C, D or F). Previous results for A and C [10] have been added. The region between rDNA and telomere is represented by a horizontal gray arrow on each extremity.
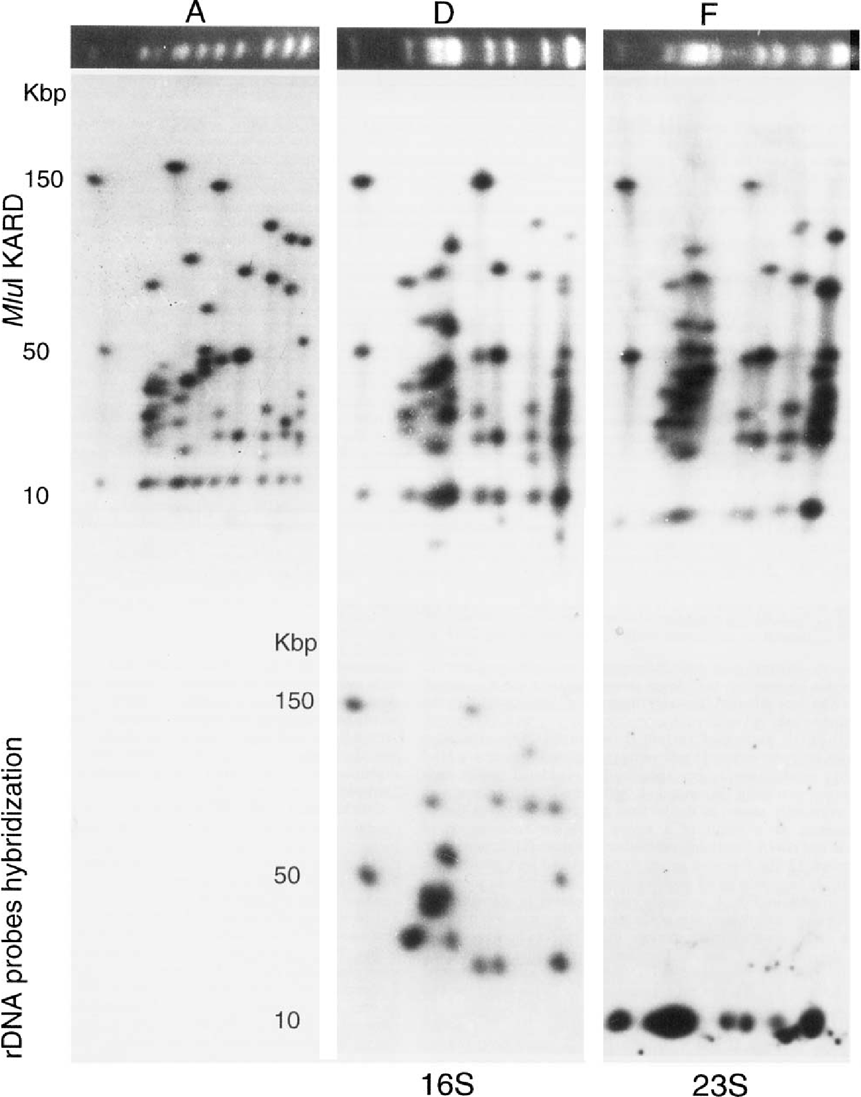
Upper photographs: karyotypes and MluI KARD spot patterns of E. cuniculi variants A, D and F. Corresponding analysis of these images is presented in Table 1. Lower photographs: hybridizations with rDNA-specific (16S and 23S regions) probes. The 16S rDNA probe revealed fragments carrying the subterminal regions for variant D. The other hybridization illustrates the terminal position of the 23S region in variant F.
KARD-PFGE pattern of variant D (strain III)
One significant difference between variant D and variant A patterns concerns a Mlul fragment which should be specifically associated with each chromosome extremity. In variant A, a 12-kbp Mlul fragment was indeed previously shown to derive from the two ends of all the 11 chromosomes, as a result of a strong sequence conservation of telomeric and rDNA-containing subtelomeric regions [8]. In variant D, the terminal 12-kbp fragment seems to be replaced by a smaller one (9.5 kbp), as suggested by its double intensity. Moreover, as shown by vertical arrows in Fig. 1, a length polymorphism of the fragment extending immediately upstream of the terminal fragment was detected in three chromosomes (length increase of 6 kbp for chromosome III; length decrease of 7 and 4 kbp for chromosomes V and VI, respectively). Three more internal InDels were also found (horizontal dashed lines in Fig. 1), one on chromosome VI (7 kbp) and the two others on the two largest chromosomes (X: 7kbp and XI: 3 kbp).
KARD-PFGE pattern of variant F (strain II)
The strong resemblance between D and F patterns (Fig. 2, upper part) reflects similarities in the presence/absence of some Mlul (see Table 1, e.g. chromosomes n and X) or BssHII (see Table 2, e.g. chromosomes I and V) restriction sites, compared to variant A. Likewise, the terminal fragment shared by all chromosome ends is smaller than in variant A. The length of this fragment is however more reduced (8.5 kbp) than in variant D. Of the three previously mentioned subterminal InDels, that in chromosome V is in fact common to D and F and that in chromosome VI is more extensive in variant F. In contrast, the subterminal InDel in chromosome III remains specific of variant D. Two other subterminal InDels are associated with chromosomes IV (7 kbp) and VTff (2 kbp) in variant F. An additional characteristic of this variant is represented by two chromosome IX homologues whose the size difference can be explained by a unique InDel (2 kbp) located in the chromosome core (dashed horizontal line in Fig. 1).
Hybridization with rDNA probes
In a previous mapping of chromosomes from strain I reference isolate, the common terminal Mlul fragment, 12 kbp in length, was demonstrated to hybridize with 23S rDNA probe, supporting a centrifuge arrangement of each rDNA unit located at the neighboring of each telomeric region [8]. To test the hypothesis that the somewhat reduced terminal fragments in strain II and III isolates still retain rDNA sequences, KARD-PFGE gels were also used for hybridizations experiments with 16S and 23S rDNA probes (Fig. 2, lower part). The data obtained with 23S rDNA probe show clearly that the 9.5-kbp (variant D) and 8.5-kbp (variant F) Mlul fragments (and corresponding smaller BssHII fragments; data not shown) are specific of conserved chromosome termini. All the subterminal fragments found to differ in size, in comparison with variant A, hybridized with 16S rDNA probe. The divergent orientation of the two subtelomeric rDNA units in each chromosome is therefore also conserved.
Analysis of a PCR-amplified subtelomeric region
We succeeded in PCR amplification of a subtelomeric DNA sequence extending downstream of the 23S rRNA gene and providing a DNA fragment with the expected size of 5 kbp in the reference variant. The same protocol was applied to chromosomal DNAs from variants D and F. The PCR product derived from variant D was about 500 bp smaller than the previous one. The size difference was confirmed through RFLP analysis with NsiI and XhoI restriction enzymes (data not shown). No amplificatian product was however obtained with DNA from variant F. Thus, the possibility remains that the size reduction of terminal regions in the two variants is not dependent on a unique InDel in a same place.
DISCUSSION
This comparative KARD-PFGE study provides more information about the multiplicity of sites of rearrangements in the small genome of the mammal-infecting microsporidian species E. cuniculi prone to a significant karyotype variability [5]. In the yeast Saccharomyces cerevisiae [4,15] or the apicomplexan parasite Plasmodiumfalciparum [20], the number of chromosomes is preserved among isolates but different karyotype forms also exist. Chromosome rearrangements in these organisms may be sometimes related to InDel events which can occur in subtelomeric regions [2,17,18]. This can be also the case in E. cuniculi, as first suggested by a KARD-PFGE comparison of two variants (A and C) from the same strain I [10]. Following the examination of the restriction patterns derived from variants D and F, it can be deduced that the total number of MluI and BssHII restriction sites is rather well conserved in spite of variations in restriction fragment size. Only, the disappearance of one BssHII site in chromosome V may be explained by an InDel event. It should be stressed that DNA rearrangements may lead sometimes to changes in the size order of the E. cuniculi chromosomes. In the variant D, not in variant F, the chromosome IV was larger than chromosomes V and VI. In fact, a careful look at the polymorphic fragments reveals that this is a consequence of deletions within the chromosomes V and VI, justifying a drastic size reduction of these chromosomes. That the size of chromosome IV is smaller than in variant A is only due to the reduction of its terminal fragment. The usual chromosome size order is restored in variant F because of the presence of a new InDel in its chromosome IV. This somewhat complex interpretation is well indicative of the power of analysis offered by the KARD-PFGE method.
Evidence for six new subterminal InDels have been brought in this study. Taking into account those previously described in two strain I isolates, the total number of different InDels attains 11 for a sample of only four E. cuniculi isolates. The mean frequency of nearly 3 new Indels per isolate strongly supports that the species E. cuniculi has been regularly subjected to chromosomal rearrangements. In P. falciparum, subtelomeric InDels have been shown to involve minisatellites or genes encoding surface antigens [1,7,17]. The relationship of InDels with specific DNA sequences and the possible incidence of InDels on phenotypic features of E. cuniculi await further investigations.
Of peculiar interest is our finding of a new type of InDel which affected all the 22 chromosome extremities of the E. cuniculi genome. This represents a major justification of the trend to a general decrease of chromosome sizes observed among strain II and III isolates [5]. Compared to that in variant A, each terminal region would have been subjected to a deletion of 2.5 kp in variant D and of 3.5 kbp in variant F. For the entire genome, this corresponds to a loss of either 55 or 77 kbp. Like in some other eukaryotic parasites, the terminal regions may include hot spots of recombination. How and why rDNA units remain associated with these regions, whatever the strain considered, are still unresolved questions. Possibly, the specific positioning of rDNA units may result from frequent mitotic interchromosomal exchanges and may contribute to the maintenance of the integrity of gene-rich chromosome cores. The InDels which have been assigned to the core regions are indeed rare and they clearly differentiate variants D and F from those of strain I (A and C). It is tempting to consider that the size of the terminal restriction fragment may represent an additional strain marker, given that three different sizes correlate with the number of strains. Further genome fingerprinting studies of other isolates from strains II and III are however needed to determine whether such variations are truly characteristic of each E. cuniculi strain. If it was the case, an insight into the evolutionary history of the small genomes of Encephalitozoon species might emerge.
ACKNOWLEDGMENTS
We thank Dr M. H6braud (URINRA 370, Theix, France) for assistance in attempts at computational data acquisition with their proteomic plateform.




