Treatment of severe von Willebrand disease with a high-purity von Willebrand factor concentrate (Wilfactin®): a prospective study of 50 patients
Abstract
Summary. Background and objectives: A plasma-derived von Willebrand factor (VWF) concentrate with low factor VIII (FVIII) content was specifically developed to treat von Willebrand disease (VWD). Efficacy and safety were investigated by merging the results of two comparable protocols conducted prospectively in 5 European and 12 French centers. Methods and results: Fifty patients with clinically severe VWD (72% had VWF ristocetin cofactor activity less than 10 IU dL–1 and 46% had FVIII < 20 IU dL–1) were treated with the concentrate as the only therapy, except for clinical situations requiring a priming dose of FVIII to rapidly correct an intrinsic coagulation defect. A total of 139 spontaneous bleeding episodes were treated; only 53 (38%) needed a concomitant FVIII dose. Outcome was excellent or good in 89% of the episodes. Forty-four patients underwent 108 surgical or invasive procedures. Outcome was excellent or good in 95 scheduled procedures (only VWF was infused) and 13 emergency procedures (a priming FVIII dose was co-administered with the first VWF infusion). There were no thrombotic complications and none of the 18 patients with type 3 VWD developed anti-VWF or anti-FVIII antibodies. Conclusions. This concentrate safely and effectively provides hemostasis in patients with clinically severe VWD.
Introduction
Von Willebrand disease (VWD) is an autosomally inherited bleeding disorder caused by a quantitative or qualitative deficiency of von Willebrand factor (VWF).[1] This multimeric glycoprotein initiates platelet adhesion and aggregation at sites of vascular injury and is the carrier of coagulation factor VIII (FVIII), protecting it from proteolysis. When patients with VWD present with quantitative VWF deficiencies, plasma FVIII levels are also low because this protection is lacking. VWD is heterogeneous and is classified into three different types.[2] Approximately 70% of patients have type 1, characterized by a mild or moderate quantitative deficiency of VWF and usually associated with a mild bleeding history. Twenty to twenty-five percent of patients have type 2, characterized by a qualitative VWF deficiency. In type 2 forms, bleeding episodes in the mucosal tract are frequent but soft tissue hemorrhages are rare because patients have relatively high levels of FVIII. Less than 5% of patients have type 3, characterized by a severe quantitative defect and very low levels of both VWF and FVIII. Treatment of VWD requires dual correction of VWF (especially in cases of mucosal bleeding) and FVIII (especially in cases of soft tissue and postoperative bleeding). Desmopressin (1-desamino-8-d-arginine vasopressin) releases endogenous FVIII and VWF from storage sites and constitutes effective treatment for the milder forms of type 1 VWD.[3] However, plasma-derived concentrates are necessary for type 2 or 3 because desmopressin is usually ineffective or contraindicated. Plasma-derived FVIII concentrates, initially developed for the treatment of hemophilia A, also contain large amounts of VWF and are often used to treat desmopressin-unresponsive patients. In contrast to hemophilia A, FVIII deficiency is secondary to VWF deficiency in VWD. Therefore, the endogenous ability to synthesize FVIII is intact in these patients. For this reason, a high-purity VWF concentrate with low FVIII content was developed in France as early as 1989.[4] This concentrate, purified by ion-exchange and affinity chromatography and called Facteur Willebrand-LFB® (LFB, Les Ulis, France), was shown to be clinically effective in a large series of patients with type 2 or type 3 VWD and in desmopressin-unresponsive type 1 patients.[5–7] An improved version of this concentrate (Wilfactin®; LFB) was recently developed in order to further reduce the risk of transmission of non-enveloped viruses and prions. [8] The pharmacokinetic profiles of Wilfactin® and Facteur Willebrand-LFB® were illustrated to be comparable in a cross-over randomized trial, indicating that the VWF molecules are not altered by the three virus inactivation/removal steps included in the Wilfactin® manufacturing process.[9] This report groups the results of two distinct but comparable prospective, non-controlled, open-label, multicenter studies (European and French studies) designed to evaluate the efficacy and safety of Wilfactin® for the prevention or treatment of bleeding in 50 patients with clinically severe forms of VWD unresponsive to desmopressin.
Material and methods
Patients
For the European study, patients were recruited from March 1999 to July 2001 in five hemophilia centers (Frankfurt, Germany; Lille, France; London, UK; Malmö, Sweden and Milan, Italy) and for the French study from January 1999 to March 2003 in 12 French centers (Besançon, Brest, Caen, Dijon, Grenoble, Lille, Nantes, Paris-Cochin, Paris-Necker, Rouen, Toulouse, Tours). For each study, patients were eligible if they had an inherited form of VWD shown to not respond adequately to a standardized test infusion of desmopressin [10] and if they had previously needed treatment with plasma-derived products or red blood cells (RBC). For the European study, patients with severe VWD were enrolled if they had: (i) a clinically significant history of bleeding (more than one lifetime bleeding episode and (ii) at least one of the following laboratory abnormalities: skin bleeding time longer than 15 min, VWF ristocetin cofactor activity (VWF:RCo) less than 10 IU dL–1 or FVIII coagulant activity less than 20 IU dL–1. For the French study, patients were enrolled if VWF:RCo was less than 30 IU dL–1 or FVIII was less than 30 IU dL–1 for type 2 N VWD. In both studies, the presence of anti-VWF or anti-FVIII alloantibodies was an exclusion criterion. Twenty patients were included in the European study and 33 in the French study. Because three French patients participated consecutively in both studies, a total of 50 patients were treated in all. The VWD types were categorized according to the revised classification of Sadler et al.[2] The study protocol was approved by the Institutional Review Boards of each participating institution for the European study and of the coordinating centre (Caen) for the French study. Written informed consent was obtained from all patients or their custodians.
VWF concentrate
Wilfactin® is labeled in VWF:RCo for dosing accuracy (100 IU mL–1). FVIII coagulant activity is equal to or less than 10 IU per 100 IU of VWF:RCo. The mean VWF:RCo to VWF:Ag ratio for 16 batches prepared between 1998 and 2002 was 0.9 ± 0.1. Viral inactivation/elimination included three steps: solvent-detergent treatment, 35 nm nanofiltration and dry heating of the lyophilized product at 80 °C for 72 h. The manufacturing process as well as the viral reduction efficacy of each step were evaluated using model or pertinent viruses as described earlier.[8] Comparison of agarose gel electrophoresis of the concentrate and normal plasma showed only a limited defect of high-molecular weight VWF multimers and no signs of enhanced proteolysis (Fig. 1). A representative multimeric postinfusion pattern from a type 3 patient is shown in the same figure. When a priming dose of FVIII was needed, recombinant or plasma-derived FVIII concentrates were used according to the choice and practice of each centre. Wilfactin® was infused first, immediately followed by the FVIII product in the same venous access.
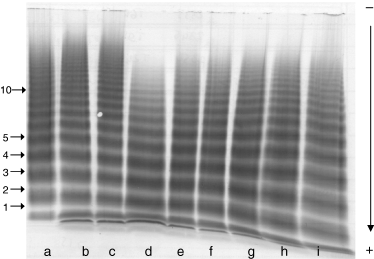
Sodium dodecylsulfate-agarose electrophoresis of von Willebrand factor (VWF) multimers (1.5% agarose gel) in a patient with type 3 von Willebrand disease treated with Wilfactin. The concentrate and normal plasma were also included for reference. (A) Wilfactin; (B, C) pooled normal plasma; (D–I) patient’s plasma obtained 24,9,6,3,1 and 0.5 h after infusion. Arrows numbered 1–10 indicate the VWF protomer and multimers. The pre-infusion patient plasma had no detectable VWF multimer (not shown)
Study design
These prospective multicenter studies were conducted to assess the efficacy and safety of the concentrate in different clinical situations: (i) treatment of spontaneous bleeding episodes (managed at home or requiring hospitalization); (ii) prevention of bleeding during scheduled or unscheduled surgical or invasive procedures; (iii) short-term prophylaxis in non-surgical situations with an increased risk of bleeding and (iv) secondary long-term prophylaxis in patients with frequently recurring bleeding episodes. In order to gather information on FVIII and VWF levels after administration of the concentrate and thus on the dosage regimens to be used for treatment, most of the patients included in the European study underwent a pharmacokinetics study beforehand (data reported in 25 patients[9]). In the framework of the French study, an in vivo recovery study was carried out in 17 of the 33 patients during a non-bleeding period. For this, the concentrate was administered at a dose of 60 IU kg–1 (100 IU kg–1 for type 3 patients). The VWF increment was calculated by measuring plasma VWF antigen (VWF:Ag) and VWF:RCo 60 min after the infusion.
Treatment regimens for spontaneous bleeding episodes Patients received Wilfactin® at an initial dose of 50 IU kg–1 in the French study and 60 IU kg–1 in the European study, depending on the current practice of the participating clinicians (Table 1). Investigators were allowed to give a concomitant priming dose of FVIII concentrate (30 to 40 IU kg–1) to patients with severe bleeding episodes and to those with baseline plasma FVIII levels below 20 IU dL–1. Subsequent infusions of the VWF concentrate, if required by the clinical situation, were also standardized (two daily infusions of 60 IU kg–1 for the first 48 h in the European study and 30–50 IU kg–1 in the French study). More details on the treatment regimens are provided in Table 1. Treatment efficacy was assessed using a subjective four-point scale (excellent, good, moderate or none). For bleeding episodes treated at home, efficacy was assessed by the patients or their custodians 6 and 24 h after the initiation of therapy. For patients in the hospital, efficacy was assessed at these same time intervals by the attending physicians.
| Clinical situation | French study | European study |
|---|---|---|
| Mucosal bleeding | ||
| 1st dose | Wilfactin® 50 IU kg–1, plus FVIII concentrate (30–40 IU kg–1) when baseline FVIII levels are < 20 IU dL–1 or in case of severe bleeding | Wilfactin® 60 IU kg–1 |
| Subsequent doses (if necessary) | Wilfactin® 30–50 IU kg–1 every 12 to 24 h, to be repeated as clinically necessary | Wilfactin® 60 IU kg–1, twice daily for 48 h, then daily or every other day to maintain VWF:RCo and FVIII levels of at least 30 IU dL–1 until clinically necessary |
| Muscle or joint bleeding | Dosing identical to mucosal bleeding | Dosing identical to mucosal bleeding plus FVIII concentrate in case of severe bleeding (dose adjusted to obtain a peak FVIII level of at least 80 IU dL–1) |
| Scheduled surgery | ||
| 1st pre-operative dose | Wilfactin® 50 IU kg–1, 12 to 24 h before surgery if baseline FVIII levels < 60 IU dL–1 | Wilfactin® 60 IU kg–1, 12 h before surgery if baseline FVIII levels are < 60 IU dL–1 |
| 2nd pre-operative dose | Wilfactin® 50 IU kg–1 1 h before surgery | Wilfactin® 60 IU kg–1, 1 h before surgery |
| Subsequent doses | Wilfactin® 50 IU kg–1, twice daily (3 times daily for type 3): dosage of injections adapted to target levels | Wilfactin® 60 IU kg–1, twice daily, then daily or every other day: dosage of injections adapted to target levels |
| Target levels | VWF:RCo and FVIII (monitoring once a day) at least 60 and 40 IU dL–1 respectively until complete healing | VWF:RCo and FVIII (monitoring once a day) at least 50 IU dL–1 until suture removal (7 to 10 days) |
| Unscheduled surgery | Same treatment regimen as above but first Wilfactin® dose infused 1 h prior to surgery together with a FVIII concentrate (dose adjusted to obtain a peak FVIII levels of at least 60 IU dL–1) | |
| Secondary prophylaxis | Wilfactin® 50–60 IU kg–1, two or three times per week | |
Treatment schedules during surgery and other invasive procedures One hour prior to surgery, all patients received Wilfactin® at a dose of 50 IU kg–1 in the French study and 60 IU kg–1 in the European study according to local clinical practices (Table 1). However, for patients with baseline FVIII levels below 60 IU dL–1, the treatment regimens differed depending on whether or not the surgery was scheduled. For scheduled procedures, patients received a VWF concentrate infusion (50 or 60 IU kg–1) 12 to 24 h prior to the procedure in order to allow endogenous FVIII to attain plasma levels of at least 60 IU dL–1 at the time of surgery. For unscheduled procedures, the pre-operative VWF dose was co-administered with a priming dose of FVIII in order to attain a FVIII peak of at least 60 IU dL–1. Subsequent infusions were scheduled and dosages were established as indicated in Table 1 and repeated as necessary at the appreciation of each investigator. Clinical efficacy was assessed by rating parameters such as per-operative blood loss, transfusion requirements and product consumption. For surgical procedures, response was evaluated twice: once by the surgeon/obstetrician upon completion of the surgery or delivery and once by the hematologist at the time of hospital discharge. Hemostasis was evaluated as follows: ‘excellent’, bleeding during surgery and the postoperative period similar to that expected for normal individuals; ‘good’, slightly excessive bleeding; ‘moderate’, moderately excessive bleeding; ‘none’, severe bleeding. Efficacy was also documented by biological assays for VWF:RCo and FVIII performed daily to ensure that the desired hemostatic levels were achieved and maintained. Invasive procedures (such as gastrointestinal endoscopies, hysteroscopies, coronary angiography, i.m. injections, intra-articular infiltrations and dental scaling) were considered minor procedures. Efficacy was defined by the absence of bleeding complications. Tranexamic acid was often used as adjuvant therapy for all type of procedures.
Short-term prophylaxis Patients with a prior history of bleeding in professional or leisure situations were treated with a single dose of 50 to 60 IU kg–1 less than 24 h before exposure to the situation associated with a risk of bleeding. Efficacy was defined by the absence of bleeding.
Long-term prophylaxis Secondary long-term prophylaxis was implemented to prevent spontaneous musculoskeletal or mucosal bleeding in patients with a history of these types of bleeds. Four patients were treated with 50–60 IU kg–1, two or three times per week. Efficacy was assessed by the reduction in the number of bleeding episodes.
Safety assessments
All patients were evaluated 1 and 3 months after the first administration, then every 6 months. Safety was assessed by routine clinical and laboratory evaluations, including serological markers for hepatitis A, B and C, HIV-1 and-2 and parvovirus B19. Screening for anti-VWF and anti-FVIII alloantibodies was only carried out in patients with type 3 VWD using a neutralization assay based upon the principle of the Bethesda assay.
Laboratory investigations
VWF:RCo, VWF:Ag and FVIII assays were performed by the laboratories of each participating center. In the great majority of them, VWF:RCo was measured by aggregometry using lyophilized platelets (von Willebrand assay; Behringwerke, Marburg, Germany) or formalin-fixed platelets from normal donors. Only one center used an enzyme immunoassay (RFF-VIII R/I; Seralabs, Loughborough, UK). VWF:Ag was measured using commercially available enzyme immunoassays. FVIII was measured by one-stage APTT-based assays in all centers, except in one that used a chromogenic assay. Skin bleeding time was measured by the Ivy horizontal incision method, except for one child who was tested with the Ivy three-point method. Anti-VWF antibody screening was based on neutralization of VWF:RCo measured using a method based on platelet agglutination/aggregometry.
Statistics
All the patients enrolled in the study who received at least one dose of VWF concentrate were included in the per protocol analysis. Descriptive statistics (mean and standard deviations or median and ranges) were used. In order to evaluate the impact of secondary long-term prophylaxis on the frequency of breakthrough bleeding episodes, the data were normalized to reflect a comparable time of prophylaxis (e.g. 1 year).
Results
General patient characteristics
Fifty patients (32 females and 18 males) were originally enrolled: 5 had type 1, 16 type 2A, 9 type 2B, 1 type 2 M, 1 type 2 N, and 18 type 3 VWD. Their median age was 37 years (range 5–81) and body weight ranged from 24 to 102 kg (median: 62). Three children were less than 15 years old. The baseline bleeding times and plasma levels of VWF:RCo, VWF:Ag and FVIII for each VWD type are shown in Table 2. The baseline levels of VWF:RCo were less than 10 IU dL–1 in 36 patients (72%) and those of FVIII were less than 20 IU dL–1 in 23 patients (46%). Bleeding time was greater than 15 min in 44 patients (88%). Three patients were lost to follow-up after surgery and did not complete the European study. Twenty-nine batches of the concentrate were used throughout the study.
| VWD type | No. of patients | Sex (Female/male) | Age median (range) | VWF:RCo (IU dL–1) median (range) | VWF:Ag (IU dL–1) median (range) | FVIII (IU dL–1) median (range) | Skin bleeding time (min) median (range) |
|---|---|---|---|---|---|---|---|
| European study | 20 | 13/7 | 40 (10–68) | < 10 (< 10–33) | 10 (< 1–52) | 24 (< 1–52) | > 20 (15–> 20) |
| type 3 | 7 | 4/3 | 59 (10–59) | < 10 (< 10–< 10) | < 1 (< 1–< 1) | 3 (< 1–4) | > 20 |
| type 1 | 4 | 3/1 | 44 (31–66) | < 10 (< 10–< 10) | 6 (2–24) | 29 (10–52) | > 20 (16–> 20) |
| type 2A | 4 | 2/2 | 38 (23–49) | < 10 (< 10–10) | 28 (18–35) | 39 (28–51) | > 20 (15–> 20) |
| type 2B | 3 | 2/1 | 41 (26–68) | 22 (14–33) | 41 (31–52) | 35 (35–37) | > 20 (19–> 20) |
| type 2 M | 1 | 1/0 | 19 | 17 | 27 | 39 | 20 |
| type 2 N | 1 | 1/0 | 24 | 12 | 12 | 6 | 15 |
| French study | 33 | 21/12 | 35 (5–81) | < 10 (< 10–29) | 20 (< 1–43) | 22 (< 1–63) | > 20 (5–> 20) |
| type 3 | 14 | 8/6 | 29 (11–66) | < 10 (< 10–< 10) | < 1 (< 1–< 1) | 3 (< 1–5) | > 20 |
| Type 1† | 1 | 0/1 | 5 | < 10 | 20 | 30 | not determined |
| type 2A | 12 | 10/2 | 46 (14–81) | < 10 (< 10–16) | 29 (20–43) | 33 (16–63) | > 20 (5–> 20) |
| type 2B | 6 | 3/3 | 34 (16–48) | < 10 (< 10–29) | 34 (23–42) | 35 (19–50) | > 20 (6–> 20) |
| Both studies* | 50* | 32/18 | 37 (5–81) | < 10 (< 10–33) | 20 (< 1–52) | 27 (< 1–63) | > 20 (5–> 20) |
- *Three type 3 patients (2 females, one male) were enrolled successively in both studies.
- †Patient classified by investigator as type 1 and reclassified subsequently as type 2A.
Recovery
The recovery study was performed in 17 of the 33 French patients upon initiation of the efficacy study (5 type 3, 1 type 1, 9 type 2A, and 2 type 2B). The median dose infused was 60 IU kg−1 (range 43–109). The mean incremental recovery per IU kg−1 infused was 1.7 ± 0.7 IU dL–1 (range 1.0–3.2) for VWF:RCo and 1.7 ± 0.4 IU dL–1 (range 1.1–2.6) for VWF:Ag.
Treatment of spontaneous bleeding episodes
Twenty-six patients (15 type 3, 1 type 1, 7 type 2A and 3 type 2B) received 733 infusions over 565 exposure days for 139 bleeding episodes (121 were treated at home and 18 in the hospital). Sixty percent of the episodes (n = 90) occurred in type 3 patients. The most frequent bleeding sites were in the musculoskeletal system (32%), the nasopharyngeal system (29%), the genital tract (21%) and gastrointestinal tract (8%), the oral cavity (9%) and others (1%). Most bleeds (41/44) in musculoskeletal sites occurred in patients with type 3 VWD. When the results of both studies were merged, the median infusion dosage was 41.8 IU kg–1 (range 14.2–74.5) and treatment required a median number of three infusions per episode (range 1–46) over 3 exposure days (range 1–37). Genital bleeding resulted in more infusions per episode than nasopharyngeal or oral bleeding (median 9.0 vs. 2.0 and 1.0), more exposure days (5.0 vs. 2.0 and 1.0) and larger doses (53.2 vs. 39.7 and 38.3 IU kg–1) (Table 3). However, 20/29 (69%) of the genital bleeding episodes were menorrhagia that occurred in one woman with type 3 VWD who did not receive contraceptive treatment. Tranexamic acid administered per os (between 500 and 5000 mg day–1 for one to several days) was a prescribed adjuvant treatment for 54 of 139 episodes. Eighteen episodes (13%) in 11 patients were severe enough to require hospitalization: six gastrointestinal and five genital bleeds (two ovarian cyst ruptures, three menorrhagia), six hematomas and one epistaxis. Four of the six gastrointestinal hemorrhages in three patients were associated with low hemoglobin levels that required transfusion of RBCs. Among the 18 severe bleeding episodes, 13 occurred in patients with baseline FVIII levels below 20 IU dL–1. The recommended concomitant priming FVIII dose was infused with the first VWF dose in seven of the 13 episodes (56%), because investigators were confident that the six remaining episodes could be managed with VWF only. Efficacy was excellent or good for 16 of the 18 (89%) episodes. The only two moderate responses involved a type 3 man who required RBCs during treatment for gastrointestinal bleeding and a type 2A woman who had severe uterine bleeding despite postsurgical treatment to prevent shedding of the cervical scab after conization of the cervix. Patients used Wilfactin® at home for the treatment of 121 minor bleeding episodes. The percentage of excellent/good responses to treatment 6 and 24 h postinfusion was 88% and 89%, respectively. Most of these episodes (86) occurred in patients with baseline FVIII levels below 20 IU dL–1 and, as prescribed, these patients self-administered a priming dose of FVIII together with the first VWF dose. However, in 40/86 episodes, the patient did not use FVIII. In this last group, efficacy was rated as excellent or good in 87% and as moderate in 13% 6 h postinfusion, then as excellent or good in 97% and moderate in 3% 24 h postinfusion. Eleven of 12 episodes of epistaxis treated with VWF alone in type 3 patients were rated as excellent or good after the first 6 h.
| Site of bleed | Musculoskeletal | Nasopharyngeal tract | Genital tract | Gastrointestinal tract | Oral cavity | Other | Total |
|---|---|---|---|---|---|---|---|
| No. patients | 13 | 8* | 6 | 6* | 8 | 1 | 26* |
| No. bleeding episodes | 44 (32%) | 41 (29%) | 29 (21%) | 11 (8%) | 13 (9%) | 1 (1%) | 139 |
| Total no. infusions | 291 | 93 | 258 | 72 | 18 | 1 | 733 |
| Median infusion dosage, VWF:RCo IU kg–1 (range) | 37.5 (24.3–57.3) | 39.7 (14.2–74.5) | 53.2 (35.6–68.3) | 41.0 (20.8–72.8) | 38.3 (24.9–71.1) | 17.1 | 41.8 (14.2–74.5) |
| Median no. of infusions per bleeding episode (range) | 4.0 (1–46) | 2.0 (1–7) | 9.0 (1–32) | 5.0 (1–18) | 1.0 (1–3) | 1 | 3.0 (1–46) |
| Median exposure days per bleeding episode (range) | 3.5 (1–37) | 2.0 (1–5) | 5.0 (1–21) | 3.0 (1–13) | 1.0 (1–2) | 1 | 3.0 (1–37) |
| No. of FVIII co-administrations with first VWF infusion | 25 | 9 | 11 | 6 | 2 | 0 | 53 |
| Excellent/good efficacy responses (%)† | 77.3 | 95.1 | 92.9 | 90.9 | 100 | 100 | 89.1 |
- *Two patients had a total of four and six hemorrhages during the European and the French study respectively.
- †Twenty-four hours postinfusion (home treatment) or at the time of hospital discharge (hospitalization).
Prevention of bleeding during surgery and invasive procedures
Forty-five patients were treated to prevent bleeding in 67 surgical and 43 invasive procedures. Two surgical procedures were excluded from the efficacy evaluation as a result of severe thrombocytopenia (23 × 109 L–1) in a patient with immune thrombocytopenic purpura and because the infusion protocol was not followed in the second case. Hence, the study product was evaluated in 108 procedures (21 in the European study and 87 in the French study) performed in 44 patients (14 type 3, 5 type 1, 14 type 2A, 9 type 2B, 1 type 2 M and 1 type 2 N). Forty-five percent of the procedures (n = 49) took place in type 3 patients. The 65 surgical procedures were orthopedic (n = 14), gynecological/obstetrical (n = 10), general (n = 7), dental (n = 14) and gastrointestinal, including transjugular liver needle biopsies (n = 20). Table 4 shows that orthopedic or gynecological/obstetrical procedures required a higher number of infusions than other procedures. Among the 108 surgical or invasive procedures, only 13 were unscheduled and co-administration of FVIII was given based on the clinical judgment of each physician. The remaining 95 scheduled procedures were performed without co-administration of FVIII. An extra dose of VWF concentrate was given 12 to 24 h before the procedure in 47/95 cases (median pre-operative FVIII level was 79 IU dL–1, range 26–210 from 33 available measurements). In the remaining 48 cases, baseline FVIII levels were considered high enough to ensure hemostasis (median pre-operative level was 59 IU dL–1, range 31–115 from 13 available measurements).
| Surgical procedures | Invasive procedures | Total | ||||||
|---|---|---|---|---|---|---|---|---|
| Orthopedic | Gynecological obstetrical | General | Dental | Gastrointestinal | Needle liver biopsy | |||
| No. patients | 11* | 8 | 6 | 12 | 9 | 8 | 17 | 44* |
| No. procedures | 14 | 10 | 7 | 14 | 12 | 8 | 43 | 108‡ |
| Total no. infusions administered** | 341 | 142 | 67 | 68 | 98 | 37 | 72 | 825 |
| Median infusion dosage†, VWF:RCo IU kg–1 (range) | 43.7 (30.4–68.7) | 41.3 (35.4–59.4) | 46.8 (33.2–69.3) | 53.2 (24.7–62.4) | 43.2 (25.5–67.6) | 46.4 (33.3–59.6) | 45.0 (11.1–100.0) | 45.5 (11.1–100.0) |
| Median no. infusions† (range) | 22.0 (3–65) | 15.0 (1–29) | 9.0 (1–18) | 2.5 (1–20) | 5.5 (3–21) | 4.0 (3–8) | 2.0 (1–4) | 3.0 (1–65) |
| Median exposure days† (range) | 19.0 (2–57) | 11.5 (1–18) | 7.0 (1–17) | 2.0 (1–10) | 4.0 (2–16) | 3.0 (2–4) | 1.0 (1–3) | 3.0 (1–57) |
| Excellent/good responses at hospital discharge (%) | 100 | 100 | 100 | 100 | 100 | 100 | no abnormal bleeding | |
| Median consumption during hospitalization (VWF:RCo IU kg–1)† | 577 | 411 | 245 | 100 | 187 | 191 | 63 | 133 |
- *Two patients underwent a total of three surgical or invasive procedures during the European and then during the French study.
- †Data included all peri-operative infusions and infusions given following surgical interventions, e.g. physical therapy after joint surgery.
- ‡Thirteen unscheduled and 95 scheduled procedures (48 were performed with only one pre-operative VWF infusion).
Pre-operative VWF:RCo levels were available from 47/108 procedures and ranged from 60 to 266 IU dL–1 (median, 134 IU dL–1). Postoperative levels (day 1 through to day 6) 12 or 24 h postinfusion (trough levels) are shown in Fig. 2. VWF:RCo ranged from 74 to 114 IU dL–1 and FVIII from 120 to 164 IU dL–1. These values were never outside the target values for the two measurements. Tranexamic acid was used as adjuvant hemostatic treatment for 27 of 65 surgical procedures, mainly for dental surgery. Antithrombotic prophylaxis with low-molecular weight heparin was implemented in 10 procedures, including seven orthopedic surgical procedures. The reported volume of blood loss ranged from 0 to 1074 mL, and peri-operative hemostasis for the 65 assessable surgeries was rated as excellent in 54 cases and good in 11 cases. Efficacy of the product in preventing excessive bleeding after nine orthopedic surgical procedures in type 3 VWD patients was evaluated as excellent in eight cases and good in one case. No abnormal bleeding was observed after 43 invasive procedures.
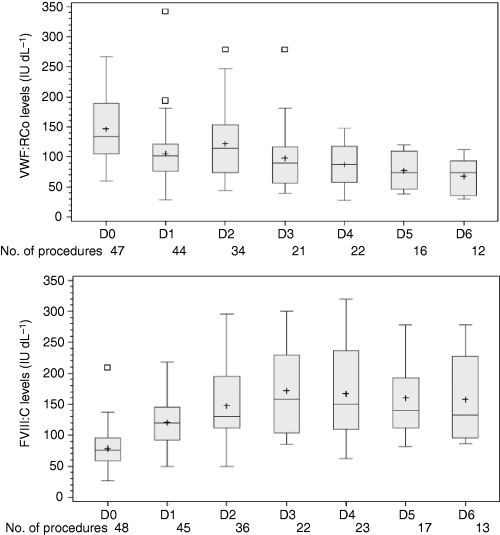
Median pre-operative levels of von Willebrand factor:ristocetin cofactor (VWF:RCo) (upper part) and factor VIII (FVIII) (lower part) (D0) and postoperative VWF trough levels from D1 (day after the surgery) to D6 after cumulating of the results of scheduled and unscheduled procedures from the French and European studies. For each day, upper and lower edges of box plots span from 25th to 75th percentiles; (–) indicates the median; (+) indicates the mean; (□) represents upper outlier (> 75th percentiles + 1.5 × IQR).
Three patients were transfused with packed RBCs. A 50-year-old patient with type 3 VWD who underwent abdominal surgery for hernia repair experienced blood loss of 500 mL and received 4 U of packed RBCs on postoperative day 9 while hemoglobin was 10.5 g dL–1. Another patient having undergone posterior spinal arthrodesis from the second thoracic vertebra to the sacrum with an autogenous tibial bone graft lost a total of 1074 mL of blood and thus received transfusion of RBCs. A third patient underwent hip arthroplasty and received 5 units of RBCs.
Short- and long-term prophylaxis
Six type 3 patients received prophylactic treatment before exposure to situations associated with a high risk of bleeding (sport activities or ovulation period). The median dose administered on 112 occasions was 45 IU kg–1 (range 14–74). Prevention was successful in each instance. Table 5 shows that four type 3 patients underwent secondary long-term prophylaxis for a median period of 17 months (range 3–25). The indications for this treatment were recurrent bleeding after ankle and knee prosthesis in two patients (age 28 and 48) and frequent joint and mucosal bleeding in two younger patients (age 11 and 15). During the regular infusions of 40 IU kg–1 (median) administered two to three times per week over 66.5 cumulative months, 17 bleeding episodes occurred (Table 5), usually more than 48 h postinfusion (59%) or after trauma (47%). Only three spontaneous hemorrhages of the 17 bleeds occurred within the first 48 postinfusion hours. Trough FVIII:C levels of 20 and 42 IU dL–1 were achieved at this interval in two patients.
| Gender/age in years | No. bleeds/year before prophylaxis | Prophylaxis duration, months | No. infusions/ week | Mean dose, IU kg–1 | Total number of bleeds during prophylaxis | No. bleeds/year during prophylaxis | |
|---|---|---|---|---|---|---|---|
| Patient 1 | Male/48 | 10 | 25.0 | 2 | 46 | 3 | 1.4 |
| Patient 2 | Female/15 | Unknown | 20.8 | 2–3 | 46–39 | 8 | 4.6 |
| Patient 3 | Female/11 | Unknown | 22.5 | 2–3 | 47–38 | 6 | 3.2 |
| Patient 4 | Male/28 | > 10 | 3.0 | 3 | 26 | 0 | 0 |
Safety
The 50 patients were followed over a median of 21 months (range, 1–42): 35 of them had at least 12 months of follow-up and 25 at least 2 years. At the end of the studies, the patients had received 6.7 million IU in 2389 infusions of the concentrate over 2046 exposure days. Of these, 15 had also received 150 000 IU of recombinant or plasma-derived FVIII. Four possible or probable treatment-related adverse events were observed in two patients (4%) and rated as mild (pruritus in opposite infused arms twice in one patient and chills twice in another). Two doubtful adverse events (vein inflammation and malaise) occurred in two additional patients. One patient discontinued treatment as a result of an accidental traumatic injury that led to death. No cases of clinically overt thrombosis were reported and no anti-VWF:RCo antibodies were detected. All patients with negative viral status for HCV (21 subjects) or HIV (48 subjects) at enrolment remained negative during the study. Expected seroconversion occurred in patients after HAV and/or HBV vaccinations. The only patient with negative parvovirus B19 status at enrolment and four previous exposure days was an 8-year-old child who developed serological signs of parvovirus B19 infection on the 30th month of follow-up after 13 exposure days. Based on biological data from a single infusion preceded by negative B19 tests performed twice after a previous infusion given months previously and followed on day 36 by negative tests for anti-B19 immunoglobulin M (IgM), the investigator judged the event to be as a result of natural infection and unrelated to the study drug.
Discussion
Wilfactin® is currently the only therapeutic concentrate containing almost exclusively VWF, the primary deficient protein in VWD. This study evaluated the efficacy and safety of Wilfactin® subjected to three viral safety steps to increase safety while preserving VWF structure and function. The concentrate complies with the goals of specific replacement therapy for the management of coagulation factor deficiencies, that is, infusion of the defective factor with the highest degree of purity while avoiding the administration of other unnecessary plasma proteins. Therefore, systematic co-administration of a FVIII product only for the first infusion of a VWF concentrate may be considered depending on the patient’s baseline plasma FVIII:C levels. This is recommended in order to avoid undue bleeding, particularly for emergency surgeries. However, if baseline FVIII:C levels are sufficient for hemostasis or if an immediate rise in FVIII:C is not necessary, particularly for mucosal bleeding, the physician may decide not to co-administer FVIII at the time of the first injection. For elective surgery, VWF treatment should start 12–24 h before surgical operation and should be repeated 1 h before the procedure. In this case, co-administration of FVIII is not required because endogenous FVIII:C levels have usually reached the general recommendation of 40 IU dL–1 before surgery, as shown in this study where physicians did not need to give extra FVIII after the second and after administrations of the VWF concentrate. When FVIII is co-administered, the VWF concentrate was infused first in order to optimize stabilization of the exogenous FVIII.
Data were gathered from a large cohort of patients with different types of VWD treated as part of two prospective studies with comparable designs and criteria to evaluate efficacy. Thus, the results could be merged. Most of the patients (72%) had clinically severe VWD with baseline VWF:RCo levels below 10 IU dL–1, including a very large number of patients with the most severe form of VWD (type 3, 15 patients treated for 90 bleeding episodes). Therefore, the patient cohort included in this prospective study is highly relevant for demonstration of the efficacy of a VWF product specially designed for VWD.
Recovery study
As expected, the results of the mean recovery analysis in 17 patients with various VWD types (1.7 ± 0.7 IU dL–1 per IU kg–1) were consistent with previous results observed in similar patients infused with the same dose during the pharmacokinetics study (1.9 ± 0.5 IU dL–1 per IU kg–1).
Treatment of spontaneous bleeding
The results of pharmacokinetics studies guided selection of the dosage regimens used for the management of spontaneous hemorrhages. These studies showed that FVIII levels rose from very low levels to hemostatic levels within approximately 6 h after VWF infusion, with sustained levels for up to 24 h, and that these levels were independent of the amount of VWF infused [9]. Therefore, in the case of soft-tissue bleeding, typically associated with relatively severe FVIII deficiency in patients with type 3, co-administration of FVIII at the time of the first VWF infusion was often required to stop bleeding and reduce the delay until hemostatic FVIII levels were reached. On the other hand, patients with qualitative VWF deficiencies (type 2) did not need co-administration because of their relatively high plasma levels of FVIII. On the whole, in spite of the fact that no FVIII product was needed in about half of the spontaneous bleeding episodes, efficacy of the VWF concentrate, even if inherently subjective, was excellent or good in the vast majority of cases and there were no reports of poor efficacy.
Prevention of surgical bleeding
For surgery, the dosage and duration of treatment were tailored to the type of procedures. In particular, orthopedic and genitourinary surgery required a larger number of infusions. The dosages of VWF:RCo administered per infusion (50 and 60 IU kg–1) were similar to those previously used in patients with VWD treated with concentrates containing both FVIII and VWF.[11–13] Mannucci et al. [12] and Thompson et al. [13] reported median doses of VWF:RCo of 40 and 53 IU kg–1 for maintenance infusions after the loading dose. In this study, for elective surgical or invasive procedures for which VWF was the sole replacement therapy, one dose of the concentrate was required 12 to 24 h before the preprocedural infusion in only half of the cases. For the remaining procedures, baseline FVIII levels were judged sufficient for the procedure. The high rate of success in the elective procedures suggests that the use of exogenous FVIII is not mandatory as the secondary rise of endogenous FVIII helped to maintain hemostatic levels (at least 50 IU dL–1) during the postoperative period.
Long-term prophylaxis
The effectiveness of secondary prophylaxis was assessed in only four patients with type 3 VWD, but three of them were treated for more than 1 year. The number of prophylactic infusions ranged from 2 to 3 per week and these regimens, guided by the bleeding pattern of each patient, yielded favorable results for the prevention of bleeding episodes. These findings concur with those of recent reports on long-term prophylaxis, indicating that, in VWD, this mode of treatment delivery is only needed in a very small proportion of patients with particularly frequent and/or severe bleeding episodes (hemarthrosis, gastrointestinal bleeding, epistaxis in children).[14,15]
Dose-response relationship
The study provides some information on the relationship between clinical response and laboratory measurements. In previous clinical studies, postinfusion plasma levels were not consistently monitored and the potential benefits or disadvantages of repeated administration of both FVIII and VWF could not be evaluated.[10] All procedures reached or exceeded pre-operative VWF:RCo levels of 60 IU dL–1. Pre-operative plasma FVIII levels of around 40 IU dL–1 or higher were attained regardless of the treatment regimen. The values after repeated VWF infusions during the postoperative period demonstrated that FVIII levels were maintained within the normal range (> 50 IU dL–1 and < 150 IU dL–1) without co-administration of additional exogenous FVIII (Fig. 2).
Adverse events
The incidence of adverse events was very low, occurring in 4% of patients. All the events reported were mild. Circulating anti-VWF antibodies are rare, but have been described in multi-transfused patients with type 3 VWD.[16] In this study, no inhibitor was reported in the 18 type 3 patients monitored for periods ranging from 6 to 42 months. Negative results from the serological test for anti-B19 IgM on day 36 after infusion is more consistent with B19 natural infection than with B19 transmission by a factor concentrate [17].
No thrombotic complications were recorded, even during major surgery. Sustained high levels of FVIII are a risk factor for venous thromboembolism[18,19] and high levels of VWF increase the risk for arterial thrombosis.[20] Venous thromboembolic complications have recently been reported in patients with VWD who attained very high levels of FVIII in association with closely spaced infusions of FVIII/VWF concentrates.[21–23] In this study, the use of a high-purity VWF concentrate containing little FVIII at the dosage chosen avoided the attainment and maintenance of very high FVIII levels while simultaneously keeping VWF:RCo within the normal range. These findings indicate that patients with VWD who previously experienced thromboembolic events during replacement therapy with FVIII/VWF concentrates, those expected to need repeated treatments and those at high risk of thrombotic complications (old age, orthopedic and cancer surgery) should preferentially be treated with a VWF concentrate with low FVIII content such as Wilfactin® at the dosages employed in this study.
Conclusions
Until recently, patients with VWD unresponsive to desmopressin were treated with concentrates containing unnecessarily high amounts of FVIII in addition to VWF, the primary deficient protein in these patients. FVIII/VWF concentrates were in general effective and safe, but had been associated with thrombotic complications in a small number of high-risk patients receiving repeated and closely-spaced infusions, particularly for major surgery [21–23]. This study provides evidence that the administration of a VWF concentrate with low FVIII content is effective and safe as it corrects both the primary VWF and secondary FVIII deficiencies, thus limiting FVIII use.
Addendum
A. Borel-Derlon, A. B. Federici, J. Goudemand, C. A. Lee, I. Scharrer, E. Berntorp, Z. Tellier, F. Bridey and P. M. Mannucci all participated in the study initiation and coordination. A. Borel-Derlon, A. B. Federici, V. Roussel-Robert, J. Goudemand, C. A. Lee I. Scharrer, C. Rothschild, E. Berntorp and C. Henriet contributed to the data collection. A. Borel-Derlon, A. B. Federici, C. Henriet and F. Bridey were involved in the analysis and interpretation of results. F. Bridey carried out the statistical analysis. A. Borel-Derlon, A. B. Federici and F. Bridey were the lead authors of the initial manuscript. A. Borel-Derlon, A. B. Federici, J. Goudemand, C. A. Lee, I. Scharrer, C. Rothschild, E. Berntorp, Z. Tellier, F. Bridey and P. M. Mannucci revised the draft manuscript. A. Borel-Derlon, A. B. Federici, V. Roussel-Robert, J. Goudemand, C. A. Lee, I. Scharrer, C. Rothschild, E. Berntorp, Z. Tellier, F. Bridey and P. M. Mannucci were responsible for the review and approval of the final manuscript.
The participating investigators in the Wilfactin® Study Group were as follows
University Hospital, Caen, France: A. Borel-Derlon; Cochin Hospital, Paris, France: N. Stieltjes, V. Roussel-Robert, N. Ounnoughène, P. Paugy; University of Lille 2, Hematology Institute, Lille, France: J. Goudemand; The Royal Free Hospital, London, United Kingdom: C. A. Lee, I. Nitu; University of Milan, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Italy: P. M. Mannucci, A. B. Federici; Necker Enfants Malades Hospital, Paris, France: C. Rothschild, M. Françoise Torchet; University Hospital, Frankfurt, Germany: I. Scharrer, G. Ludwig; University Hospital, Rouen, France: J. Yvonne Borg; University Hospital, Besançon, France: M. A. Bertrand; University Hospital, Toulouse, France: S. Claeyssens; Malmö University Hospital, Malmö, Sweden: E. Berntorp, S. Lethagen; University Hospital, Nantes, France: E. Fressinaud; University Hospital, Dijon, France: J. L. Lorenzini, F. Volot; University Hospital, Tours, France: C. Guérois; University Hospital, Grenoble, France: B. Polack; University Hospital, Brest, France: M. Vicariot, B. Pan-Petesch; University Hospital, Marseille, France: H. Chambost; University Hospital, Lyon, France: C. Négrier; University Hospital, Kremlin-Bicêtre, France: R. d’Oiron; University Hospital, Versailles, France: J. Peynet.
Acknowledgements
We thank B. Samor for supplying the gel electrophoresis of Wilfactin® and Claudine Mazurier for stimulating discussions.
Disclosure of Conflict of Interests
Supported by European Community grant BMH4-972 256 for a BIOMED 2 Project entitled “Optimizing Orphan Drug Therapy of Severe Forms of von Willebrand Disease” (4th Framework program for Research and Technical Development and Demonstration) (Coordinator: P. M. Mannucci). Three of the authors, F. Bridey, C. Henriet and Z. Tellier are employees of LFB but not investigators of the clinical study. LFB was the sponsor of this clinical research investigation.




