Local tissue-type plasminogen activator release in patients with ischemic stroke
The intravascular endogenous fibrinolytic system, with the key enzyme tissue-type plasminogen activator (t-PA), constitutes a potential protective system against atherothrombosis, as illustrated by the occurrence of spontaneous reperfusion in both coronary and cerebrovascular beds [1,2]. t-PA is released from endothelial cells both by a constitutive and a regulated pathway [3]. The latter is triggered by platelet and coagulation products formed during the clotting process, resulting in a rapid and large increase of local t-PA plasma concentrations [3]. It follows that if the capacity for regulated t-PA release is defective, in terms of either rapidity of onset or magnitude, the likelihood of timely spontaneous thrombolysis may be reduced.
Endothelial t-PA release rates cannot be inferred from systemic levels [4], as plasma levels of t-PA are highly dependent on hepatic clearance as well as on the plasma levels of its main inhibitor, plasminogen activator inhibitor type 1 (PAI-1) [5]. Our group has therefore developed experimental models, both in humans [6–9] and in animals [10,11], by which t-PA release can be monitored in vivo. Using this model, we have demonstrated markedly impaired t-PA release in patients with hypertension, a well-recognized risk factor for both ischemic stroke and myocardial infarction [12–14]. A similar impairment has been reported for smokers [15,16]. In the present study, we investigated the capacity for acute t-PA release in patients with ischemic stroke.
For this purpose, nine patients with ischemic stroke were recruited from the Sahlgrenska Academy Study on Ischemic Stroke [17]. Patients with aphasia were excluded because of difficulties in obtaining informed consent, and patients with paresis and/or sensory sequelae in the upper extremity after stroke event were excluded, as this could possibly influence local blood flow and t-PA release. Patients were selected so as to minimize confounding factors. Thus, only non-smoking male patients without diabetes and potential sources of embolism in the heart and/or the large cervical vessels were investigated. Patients with uncommon stroke etiology, such as vasculitis or hematologic diseases, were excluded. Patients were examined 6–40 months after the stroke incident. The etiologic subtype according to TOAST criteria [17,18] was small vessel disease in four patients, and cryptogenic stroke in five patients.
For each case, one male control without clinical atherothrombotic disease was recruited from the control group in SAHLSIS and from a study of endothelial t-PA release in subjects with hypertension [14]. Smokers and diabetics were excluded. Patients and controls were matched for age and presence/absence of hypertension. The study protocol was approved by the Ethics Committee of Göteborg University and conducted according to the Declaration of Helsinki. The nature, purpose and potential risks of the study were carefully explained to each subject before informed consent was obtained.
The experimental procedure has been described in detail previously [8,14]. In brief, regulated endothelial t-PA release was induced over the forearm vascular bed through constant infusion of substance P (8 pmol mL−1 at a rate of 1 mL min−1) in the brachial artery during 20 min. During pre-infusion and postinfusion baseline periods, blood samples were collected simultaneously from the brachial artery and vein. Blood samples were obtained from the brachial vein during substance P infusion at 1.5, 3, 6, 9, 12, 15 and 18 min. To avoid interruption of the infusion, arterial blood was obtained only at baseline and at the end of infusion. In-between arterial values were interpolated from these values [6]. Forearm blood flow (FBF) was determined after each venous blood sampling by venous occlusion plethysmography.
Arterial and venous plasma concentrations of total t-PA and PAI-1 antigen were determined by enzyme-linked immunosorbent assays (TintElize t-PA, Biopool International, Umeå, Sweden, and COALIZE PAI-1, Chromogenix, Haemochrom Diagnostica AB, Mölndal, Sweden). Plasma t-PA activity, i.e. the uncomplexed fraction of t-PA, was measured by a biofunctional immunosorbent assay (BIA, Chromolize tPA, Biopool International) calibrated against the international standard for t-PA (lot 86/670) and expressed in ng mL−1 using a specific activity of 0.60 IU ng−1 [9].
Forearm vascular resistance was calculated as the ratio of mean arterial pressure to FBF, and expressed in arbitrary resistance units. FBF was converted to forearm plasma flow (FPF) using individual hematocrit values. Net release or uptake rates for t-PA were calculated as the venoarterial concentration gradient multiplied by FPF. Total cumulative t-PA release in response to substance P was estimated for each individual as area-under-the-curve (AUC) from baseline until 20 min after terminatation of the infusion. In this analysis, negative areas (i.e. net uptake) were ignored.
Clinical and baseline characteristics for cases and controls are given in Table 1. Cholesterol levels were significantly lower in cases than in controls, probably because four cases, but none of the controls, were on lipid-lowering treatment with a statin. In both cases and controls, infusion of substance P induced a regulated release of t-PA, as reflected by a significantly increased forearm net release of t-PA antigen and t-PA activity (anova, P < 0.0001) (Fig. 1A,B). Net release rates of t-PA antigen and t-PA activity did not differ between the two groups. In controls, the average peak net release rates were 261.2 (61.5) and 146 (41.9) ng min–1 L–1 forearm tissue for t-PA antigen and activity, respectively, and in cases they were 258.7 (48.6) and 107.3 (24.5) ng min–1 L–1. Furthermore, no significant difference between the two groups was observed with regard to the cumulative amount (AUC) of t-PA secreted during stimulation. In controls, the mean AUCs were 2452 (534) and 1159 (316) ng L−1 tissue for t-PA antigen and activity, respectively, as compared to 2728 (501) and 1265 (272) ng L−1 tissue in cases.
| Cases, n = 9 | Controls, n = 9 | P-value | |
|---|---|---|---|
| Age (years) | 55 (4) | 55 (4) | 0.90 |
| Body mass index (kg m–2) | 28.1 (1.4) | 26.4 (1.0) | 0.28 |
| Serum cholesterol (mmol L−1) | 4.5 (0.2) | 5.9 (0.2) | < 0.001 |
| Plasma glucose (mmol L−1) | 5.9 (0.2) | 5.4 (0.2) | 0.19 |
| Systolic blood pressure† (mmHg) | 139 (7) | 141 (4) | 0.67 |
| Diastolic blood pressure† (mmHg) | 70 (3) | 70 (2) | 1.00 |
| Mean arterial pressure† (mmHg) | 97 (4) | 99 (2) | 0.75 |
| FBF (mL L−1 tissue) | 40 (7) | 36 (5) | 0.68 |
| FVR (arbitrary units) | 3.0 (0.5) | 3.3 (0.5) | 0.75 |
| Arterial t-PA antigen (ng mL−1) | 8.3 (1.2) | 8.2 (1.1) | 0.88 |
| Arterial t-PA activity (ng mL−1) | 0.51 (0.06) | 0.51 (0.08) | 0.69 |
| Arterial PAI-1 antigen (ng mL−1) | 26.6 (5.8) | 38.5 (13.7) | 0.70 |
| Net release of t-PA antigen (ng min−1 L−1 tissue) | 11.8 (5.9) | 14.4 (4.0) | 0.67 |
| Net release of t-PA activity (ng min−1 L−1 tissue) | 2.07 (0.36) | 3.65 (0.31) | 0.52 |
- Values are presented as mean and standard error of the mean (SEM). †Blood pressure was measured intra-arterially at the beginning of the experiment.
- FBF, forearm blood flow; FVR, forearm vascular resistance; t-PA, tissue-type plasminogen activator; PAI-1, plasminogen activator inhibitor type 1.
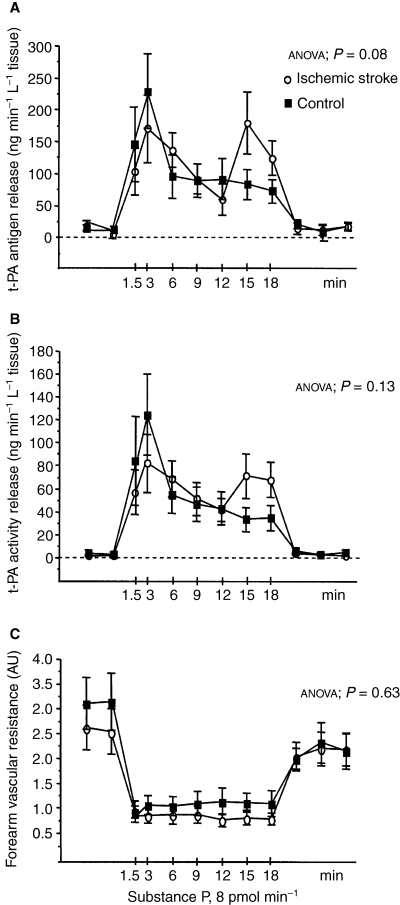
Net forearm vascular release of tissue-type plasminogen activator and vascular resistance in response to substance P stimulation. Net release rate of tissue-type plasminogen activator (t-PA) antigen (A), net release of t-PA activity (B) and forearm vascular resistance (C) at baseline and during substance P infusion in cases (open circles) and controls (closed squares). Mean and SEM values are given. P-values refer to statistical analysis by two-way anova (time × group interaction).
Temporal patterns of response differed between cases and controls. In contrast to controls, cases displayed a biphasic release pattern with a somewhat weaker initial rapid response (Fig. 1A,B). This resulted in a significantly prolonged average time to peak secretion for both t-PA antigen and activity in cases as compared to controls. The average time to peak secretion for t-PA antigen was 9.3 (2.1) min in cases vs. 4.3 (1.4) min in controls (P < 0.05), and the corresponding values for t-PA activity were 8.3 (2.0) min in cases vs. 4.0 (1.8) min in controls (P < 0.01).
This is, to the best of our knowledge, the first study on local t-PA release in patients with ischemic stroke. For ethical reasons and because of the nature of the perfused-forearm model, stroke patients with more severe sequelae could not be included. Hence, it is theoretically possible that subjects included in the study represent a subgroup that, because of preserved endothelial function and t-PA release, did not develop extended brain lesions. A reduced capacity for t-PA release in more severe cases of ischemic stroke cannot, therefore, be excluded.
Baseline characteristics were similar in cases and controls, except for s-cholesterol levels, which were significantly lower in cases. The effect of hypercholesterolemia and pravastatin treatment on local t-PA release has previously been investigated by Newby et al. [19]. In this study, neither hypercholesterolemia nor lipid-lowering treatment showed effects on local t-PA release over the forearm. Therefore, a major influence of the difference in cholesterol on our results is unlikely. However, it should be pointed out that patients were investigated 6–40 months after the acute event. One could therefore speculate that other pharmacologic and/or lifestyle interventions aimed at secondary prevention may have restored a reduced capacity for t-PA release that was present at the time of the acute event.
Despite this, we found differences between cases and controls with respect to temporal patterns of response. Patients with ischemic stroke appeared to display a biphasic release pattern characterized by a somewhat prolonged delay until peak secretory t-PA response as compared to controls. Could this finding possibly be of any biological importance? There is evidence indicating that t-PA-induced thrombolysis is more effective when it is initiated at an early stage during clot formation rather than for dissolving an existing thrombus [20–22]. It is therefore possible that not only the magnitude but also the temporal pattern of t-PA release upon stimulation is of importance. Thus, a slow onset with a prolonged delay until peak of the secretory response, as in the patients with ischemic stroke investigated here, might, despite a preserved magnitude of t-PA release, reduce the potential for a well-timed activation of the fibrinolytic response. However, the relative importance of the temporal pattern of regulated t-PA release has received little attention, and needs to be further explored, as many published studies on regulated t-PA release rely on single, rather than serial, blood samples after stimulation. Our finding of biphasic t-PA release in patients with ischemic stroke may therefore have important implications for the design of future studies, as it suggests that single blood samples taken during or after stimulation may be insufficient to characterize the t-PA response. Interestingly, our group recently reported that antihypertensive therapy not only restores the capacity for regulated t-PA release, but also improves the rapidity of the response in patients with primary hypertension [14].
In conclusion, this is, to the best of our knowledge, the first study on local t-PA release in patients with ischemic stroke. We found a preserved, but biphasic and somewhat delayed, t-PA response upon stimulation with substance P, possibly indicating a reduced ability for rapid activation of the fibrinolytic system in patients with ischemic stroke. However, the number of subjects included in the present study was small, and, because of experimental procedures, patients were limited to those with minor sequelae. Further studies in this area are encouraged.
Acknowledgements
The authors wish to thank H. Korhonen, C. Lundholm, A.-C. Lindahl and I. Eriksson for excellent technical support. This study was supported by the Swedish Research Council (K2005-71X-14 605-03A), grants from the Swedish state under the LUA/ALF agreement (ALFGBG 2964), the Swedish Heart Lung Foundation (20 050 603), the Swedish Stroke Association, the Swedish Hypertension Society, the Swedish Society of Medicine, the Göteborg Medical Society, the Rune and Ulla Amlövs Foundation for Neurological Research, the John and Brit Wennerström Foundation for Neurological Research, the Per-Olof Ahl Foundation for Research regarding Cerebrovascular diseases, and the Yngve Land Foundation for Neurological Research.
Disclosure of Conflict of Interests
The authors state that they have no conflict of interest.




